Clinically-suspected cast nephropathy: A retrospective, national, real-world study
Abstract
Presentation with severe acute kidney injury due to cast nephropathy (CN) is a medical emergency in multiple myeloma (MM), with high risk of dialysis-dependent renal failure and death. Accrual of patients with CN into interventional studies is difficult, while phase III trials exclude patients with severe renal insufficiency. Real-world data are warranted. We assessed 2252 patients from the population-based Danish Multiple Myeloma Registry (DMMR) who were diagnosed between 2013 and 2017. We identified 204 patients with clinically-suspected CN, defined as serum creatinine concentration >177 μmol/L and serum free light chain (sFLC) concentration >1000 mg/L at the time of diagnosis. The median age was 72 years. Thirty-one percent of patients presented with dialysis-dependent renal failure. Kidney biopsies were performed in 19% of patients and showed CN in 74% of cases. Despite prompt initiation of bortezomib-based therapy in 94% of patients, 33% of patients died in the first year after diagnosis. Compared with the rest of the patients in the DMMR with symptomatic MM, patients with clinically-suspected CN had worse overall survival (OS) irrespective of transplant eligibility. Achievement of renal recovery was associated with deep reductions of involved sFLC. Achievement of very good partial response or better in the first line of therapy and/or deep reduction of involved sFLC at 3 months after initiation of therapy were associated with superior OS. In conclusion, MM patients presenting with clinically-suspected CN have an alarmingly high one-year mortality when treated with current standards of care. Early and deep hematologic response is crucial for survival.
1 INTRODUCTION
Renal failure, defined by serum creatinine higher than 177 μmol/L, occurs in approximately 20%-30% of patients with multiple myeloma (MM) at the time of diagnosis.1-4 Severe renal impairment requiring hemodialysis occurs in 3%-9% of all patients with newly-diagnosed MM, and unless renal function is recovered, it leads to considerable mortality during the first months after diagnosis.1, 2, 5-10 Myeloma cast nephropathy (CN) is the most common form of monoclonal immunoglobulin-mediated kidney disease.11, 12 It results from the interaction of excessive amounts of monoclonal light chains with Tamm-Horsfall proteins in the distal tubule, leading to precipitation of light chains and obstruction of the lumen of the distal nephron.13, 14 Cast nephropathy causes around 70% of the cases of dialysis-dependent renal failure in MM and is a medical emergency requiring prompt intervention to rescue the kidneys from irreversible damage.12, 14-19
The serum free light chain (sFLC) assay is useful for identifying and monitoring patients with light chain myeloma, and is an important tool during the initial screening of patients with unexplained acute renal failure.14, 20-22 In such cases, the finding of a high sFLC concentration with an abnormal sFLC ratio raises the clinical suspicion of CN.14 Although the histopathologic diagnosis of CN is established by a percutaneous kidney biopsy, in routine clinical practice the diagnostic yield of this procedure is often outweighed by the urgent need of anti-myeloma treatment and the risk of procedure-related complications.14, 23, 24
Accrual of patients with clinically-suspected CN into clinical trials is challenging. This was demonstrated by two randomized clinical trials that had the objective of assessing renal recovery and survival of newly-diagnosed patients with MM and CN who were treated with or without high cut-off hemodialysis.25, 26 Both trials required dialysis-dependent renal failure and histopathologic diagnosis of CN for inclusion. The MYRE study was conducted between 2011 and 2016 at 48 French centers and recruited 98 patients.25 The EULITE trial was conducted between 2008 and 2013 in 14 British and two German centers and recruited 90 patients.26 While interventional studies struggle to include patients with CN, patients with severe renal failure are excluded from most phase III clinical trials.27 Real-world data in patients with clinically-suspected CN are warranted.
We conducted a retrospective, national, patient chart review in patients with newly-diagnosed MM and clinically-suspected CN. The aims of our study were to assess baseline characteristics, biopsy results, anti-myeloma treatment, renal recovery and overall survival in this patient group.
