Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: A retrospective analysis by the chronic malignancies working party on behalf of European society for blood and marrow transplantation (EBMT)
Nicola Polverelli and Katya Mauff have contributed equally. Donal McLornan and Ibrahim Yakoub-Agha have senior joint authorship.
Abstract
The role of spleen size and splenectomy for the prediction of post-allogeneic hematopoietic stem cell transplant (allo-HCT) outcome in myelofibrosis remains under debate. In EBMT registry, we identified a cohort of 1195 myelofibrosis patients transplanted between 2000-2017 after either fludarabine-busulfan or fludarabine-melphalan regimens. Overall, splenectomy was performed in 202 (16.9%) patients and its use decreased over time (28.3% in 2000-2009 vs 14.1% in 2010-2017 period). By multivariate analysis, splenectomy was associated with less NRM (HR 0.64, 95% CI 0.44-0.93, P = .018) but increased risk of relapse (HR 1.43, 95% CI 1.01-2.02, P = .042), with no significant impact on OS (HR 0.86, 95% CI 0.67-1.12, P = .274). However, in subset analysis comparing the impact of splenectomy vs specific spleen sizes, for patients with progressive disease, an improved survival was seen in splenectomised subjects compared to those patients with a palpable spleen length ≥ 15 cm (HR 0.44, 95% CI 0.28-0.69, P < .001), caused by a significant reduction in NRM (HR 0.26, 95% CI 0.14-0.49, P < .001), without significantly increased relapse risk (HR 1.47, 95% CI 0.87-2.49, P = .147). Overall, despite the possible biases typical of retrospective cohorts, this study highlights the potential detrimental effect of massive splenomegaly in transplant outcome and supports the role of splenectomy for myelofibrosis patients with progressive disease and large splenomegaly.
1 INTRODUCTION
Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by JAK-STAT hyperactivation leading to splenomegaly, constitutional symptoms, variable degrees of cytopenias and an inherent tendency to evolve into acute leukemia, associated with reduced survival.1-3 Despite many advances in novel therapies, allogeneic hematopoietic stem cell transplantation (allo-HCT) remains the only curative approach, able to overcome the adverse survival effect of negative biological features.4
The potential role of spleen size and splenectomy in modulating allo-HCT outcome has long been under debate. Previous work has suggested that “huge” splenomegaly may be associated with a higher risk of graft failure, a significant delay in hematopoietic recovery and an increased occurrence of poor graft function.5, 6 However, conflicting results have been reported in terms of both overall survival (OS) and relapse rates.7-10 By way of example, Bacigalupo et al documented a lower non-relapse mortality (NRM) and improved survival in patients undergoing allo-HCT with spleen diameters <22 cm.8 In 2017, Robin et al published a single center experience incorporating 85 MF allo-HCT recipients, demonstrating a 50% decrease in the risk of death and a slight, although non statistically significant, decrease in relapse incidence in splenectomised patients. In contrast, a European multicenter phase-II MF allo-HCT study demonstrated no impact of splenectomy on OS yet there was a 3.5-fold increase in relapse incidence in splenectomised compared to non-splenectomised subjects.9
Ruxolitinib (RUX) (Jakavi, Novartis, Basel, Switzerland), a JAK1/JAK2-inhibitor, has proven to be effective in reducing spleen size, and improving disease-related symptoms in the majority of MF patients, and is therefore frequently utilized in patients with symptomatic splenomegaly and/or constitutional symptoms prior to allo-HCT.2, 11-14 Positive benefits of therapeutic JAK-inhibition, together with the advances in transplant procedure, have likely indirectly contributed to the increasing number of MF patients undergoing allo-HCT in recent years.15 However, little information is available regarding patients who fail or progress while on RUX.16 Whether splenectomy in this category of patients could affect transplant outcome remains unknown. Based on available evidence, the 2015 ELN/EBMT expert panel did not recommend routine pre-transplant splenectomy but suggested a potential role in refractory splenomegaly on a case-by-case basis, taking into account also the potential significant morbidity and mortality of the surgical procedure.2, 17
Indeed, the risks of splenectomy are not negligible and the preclusion of subsequent transplant is a real concern.2, 17 In this respect, a reassuring message comes from the study presented by Bossard et al. The authors herein report a French nationwide experience on 530 MF patients registered in French bone marrow transplantation registry for unrelated donor search. In that cohort, splenectomy was associated to a higher probability of undergoing allo-HCT, particularly within 4 months from surgery, with no significant risk for pre-transplant death.18 With this in mind, the knowledge of long-term effects of splenectomy on allo-HCT outcome become crucial in pre-transplant MF management.
