Involved free light chains <10 mg/L with treatment predict better outcomes in systemic light-chain amyloidosis
Funding information Amyloidosis and Myeloma Research Fund at Tufts; Amyloidosis Foundation; Cam Neely and John Davis Myeloma Research Fund; David and Barbara Levine (in memoriam); Demarest Lloyd Jr Foundation; John C. Davis Program for Myeloma and Amyloid at Tufts; Lavonne Horowitz Trust; Sidewater Family Fund; Werner and Elaine Dannheiser Fund for Research on the Biology of Aging of the Lymphoma Foundation
Systemic light-chain (AL) amyloidosis involves a marrow-based plasma cell clone which secretes immunoglobulin light chains that form toxic intermediates and complex deposits causing organ damage.1 Targeting the clone to reduce the production of light chains remains the cornerstone of treatment. Among the assays for detection of clonal light chains, measurement of serum free light chains (FLC) is the most sensitive and relevant tool. Hematologic response criteria for AL were re-evaluated in 2012 defining amyloid complete response (aCR) as the absence of monoclonal protein in serum and urine with normalization of FLC levels and ratio, and a very good partial response (VGPR) as a decrease in the difference between involved and uninvolved FLC (iFLC, dFLC) of <40 mg/L. Both aCR and VGPR were associated with significantly better survival compared to partial or no hematologic responses. The depth of the iFLC response played no role.2 Currently the optimal post-treatment iFLC remains undefined. Of relevance is that patients at diagnosis with a dFLC <50 mg/L have a lower plasma cell burden, less frequent cardiac involvement and better survival when post-treatment dFLC is <10 mg/L.3 Recent studies have also indicated that attaining a lower iFLC with treatment is beneficial.4, 5 With these observations in mind, we analyzed survival and organ-related outcomes by iFLC levels attained post-treatment in patients with AL who achieved aCR or VGPR irrespective of the type of therapy.
In a study approved by the Institutional Review Board (IRB), patients diagnosed with AL amyloidosis between 2005 and 2017 formed the derivation cohort if they achieved an aCR or VGPR with treatment. Laboratory evaluations were done monthly during therapy and every 3 months when not on treatment, and included serum and urine protein electrophoresis with immunofixations, serum FLC, NT-pro BNP, troponin-I and 24-hour urine. Hematologic response was assessed per the 2012 response criteria2 and organ responses were based on consensus criteria.2, 6 The Memorial Sloan Kettering (MSK) Cancer Center validation cohort included patients with AL diagnosed between 2007-2015 who achieved aCR or VGPR. Patients in both cohorts were separated based on their post-treatment iFLC best responses: <10 mg/L, 10-20 mg/L or > 20 mg/L. Descriptive statistics, Cox proportional-hazards and Kaplan-Meier survival analyses, were performed using MedCalc version 19.1.3 (MedCalc, Ostend Belgium).
One hundred and thirty-three patients with AL amyloidosis met inclusion criteria for the derivation cohort. Median age was 61 years (range, 35-81) and 59% were males (Table S1). Median bone marrow plasma cell burden at diagnosis was 10% (1-50), iFLC 135 mg/L (29-9780) and dFLC 123 mg/L (4-9770). Eighty-nine patients (66%) had cardiac and 108 (81%) had renal involvement. Overall, 63 patients (47%) underwent SCT, 90 (68%) had bortezomib-based initial therapy and 22 (17%) received daratumumab.
With a median follow up of 63 months, median OS (Figure 1A) was not reached for iFLC <10 mg/L and significantly exceeded the median OS of 96 and 121 months for iFLC 10-20 mg/L and > 20 mg/L groups, respectively. The independent verification cohort of patients (N = 48, Table S2) from MSK confirmed that overall survival was significantly longer with iFLC <10 mg/L (Figure 1B). In multivariate analysis, iFLC >10 mg/L, age > 60, and stage three cardiac or renal involvement (Figure 1C) were identified as independent predictors of shorter survival. Gender, dFLC or iFLC at diagnosis, post-treatment dFLC, or prior stem cell transplant did not independently impact survival. The impact of age on survival can be appreciated in Figure S1.
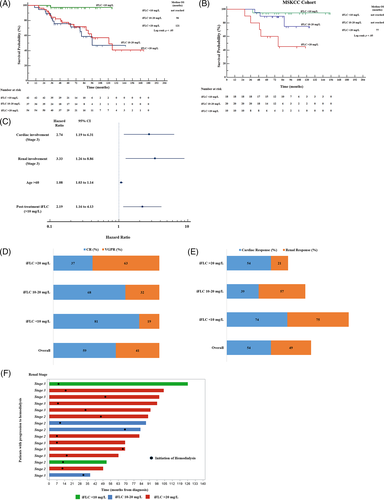
In the derivation cohort, the best iFLC response was <10 mg/L in 42 patients (32%), 10-20 mg/L in 37 patients (28%) and > 20 mg/L in 54 patients (40%). Median iFLC levels at the time of diagnosis were lower in the group with post-treatment iFLC <10 mg/L in comparison to the two other groups (75 mg/L vs 198 mg/L vs 250 mg/L, respectively, P < .01, Table S3). Stem cell transplant utilization was also higher in the iFLC <10 mg/L group (67% vs 43% vs 35%, P < .01). No age or gender differences were noted among the three groups. Of the 42 patients with iFLC <10 mg/L (Table S4), 25 patients achieved it in a median of 4 months (1–9) and 17 in a median of 31 months (12-80).