2 METHODS
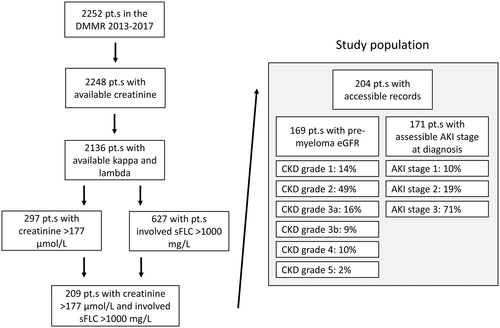
We used five inclusion criteria to define the study population: (a) Patients had to be registered in the Danish Multiple Myeloma Registry (DMMR) with (b) newly-diagnosed MM according to the International Myeloma Working Group (IMWG) criteria28 between (c) 1 January 2013, and 31 December 2017, with (d) a serum creatinine concentration higher than 177 μmol/L, and (5) an involved sFLC concentration higher than 1000 mg/L at the time of diagnosis (Figure 1 and Figure S1A). The sFLC cutoff of 1000 mg/L was chosen based on kernel density analysis of the percentage of renal failure in relation to involved sFLC concentrations in patients from the DMMR diagnosed between 2013 and 2017 (Figure S1B). The DMMR is a national registry that collects baseline and treatment-related data from myeloma patients diagnosed since the 1 January 2005. The coverage of the population is close to 100%.27, 29-35 Serum free light chain measurements have been routinely reported to this registry since 1 January 2013. After identification of patients, investigators from 10 Danish centers reviewed patient charts and retrospectively-registered predefined data in a designated Research Electronic Data Capture (REDCap) database between 1 October 2018 and 1 September 2019. All biopsy reports were reviewed by a nephropathologist for the purposes of this study. Lines of therapy were registered in the first 12 months after MM diagnosis. A line of therapy was defined according to the IMWG guidelines for the determination of the number of prior lines of therapy in MM.36 The best response to a given line of therapy was defined according to the IMWG uniform response criteria for MM.37 Time to next treatment (TNT) was defined as the length of time between the date of initiation of a line of therapy and the subsequent line of therapy. Pre-myeloma kidney function was defined based on the latest serum estimated glomerular filtration rate (eGFR) assessment prior to myeloma diagnosis. Grades of chronic kidney disease (CKD) were defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group 2012 Clinical Practice Guidelines; grades 1, 2, 3a, 3b, 4 and 5 were defined by eGFR values of ≥90, 60-89, 45-59, 30-44, 15-29 and <15 mL/min/1.73 m2, respectively.38, 39 Acute kidney injury (AKI) stage at diagnosis was classified according to the KDIGO 2012 Clinical Practice Guideline for acute kidney injury; stages 1, 2 and 3 were defined as serum creatinine 1.5-1.9 times baseline or ≥25.5 μmol/L increase, 2.0-2.9 times baseline, and 3.0 times baseline or increase in serum creatinine to ≥353.6 μmol/L or initiation of renal replacement therapy, respectively.40 Kidney function and sFLC concentrations were assessed at the following time points: the date of first sFLC measurement, the end of the first cycle of anti-myeloma therapy, 3 months after initiation of therapy, 6 months after initiation of therapy and 12 months after initiation of therapy. Renal response was assessed according to the IMWG criteria for the definition of renal response to anti-myeloma therapy.41 Renal recovery at the predefined time points was assessed according to the following simplified criteria: In patients with dialysis-dependent renal failure at diagnosis, renal recovery was defined as independence from dialysis; in patients without dialysis-dependent renal failure at diagnosis, renal recovery was defined as an improvement of at least one CKD grade: a rise in eGFR from <15 to ≥15, from 15-29 to ≥30, from 45-59 to ≥60 or from 60-89 to ≥90 mL/min/1.73 m2. Overall survival (OS) was defined as the length of time between the date of diagnosis and the date of death or last contact. Overall survival data were acquired from the DMMR, which automatically receives updated survival status from the Danish population registry.42
2.1 Statistics
Continuous variables were described with median, range, interquartile range (IQR) and confidence interval (CI). Categorical variables were summarized using the number of observations and percentages as appropriate. Overall survival was estimated and visualized using the Kaplan-Meier method, and log-rang tests were used to assess difference between survival curves. Associations with OS were assessed by Cox proportional hazards regression models, with estimates reported as Hazard Ratios. Statistical analysis was performed with the statistical software R v. 3.6.1 and the add-on packages “tidyverse,” “ggplot2” and “ggkm.”43-46 Data visualizations were performed with R version 3.6.1 and Microsoft Excel for Office 365.