Therefore, these unresolved questions provided rationale for investigating the role of spleen size and splenectomy in determining outcome of a large cohort of MF patients undergoing allo-HCT and to understand if there is still a role for splenectomy prior to allo-HCT in the era of JAK-inhibitors.
2 METHODS
2.1 Study design
This is a registry-based study conducted on behalf of the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Information on the process of data registration in EBMT registry, management and extraction has been previously reported.19 Inclusion criteria were represented by (a) allo-HCT for chronic phase primary or post-polycythaemia vera (PPV)/post-essential thrombocythaemia (PET)-MF between 2000 to 2017, (b) age ≥18 years, (c) fludarabine-busulfan (FluBu) or fludarabine-melphalan (FluMel)-based conditioning regimens, and (d) availability of splenectomy status at time of allo-HCT. Patients with accelerated or blast phase disease20 were excluded from the analysis. The decision to include only patients receiving the above conditioning regimens relates to their extensive use for MF allo-HCT in Europe and to avoid heterogeneity across conditioning platforms which may compromise subsequent analyses.15, 21 Primary endpoint was characterization of the proportion and characteristics of patients receiving splenectomy before allo-HCT for MF in Europe and to evaluate the impact of splenectomy and spleen size on OS, NRM and cumulative incidence of relapse (CIR); secondary endpoints incorporated engraftment and cumulative incidence of acute (aGVHD) and chronic (cGVHD) GVHD. The study was conducted according to the declaration of Helsinki.
2.2 Study cohort
In the PROMISE EBMT registry, a total of 1195 MF patients were identified who fulfilled inclusion/exclusion criteria. The decision to undergo splenectomy was taken by the transplant physicians, according to local policy. Spleen length was measured by palpation as cm below left costal margin (LCM). Response to MF-directed treatment was assessed by the local center; patients were defined as having progressive disease if they demonstrated increasing splenomegaly, worsening of constitutional symptoms or new onset cytopenias. Myeloablative conditioning was defined as busulfan dose >8 mg/kg, or melphalan >140 mg/m2;22 the definitions of engraftment, aGVHD and cGHVD were as previously reported.23 No universal definition on relapse after transplant is still available for MF, all the patients recorded as relapsed in the Registry were taken into account for CIR analysis, as previously published.24 Donor chimerism analysis was not evaluated in the present study.
2.3 Statistical analysis
Categorical variables were summarized as counts and percentages and compared using the chi-square or Fisher's Exact test; continuous variables as median and IQR and compared using the Wilcoxon Rank-sum or Kruskal-Wallis tests, as appropriate. The OS was calculated from the date of transplant to the date of last follow-up or death, using the Kaplan-Meier method; the log-rank test was used to evaluate differences among subgroups. Cumulative incidence curves, based on competing risks models, were used to assess aGVHD, cGVHD, NRM and relapse, and subgroup comparisons were performed using Gray's test. Death without the event of interest was considered as a competing event in each case, together with second transplant for cGVHD. Median follow up was calculated as per the reverse Kaplan-Meier Method. Multivariate analysis was performed using Cox proportional hazards models for OS and cause-specific Cox models for all other outcomes. Primary multivariate models considered the role of splenectomy on the OS, CIR and NRM. Additional multivariate models were used to examine the impact of splenectomy vs specific spleen sizes (<5, 5-14, 15+) in non-splenectomised subjects for whom this information was available (a subset analysis). So, RUX exposure, disease stage (progressive vs responsive or stable), stem cell source (peripheral blood vs bone marrow), disease classification (secondary vs primary MF), T cell depletion, donor type (matched related donor [MRD] vs mismatched related donor [MMRD], MRD vs matched unrelated donor [MUD] and MRD vs mismatched unrelated donor [MMUD]), conditioning intensity (RIC vs MAC), patient age, interval from diagnosis to transplant and year of transplant were included in the models in each case. Interactions between splenectomy status and disease stage/RUX exposure/conditioning intensity were also considered. Statistical analysis was performed in R version 3.6.3 (29 February 2020), using the packages survival (version 3.1.12), cmprsk (version 2.2.9) and prodlim (version 13 November 2019).