Of the 133 patients, 79 (59%) achieved aCR and 54 (41%) a VGPR at best response (Figure 1D). The aCR rates in the three iFLC groups were 81%, 68% and 37% (P < .05) respectively. Cardiac responses (Figure 1E) were seen in 54% of the patients and the rate was highest in the lowest iFLC group (74% vs 39% vs 54%, P = .08). Renal response (Figure 1E) was seen in 49% of patients and was similarly highest in the lowest iFLC group (75% vs 57% vs 21%, P < .05 respectively). While such responses are useful markers of therapeutic efficacy, it is also critical to note that the frequency of progression to dialysis dependence was 5%, 8% and 19% in the low, intermediate and high iFLC groups (Figure 1F).
Our results, validated externally, provide evidence that attaining a deeper iFLC response, ideally an iFLC level < 10 mg/L, permits recovery of involved organs and achieves over 95% survival at 120 months.7 The iFLC <10 mg/L group also had the highest rates of both cardiac and renal responses. The majority of patients who reached iFLC <10 mg/L did so within 12 months; an ad-hoc survival analysis of patients with early vs late iFLC <10 mg/L did not show a significant difference.
The impact of treatment on the pathologic protein in AL has been a major feature of response criteria as therapies have evolved over three decades. In the first major phase three trial to show a benefit in survival with anti-plasma cell therapy the response criteria included “disappearance of serum monoclonal protein or a reduction of more than 50 percent; disappearance of urinary monoclonal protein or a decrease of more than 50 percent”.8 The response criteria in that trial, conducted over a 15-year period of accrual with results published in 1997, also included organ responses. Subsequently, with the application of high-dose melphalan and mobilized peripheral blood stem cells to AL, the response criteria for hematologic and organ disease were separated.9 Hematologic response required scoring of serologic and marrow studies. Complete response followed the criteria then newly defined for myeloma, including negative serum and urine immunofixations and less than 5% marrow plasma cells. The FLC assay was first employed in response criteria without validation.6 Subsequently, with a test cohort of over 800 patients, the definitions of hematologic response in AL were refined with emphasis on FLC differentials and ratios; complete responses still, however, required negative immunofixations.2
Clearly the data we present evolve from these prior efforts at defining hematologic response in AL. Our emphasis, however, is on the pathologic light-chain protein and on its maximal reduction with therapy. Although a limitation of this study is the lack of prospective validation, a strength is that we include patients from the two best response categories as defined in the most recent revision of response criteria, the aCR and VGPR. We focus on the lowest iFLC at best response in these patients, noting that they were treated with a variety of therapies. The mix of categories and therapies in these cases is a strength of this analysis.
The basis of improvement in survival with low iFLC responses of limited duration is unclear; lower iFLC levels at the time of diagnosis plays a role, but iFLC <10 mg/L could also be a surrogate for sufficient reduction of clonal plasma cells to enable restoration of more normal humoral activity. The interplay between malignant and normal marrow-based plasma cells has been shown to be relevant to the evolution to myeloma from MGUS, and may be equally relevant to the therapeutic resolution of the hematologic aspects of AL.10 Persistence of iFLC >10 mg/L despite achieving aCR or VGPR with treatment might be linked to residual clonal bone marrow disease competing with restorative aspects of normal humoral activity. Correlative studies of iFLC with mass spectrometry and marrow minimal residual disease assessments may clarify this issue and enable further clinical research regarding novel transplant approaches as well as post-induction consolidation and maintenance therapies.
In conclusion, achieving iFLC <10 mg/L increased organ responses and extended survival in two independent cohorts. With the use of effective anti-plasma cell therapies such as anti-CD38 monoclonal antibodies, it is likely that more AL patients will achieve low iFLC levels post-treatment and will less frequently progress to organ failure. Therefore, re-evaluation of the hematologic response criteria and perhaps increased use of both organ response, and organ failure as endpoints in upcoming clinical trials should be considered.
ACKNOWLEDGMENTS
The authors thank the Division of Hematology-Oncology and Departments of Medicine and Pathology and Laboratory Medicine at Tufts.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
R.L.C., C.V. and A.G. designed the research; D.T., A.G., J.A., B.R., H.J.L., and R.L.C. collected data; A.G., H.J.L. and R.L.C. analyzed and interpreted the results; R.L.C., A.G. and N.S.S. designed the figures; A.G., and R.L.C wrote the manuscript. All authors critically reviewed the final draft prior to submission.
Open Research
DATA AVAILABILITY STATEMENT
Data are not publicly archived.