3 RESULTS
3.1 Study population
In the study period, 2252 patients were registered in the DMMR. Diagnostic serum creatinine concentrations were available in 2249 patients. In addition to serum creatinine, diagnostic sFLC kappa and lambda concentrations were available in 2136 patients. Of these, 297 (14%) patients had a serum creatinine concentration higher than 177 μmol/L and 627 (29%) patients had an involved sFLC concentration higher than 1000 mg/L. Two-hundred nine patients had both a serum creatinine concentration higher than 177 μmol/L and an involved sFLC concentration higher than 1000 mg/L and were included in the study. Medical records in 5 patients were not accessible. The study population thus consisted of 204 patients. The flow-chart for inclusion and identification of the study population are presented in Figure 1 and Figure S1, respectively. Two patients had previously been diagnosed with monoclonal gammopathy of unknown significance (MGUS). Five patients did not receive treatment for MM. Of these, one patient had advanced renal cell carcinoma, one patient had prostate cancer, one patient had pre-existing dialysis-dependent hypertensive nephropathy and two patients chose not to receive treatment for MM due to advanced age. One-hundred ninety-nine patients received therapy. The median age at diagnosis was 72 (IQR: 65-79) years. One hundred and thirty-six (67%) patients were men. The median serum creatinine and involved sFLC concentrations at diagnosis were 340 (IQR: 243-530) μmol/L and 5677 (IQR: 2495-11 918) mg/L, respectively. Baseline characteristics are presented in Table 1. Pre-myeloma serum creatinine concentrations were available in 169 (83%) patients and 165 (81%) patients had a pre-myeloma eGFR registered. The median pre-myeloma serum creatinine concentration and eGFR were 89 (IQR: 77-115) μmol/L and 66 (IQR: 47-80) ml/min/1.73 m2, respectively. Nineteen (11%) patients had a pre-myeloma serum creatinine concentration higher than 177 μmol/L. Based on pre-myeloma eGFR, there were 23 (14%) patients with grade 1, 80 (49%) with grade 2, 27 (16%) with grade 3a, 15 (9%) with grade 3b, 16 (10%) with grade 4 and five (2%) with grade 5 CKD. Acute kidney injury stage at diagnosis was assessable in 171 patients. Of these, 17 (10%) had AKI stage 1, 32 (19%) had AKI stage 2 and 122 (71%) had AKI stage 3.