3 RESULTS
3.1 Study cohort
Patients' characteristics are detailed in Table 1. The median follow-up was 55.3 months (interquartile range [IQR] 35.6-83.3). Information on International Prognostic Scoring System (IPSS)1 at MF diagnosis was available in 319/837 patients with Primary-MF (38.1%) and was stratified as low risk in 50 (6.0%), intermediate-one in 73 (8.7%), intermediate-two in 128 (15.3%) and high in in 68 (8.1%), missing in 518 cases (61.9%). Information on DIPSS for PMF25 and MYSEC-PM for PET/PPV-MF26 at transplant were not available in the large majority of patients, and therefore these scores were not evaluated in the present analysis. Karyotype was abnormal in 368 (42.6%) out of 863 patients with available cytogenetic analysis, in 332 patients the cytogenetic analysis was missing or failed. A driver mutation was present in 619 out of 950 analyzed cases (65.1%): JAK2 mutation was detected in 562 patients (80.3%), CALR in 50 cases (7.1%), MPL in seven cases (1.0%). A total of 569/1165 (48.8%) patients were defined as having progressive disease in the registry.
| Total cohort (n = 1195) | |
|---|---|
| Median age, y (IQR) | 57.9 (51.4-63.0) |
| Male sex, n (%) | 735 (61.5%) |
| Primary myelofibrosis, n (%) | 837 (70.0%) |
| IPSS category at diagnosis, n on 837 (%) | |
| Low/Intm-1 | 123 (14.7%) |
| Intm-2/High risk | 196 (23.4%) |
| Missing information | 518 (61.9%) |
| Splenectomy status pre-transplant, n (%) | 202 (16.9%) |
| Spleen size known, n on 546 (%) | |
| Spleen <5 cm below LCM | 145 (26.6%) |
| Spleen 5–14 cm below LCM | 266 (48.7%) |
| Spleen ≥15 cm below LCM | 135 (24.7%) |
| Ruxolitinib pre-transplant, n on 1067 (%) | 333 (31.2%) |
| Karnofsky >80, n (%) | 1059 (88.6%) |
| HCT-CI distribution, n on 888 (%) | |
| HCT-CI 0 | 485 (54.6%) |
| HCT-CI 1–2 | 199 (22.4%) |
| HCT-CI ≥3 | 204 (23.0%) |
| Conditioning regimens, n (%) | |
| FluMel-based | 931 (87.9%) |
| FluBu-based | 264 (22.1%) |
| Conditioning intensity, n on 1189 (%) | |
| Reduced intensity | 842 (70.8%) |
| Myeloablative | 347 (29.2%) |
| Donor relationship, n on 1151 (%) | |
| Identical sibling | 385 (33.4%) |
| Mismatched relative | 54 (4.7%) |
| Matched unrelated | 395 (34.3%) |
| Mismatched unrelated | 317 (27.6%) |
| Graft source, n (%) | |
| Bone marrow | 116 (9.7%) |
| Peripheral blood | 1075 (90.0%) |
| Cord blood | 4 (0.3%) |
| T cell depletion, n on 1183 pts (%) | 953 (80.6%) |
| In vivo T cell depletion | 935/953 (98.1%) |
| ATG | 867/935 (92.7%) |
| Alemtuzumab | 53/935 (5.7%) |
| Others combinations | 15/935 (1.4%) |
| Ex vivo T cell depletion | 18/953 (1.9%) |
| GVHD prophylaxis in non T cell depleted transplant, n on 228 (%) | |
| CnI + Methotrexate | 67/228 (29.1%) |
| CnI + Mycophenolate | 84/228 (36.5%) |
| Post-transplant cyclophosphamide | 47/228 (20.4%) |
| Others combinations | 30 (13.0%) |
| Interval between dx and allo-SCT, mo (IQR) | 44.8 (12.6-128.4) |
| Median follow-up, mo (IQR) | 55.3 (35.6-83.3) |
- Abbreviations: ATG, anti-thymocyte globulin; CnI, Calcineurin inhibitors; FluBu, Fludarabine-Busulfan; FluMel, Fludarabine-Melphalan; HCT-CI, Hematopoietic-stem cell transplantation-specific comorbidity index; Intm-1, intermediate-1; Intm-2, intermediate-2; IPSS, International Prognostic Score System; IQR, inter-quartile range.
3.2 Allo-HCT outcome
At last follow up, a total of 559 patients (46.8%) had died. Cause of death was known in 543 cases (97.1%) and was related to relapse/progression in 93 (17.1%) and NRM in 450 (82.9%) cases, respectively, namely: GVHD (n = 163, 36.2%), infection (n = 158, 35.1%) and others (n = 129, 28.7%).
Estimated 12-month, 36-month and 60-month OS was 70% (95% CI 68%-73%), 57% (54%-60%) and 51% (48%-54%) for the whole cohort, respectively. In 1131 (94.6%) evaluable patients, the CIR and NRM at 12, 36, and 60 months was 14% (12%-16%), 21% (18%-23%), 24% (21%-26%) and 22% (19%-24%), 29% (27%-32%) and 32% (29%-35%), respectively. Cumulative incidence of neutrophil (≥0.5 × 109/L) and platelet (≥20 × 109/L) recovery was 85% (83%-87%) at 28 days and 80% (77%-82%) at 100 days, respectively. Primary graft failure was encountered in 4.9% of cases, respectively. The cumulative incidence of grade 2-4 and grade 3-4 aGVHD was 30% (27%-33%) and 14% (12%-16%) at day 100; 36-month cumulative incidence of cGVHD was 44% (41%-47%). A total of 119 (10.0%) patients received a second transplant, donor lymphocytes were infused to 164 patients (13.7%).
3.3 Splenectomy prior to Allo-HCT - Cohort details and outcome
Overall, 202 patients (16.9%) underwent splenectomy before transplant. Table 2 summarizes clinical characteristics according to splenectomy status. Notably, the proportion of patients undergoing splenectomy prior to allo-HCT decreased over time (28.3% from 2000 to 2009 and 14.1% from 2010 to 2017, the numbers decreased further after year 2012 when RUX was widely available) (Figure S1 in Appendix S1). Patients undergoing splenectomy were significantly younger (P = .001) and had a longer interval from diagnosis to transplant (P = .009) compared to the non-splenectomised cohort. A greater number of splenectomised patients had a lower IPSS score at diagnosis (P = .001), and had both a lower Karnofsky Performance Status (P = .005) and higher hematopoietic stem cell transplantation-specific comorbidity-index (HCT-CI) score (P = .002) at transplant. T cell depletion was employed more in the non-splenectomised group (P < .001). Prior RUX-exposure was present only in 20.0% of splenectomised compared to 33.5% of non-splenectomised patients (P = .001).
| No Splenectomy (n = 993) | Splenectomy (n = 202) | P | |
|---|---|---|---|
| Median age, y (IQR) | 58.3 (51.9-63.4) | 56.6 (49.4-61.2) | .001 |
| Male sex, n (%) | 609 (61.3%) | 126 (62.4%) | .842 |
| Primary myelofibrosis, n (%) | 687 (69.2%) | 150 (74.3%) | .177 |
| IPSS category at dx, n (%) | |||
| Intm-2/High risk | 180 (18.1%) | 16 (7.9%) | .001 |
| Ruxolitinib pre-transplant, n on 1067 (%) | 297 (33.5%) | 36 (20.0%) | .001 |
| Karnofsky >80, n (%) | 892 (89.8%) | 167 (82.7%) | .005 |
| HCT-CI distribution, n on 888 (%) | |||
| HCT-CI 0 | 423 (55.9%) | 62 (47.3%) | .002 |
| HCT-CI 1–2 | 176 (23.2%) | 23 (17.6%) | |
| HCT-CI ≥3 | 158 (20.9%) | 46 (35.1%) | |
| Conditioning regimens, n (%) | |||
| FluMel-based | 765 (77.1%) | 166 (82.2%) | .245 |
| FluBu-based | 228 (22.9%) | 36 (17.8%) | |
| Conditioning intensity, n on 1189 (%) | |||
| Reduced intensity | 699 (70.8%) | 143 (70.8%) | .999 |
| Myeloablative | 288 (29.2%) | 59 (29.2%) | |
| Donor relation, n on 1151 (%) | 197 | ||
| Identical sibling | 306 (32.1%) | 79 (40.1%) | .120 |
| Mismatched relative | 43 (4.5%) | 11 (5.6%) | |
| Matched unrelated | 334 (35.0%) | 61 (30.9%) | |
| Mismatched unrelated | 271 (28.4%) | 46 (23.3%) | |
| Graft source excluding cord, n (%) | |||
| Bone marrow | 90 (9.1%) | 26 (13.1%) | .109 |
| Peripheral blood | 902 (90.9%) | 173 (86.9%) | |
| T cell depletion, n on 1183 pts (%) | 820 (83.5%) | 133 (66.52%) | <.001 |
| Interval between dx and allo-HCT, months (IQR) | 42.7 (11.6-123.5) | 51.7 (18.5-155.5) | .009 |
- Note: Chi-square test was used to assess differences among categorical variables and Wilcoxon Rank sum test for continuous variables.
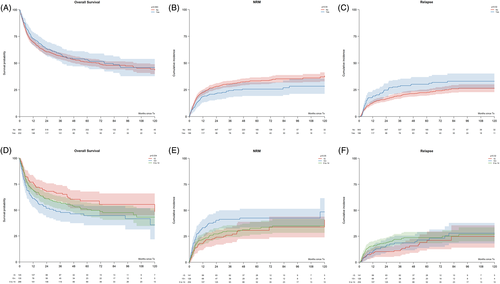
No significant differences were observed in OS between splenectomised and non-splenectomised patients (P = .563) (Figure 1A). The NRM was significantly lower in splenectomised patients (P = .04) (Figure 1B), even though the causes of NRM were reasonably similar according to splenectomy status (GVHD 24.3% vs 38.4%, infection 41.4% vs 33.9%, others 34.3% vs 27.6%, in splenectomy and non-splenectomy cohorts, respectively, P = .08).
The lower NRM was confirmed in patients receiving RIC but not MAC regimens (36-month NRM of 21% [14%-27%] vs 31% [27%-35%], P = .01 and 34% [21%-46%] vs 29% [23%-35%], P = .85, in splenectomised and non-splenectomised patients, in the RIC and MAC setting, respectively). Among the RIC patients, no significant differences were recorded according to splenectomy status in terms of infections (P = .999) or primary graft failure-related deaths (P = .13).
Of note, CIR was higher in the splenectomised cohort (P = .02) (Figure 1C). The 36-month CIR was 29% (21%-37%) vs 20% (17%-23%) (P = .09) in RIC and 26% (14%-38%) and 17% (12%-21%) (P = .08) in MAC setting for splenectomised and non-splenectomised patients, respectively. Splenectomised patients had faster neutrophil and platelet engraftment. The cumulative incidence of neutrophil and platelet recovery was 89% (84%-93%) vs 84% (81%-86%) at 28 days (P < .001) and 85% (79%-91%) vs 79% (76%-81%) at 100 days (P < .001) in the splenectomised and non-splenectomised cohorts, respectively. On the contrary, the rate of primary graft failure (4.9% vs 5.0%, P = .98) was almost identical between the splenectomy and non-splenectomy categories, respectively.
Splenectomy had no apparent effect on either aGVHD or cGVHD occurrence. Three-month cumulative incidence of grade 2-4 and grade 3-4 aGVHD was 25% (19%-31%) vs 31% (28%-34%) (P = .20) and 10% (6%-15%) vs 15% (13%-17%) (P = .11) in splenectomised vs non-splenectomised subjects, respectively. Similarly, the 36-month cumulative incidence of cGVHD was likewise in both cohorts (45% [38%-53%] vs 43% [40%-47%], P = .70).
3.4 Spleen size before transplant - Characteristics and outcome
Overall, 546 out of 993 (55.0%) non-splenectomised patients had information on spleen length at the time of transplant. Table S1 in Appendix S1 summarizes the characteristics of the cohort according to spleen size at transplant. A greater proportion of patients with spleen ≥15 cm received T cell depletion (P = .017) and were classified with progressive disease at time of transplantation (P = .005). More patients with less voluminous splenomegaly belonged to the higher HCT-CI category (P = .053). Notably, no other significant differences were observed in terms of disease, RUX exposure and transplant features.
Increasing spleen size at the time of transplant was significantly associated with worse OS (P = .034) (Figure 1D). Of note, this finding was related to an excess of NRM noted in the larger spleen category (P = .05) (Figure 1E), while no significant differences were observed between different spleen size groups in term of CIR (P = .32) (Figure 1F).
The effect of spleen size on OS was no longer significant in subset analysis of patients undergoing MAC. In this setting (n = 162), the 36-month OS probability was 73% (59%-87%) in the <5 cm group, compared to 65% (55%-76%) and 62% (45%-79%) of patients in the 5-14 cm and ≥15 cm groups respectively (P = .917). In contrast, for patients undergoing RIC (n = 382), the 36-month OS probability was 64% (54%-73%) vs 54% (46%-61%) vs 44% (34%-55%) for increasing spleen size (P = .021). Moreover, patients submitted to transplant with less bulky splenomegaly had faster engraftment: the 28-day cumulative incidence of neutrophil recovery was 87% (82%-93%) for patients with spleen <5 cm compared to 81% (76%-86%) and 82% (75%-89%) in patients with larger splenomegaly (P = .02); similarly, 100-day cumulative incidence of platelet recovery was 85% (79%-91%) vs 79% (74%-84%) vs 66% (57%-74%) (P < .001) for increasing spleen size. Splenomegaly had no significant effect on the cumulative incidence of grade 2-4 aGVHD (P = .37), grade 3-4 aGVHD (P = .17), or cGVHD (P = .42).
3.5 Comparison of splenectomy and spleen categories
As splenectomy is usually reserved for patients with massive splenomegaly, we decided to compare allo-HCT outcomes in splenectomised patients and patients undergoing transplant with a spleen palpable ≥15 cm. The 36-month OS probability was 59% (52%-66%) in splenectomised patients compared to 49% (40%-57%) in patients with spleen palpable ≥15 cm (P = .064). Comparing splenectomy vs the largest spleen category, we observed a mild increase in relapse incidence among splenectomised patients (36-month CIR 28% [22%-35%] vs 19% [11%-26%], P = .10), counterbalanced by a significant reduction of NRM (36-month NRM 24% [18%-30%] vs 41% [33%-50%], P < .001) (Figure S2A-C in Appendix S1).
3.6 Multivariable analysis of variables associated with overall survival, RI and non-relapse mortality
In the multivariate analysis, MRD vs MMRD (HR 0.57, 95% CI 0.34-0.96, P = .036), MRD vs MMUD (HR 0.67, 95% CI 0.52-0.85, P = .001), stable/responsive disease stage (HR 0.83, 95% CI 0.68-0.99, P = .047) and younger patient age (HR 0.75, 95% CI 0.67-0.85, P < .001) were associated with improved survival, while splenectomy was not found to affect OS (HR 0.86, 95% CI 0.67-1.12, P = .274) (Figure 2A). NRM was reduced by splenectomy (HR 0.64, 95% CI 0.44-0.93, P = .018), T cell depletion (HR 0.63, 95% CI 0.46-0.87, P = .004), MRD compared to MUD and MMUD (HR 0.69, 95% CI 0.51-0.94, P = .021; HR 0.56, 95% CI 0.41-0.76, P < .001, respectively) and younger patient age (HR 0.79, 95% CI 0.68-0.91, P = .001) (Figure 2B). Conversely, splenectomy was associated with a higher risk for disease relapse (HR 1.43, 95% CI 1.01-2.02, P = .042), together with T cell depletion (HR 1.87, 95% CI 1.20-2.93, P = .006), older age (HR 1.26, 95% CI 1.07-1.48, P = .007) and earlier transplant year (HR 1.05, 95% CI 1.01-1.10, P = .008) (Figure 2C).

With the aim to evaluate the impact of splenectomy compared to different spleen sizes at transplant, we undertook a subset analysis including only non-splenectomised patients with available information on spleen size at transplant and splenectomised patients. In this cohort of 748 patients (62.3%), T cell depletion (HR 0.70, 95% CI 0.51-0.96, P = .027) and younger age (HR 0.78, 95% CI 0.68-0.88, P < .001) were associated with superior survival; in contrast MMUD was associated with a worse outcome compared to MRD (HR 1.53, 95% CI 1.11-2.09, P = .009). For patients with progressive disease, there was a significant effect of spleen size ≥15 cm compared to <5 cm (HR 2.57, 95% CI 1.49-4.44, P = .001), OS was significantly improved in splenectomised patients compared to those with spleen ≥15 cm (HR 0.44, 95% CI 0.28-0.69, P < .001) (Figure 2D). For NRM, as for OS, patients with progressive disease had significantly worse outcome when spleen was palpable ≥15 cm compared to <5 cm (HR 2.54, 95% CI 1.34-4.81, P = .004). Splenectomised patients had a significantly improved outcome compared to spleen size ≥15 cm (HR 0.26, 95% CI 0.14-0.49, P < .001), and 5-14 cm cohorts (HR 0.51, 95% CI 0.28-0.94, P = .03) (Figure 2E). The risk for relapse was higher in splenectomised patients compared to those undergoing transplant with spleen size <5 cm (HR 2.08, 95% CI 1.21-3.59, P = .008); however, no significant differences were observed when splenectomy was compared to spleen sizes 5-14 cm and ≥15 cm (HR 1.39, 95% CI 0.90-2.12, P = .134, and HR 1.47, 95% CI 0.87-2.49, P = .147, respectively). (Figure 2F).
3.7 Role of splenectomy and spleen size in ruxolitinib-exposed patients
Overall, 333 (31.2%) evaluable patients included in this analysis received RUX prior to allo-HCT. By multivariate analysis, a modest survival benefit was seen in RUX-exposed subjects (HR 0.81, 95% CI 0.64-1.02, P = .067), with no substantial differences in NRM (HR 0.80, 95% CI 0.61-1.07, P = .13), and relapse incidence (HR 0.95, 95% CI 0.67-1.35, P = .772).
A total of 36 (10.8%) out of 333 RUX-exposed patients were splenectomised and 297 underwent transplant without splenectomy. Spleen size information was available in 184 out of 297 (62.0%) RUX-treated patients: specifically, 49 (26.6%) patients had spleen palpable <5 cm, 89 (48.4%) 5-14 cm and 46 (25.0%) ≥15 cm. Interestingly, patients with a spleen length <5 cm had the best outcome with a 36-month OS of 72% (59-85%) vs 54% (44%-65%) of 5-14 cm and 47% (32%-61%) of spleen ≥15 cm cohorts (P = .035). In patients on RUX, the 36-month OS was 71% (55%-86%) for patients undergoing splenectomy compared to 47% (32%-61%) in patients with spleen size ≥15 cm (P = .042). The 36-month NRM was 24% (9%-38%) in splenectomised patients vs 42% (28%-57%) in those with spleen size ≥15 cm (P = .08), with almost identical CIR (36-month CIR 18% [5%-31%] in splenectomy vs 19% [7%-31%] in spleen size ≥15 cm, P = .66) (Figure S3A-C in Appendix S1).
4 DISCUSSION
The presence of huge splenomegaly in MF candidates prior to allo-HCT is a frequent cause of concern for transplant physicians.2, 15 Fear of delayed hematological recovery with potential increase in NRM leads a number of transplant physicians to consider splenectomy as a fundamental step prior to allo-HCT. However, no definitive nor prospective data supports this approach before transplant and the impact of spleen size and splenectomy on overall allo-HCT outcome requires clarification.
Despite the considerable surgical risk,27 European transplant physicians considered splenectomy in a significant proportion of patients (around 17%), likely in an attempt to improve engraftment and possibly influence survival. Interestingly, we documented a marked decrease of the use of splenectomy over time. This observation could be partially related to the availability of novel therapeutics: the JAK-inhibition mediated spleen response could have influenced the decision to accept the risks of splenectomy only in a minority of refractory and symptomatic patients,11, 28, 29 as potentially indicated by a longer interval diagnosis-transplant and worse KPS distribution in the splenectomised cohort. Moreover, a higher proportion of non-splenectomised patients belonged to intermediate-2/high risk IPSS categories at diagnosis, therefore it could be hypothesized that such patients were referred to transplant procedure earlier, before the development of voluminous splenomegaly.
In our population, splenectomy was associated with 36% decrease of NRM. This beneficial effect of splenectomy was observed in the overall cohort despite a higher proportion of splenectomised patients having higher HCT-CI and lower KPS, two recognized poor prognostic factors for allo-HCT outcome.30-32
However, an increased relapse incidence in the splenectomised cohort was encountered, with no resultant beneficial effect on OS, irrespective of conditioning intensity. Relapse was documented in multivariate analysis to be influenced by spleen removal, older recipient age and T cell depletion together with transplants occurring in the earlier era. Conversely, OS was influenced by recipient age, presence of sibling donor and disease status, as has been shown previously.33-40
Taking into account the prominent use of splenectomy in massive splenomegaly patients, we conducted a subset analysis by stratifying the study cohort according to spleen size at time of allo-HCT and then comparing these different spleen size categories with the splenectomy cohort. In accordance with Bacigalupo report,8 we confirmed the negative significance of enlarged splenomegaly. More bulky splenomegaly was associated with higher NRM with survival disadvantage, without an impact on relapse. Notably, MAC regimens seemed to overcome the negative effect of splenomegaly, thus indirectly supporting the ELN/EBMT recommendation to use more intensive conditioning in patients with advanced disease and good performance status.2 In the subset analysis evaluating splenectomy vs specific spleen size groups, we documented, among progressive disease patients, lower NRM in those receiving splenectomy before transplant compared to the ones with spleen size >5 cm. Importantly, we did not observe a significant increase of relapse incidence in splenectomy vs spleen size >5 cm categories. Lastly, a survival benefit among progressive patients was seen for splenectomised compared to patients undergoing transplant with spleen ≥15 cm, thus providing evidence of potential beneficial role of splenectomy in progressive patients with massive splenomegaly. However, caution is needed when these results are extended to conventional nonTcell depleted RIC transplants, taking into account the high prevalence of T cell depleted transplants in our cohort.
Finally, we considered the impact of splenectomy and spleen size according to RUX use prior to transplant. As documented in the non-transplant setting for RUX responding patients,14, 41, 42 patients achieving spleen length <5 cm had an improved transplant outcome, with a borderline, although not-statistically significant, survival benefit in RUX-treated patients. The potential positive impact of JAK-inhibition on splenomegaly and disease-related symptoms,13, 43 may well have led to improvement of performance status and linked with this observation. Globally, these findings could suggest titration of RUX to maximum tolerated dose in the effort to augment splenic responses before transplant. Also, timing of transplant should be considered, possibly at the time of best response while on RUX, and not delaying allo-HCT at the loss of response or disease progression. Indeed, the prognosis after RUX failure is extremely poor,44 and transplant efficacy is significantly impacted in this setting.16 For those progressive patients on RUX with huge splenomegaly, splenectomy might represent a useful option. Splenic irradiation could be a feasible alternative to surgery in patients with comorbidities and high operative risk, however its efficacy still needs to be further evaluated.45, 46
One relevant finding of this study is the possible association between splenectomy and relapse risk. This topic has been under frequent debate. A previous French experience showed a modest favorable effect of splenectomy on relapse risk;7 conversely, in a prospective phase-II EBMT trial investigating FluBu+ATG RIC platform, splenectomy was associated with a >3-fold increase in relapse incidence,9 whereas other research groups have demonstrated no effect on relapse risk.6, 8, 10 Certain biological and clinical evidence can sustain the hypothesis that splenectomy per se can affect disease recurrence and progression after transplant. In MF, the spleen plays a pivotal role in disease pathogenesis and maintenance: additional cytogenetic and molecular abnormalities occurring in post-splenectomy spleen compared to bone marrow analysis were detected,47, 48 and leukemic transformation arising primarily from spleen hematopoiesis has been published.49 Certain groups have suggested that spleen removal could augment the circulation of MF cells harboring higher genetic complexity and their homing in the bone marrow.47 In 1998, an Italian cooperative group reported by propensity score analysis a >2-times increased risk of blastic transformation after splenectomy in MF.50 Moreover, considering the allo-HCT setting, animal models have given some evidence that the spleen contribute to hematopoiesis and the production of specific antigen-presenting cells.51 A preclinical study of murine allo-HCT reported the influence of splenectomy on stem cell trafficking and hematopoietic reconstitution in both thymus and bone marrow.52 It is therefore possible that such alterations occurring after splenectomy, could impair long-term donor immune-surveillance potentially contributing to a reduction in a graft-vs-myelofibrosis effect. In considering our results, we must also recognize the large number of patients receiving RIC conditioning regimens and in particular, the extensive use of T cell depletion (>80%) in our cohort, compared to 17.6% in the French study,7 a variable independently associated with relapse incidence. Cumulatively, these findings can support the idea that splenectomy could affect the protective allo-HCT immunological activity, particularly after T cell depleted RIC conditioning, as previously documented by the EBMT study.9 Nonetheless, the characteristics of this cohort and the association of splenectomy with T cell depletion, older age and earlier transplant era can raise concern for potential selection bias.
Indeed, our study has some evident limitations: the nature of registry-based study with incomplete details on spleen size prior to splenectomy, unavailability of complete clinical (eg, IPSS/DIPSS at the time of transplant) and biological prognostically-relevant disease features (eg, ASXL1 mutational status),33, 40, 53 as well as the definition of progressive disease according to physician opinion rather than IWG-MRT criteria,54 reduce the power of the present analysis. No information on the intent of the surgery was available in Promise registry. Lastly, since no splenectomy-related complications were recorded we are not aware of how many patients have had a planned allo-HCT procedure deferred or canceled, thus contributing to a possible selection of a fitter cohort of patients. However, in this regard, the recent publication of the French cooperative study group seems to reassure about the safety of pre-transplant splenectomy.18
Despite these limitations, this study has many strengths: the availability of splenectomy status in a very large study population undergoing allo-HCT in the last 20 years, homogeneous conditioning platforms, detailed information on spleen size in a vast majority of non-splenectomised patients and robust follow up.55
In conclusion, our experience highlights the adverse effect of bulky splenomegaly on MF allo-HCT outcome associating with a delay in hematologic recovery, higher NRM rates and lower survival. Splenectomy was associated with more rapid engraftment and lower NRM counterbalanced by a higher relapse incidence, with no resultant OS advantage. However, in those patients with progressive disease and massive splenomegaly, splenectomy may remain an option. Future prospective randomized studies focusing on an evaluation of the role of splenectomy in patients who fail JAK inhibitors prior to allo-HCT would be ideal but are likely difficult due to the rarity of disease and heterogeneic allo-HCT practice.
FINANCIAL DISCLOSURES
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