| Baseline variable | Category present (%) | Median (IQR) | n obs. |
|---|---|---|---|
| Age | 72 (64; 79) | 204 | |
| Age ≤65 y | 55 (27) | 204 | |
| Male sex | 136 (67) | 204 | |
| Hemoglobin (mmol/L) | 5.9 (5.3; 6.5) | 203 | |
| Hemoglobin <6.2 mmol/L | 123 (61) | 203 | |
| Creatinine (μmol/L) | 340 (243; 530) | 204 | |
| Ionized calcium (mmol/L) | 1.26 (1.2; 1.49) | 174 | |
| Ionized Calcium ≥1.345 (mmol/L) | 59 (34) | 174 | |
| β2 microglobulin (mg/L) | 14 (10; 20) | 176 | |
| Albumin (g/L) | 34 (29; 39) | 204 | |
| LDH (U/L) | 214 (178; 260) | 198 | |
| Bone marrow clonal plasma cell infiltration (%) | 50 (30; 66) | 199 | |
| Serum M-protein present | 175 (86) | 204 | |
| Serum M-protein (g/L) | 12 (3; 30) | 160 | |
| Involved sFLC (mg/L) | 5677 (2495; 11 918) | 204 | |
| Serum M-protein isotype: IgA | 38 (22) | 177 | |
| Serum M-protein isotype: IgG | 73 (41) | 177 | |
| Serum M-protein isotype: light chain | 63 (36) | 177 | |
| Serum M-protein isotype: other | 3 (2) | 177 | |
| Urine M-protein present | 104 (51) | 203 | |
| Urine M-protein (g/L) | 1.01 (0.45; 1.7) | 62 | |
| Urine M-protein (g/d) | 1.9 (1.08; 3.99) | 14 | |
| Urin M-protein isotype: kappa | 52 (54) | 96 | |
| Urin M-protein isotype: lambda | 44 (46) | 96 | |
| Osteolytic lesion | 122 (60) | 204 | |
| Spinal cord compression | 3 (2) | 204 | |
| Amyloidosis | 6 (3) | 204 | |
| Dialysis-dependent renal failure | 64 (31) | 204 | |
| PS 0 | 57 (29) | 197 | |
| PS 1 | 67 (34) | 197 | |
| PS 2 | 45 (23) | 197 | |
| PS 3 | 19 (10) | 197 | |
| PS 4 | 9 (5) | 197 | |
| ISS I | 2 (1) | 176 | |
| ISS II | 9 (5) | 176 | |
| ISS III | 165 (94) | 176 | |
| FISH with del17p/t(4;14)/t(14;16) | 29 (21) | 139 | |
| AKI stage 1 | 17 (10) | 171 | |
| AKI stage 2 | 32 (19) | 171 | |
| AKI stage 3 | 122 (71) | 171 |
- Abbreviations: AKI, acute kidney injury; FISH, Fluorescence in situ hybridization; IQR, interquartile range; ISS, International Staging System; LDH, lactatdehydrogenase; n obs., number of observations; PS, Eastern Cooperative Oncology Group Performance Status; sFLC, serum free light chain; %, percentage.
3.2 Kidney biopsies
A kidney biopsy was carried out in 38 (19%) patients. In 25 (66%) of these patients, the kidney biopsy was performed prior to the diagnosis of MM. The median time from kidney biopsy to MM diagnosis was 4 (IQR: 3-8) days. In 13 (34%) patients, the kidney biopsy was done after the diagnosis of MM. The median time from MM diagnosis to kidney biopsy was 5 (IQR: 2-7) days. The histopathologic diagnosis based on the kidney biopsy was CN in 28 (74%) cases. Besides CN, additional diagnoses were reported in 12 patients. These diagnoses were acute tubular necrosis (n = 3), acute interstitial nephritis (n = 2), pyelonephritis (n = 2), interstitial fibrosis (n = 2), amyloidosis (n = 1), hypertensive blood vessel changes (n = 1), chronic interstitial nephritis (n = 1), mesangioproliferative glomerulonephritis (n = 1) and unspecific chronic changes (n = 1). In the 10 biopsied patients who did not have CN, the following diagnoses were reported: renal cell carcinoma (n = 2), amyloidosis (n = 1), acute tubulointerstitial nephritis (n = 1), chronic inflammation with fibrosis (n = 1), unspecific changes (n = 1), normal tissue (n = 1), and unsuitable biopsy (n = 3). Baseline clinical characteristics in biopsied patients with and without CN were similar (Table S1).
3.3 Time from first serum free light chain measurement to initiation of therapy
The first line of anti-myeloma therapy was initiated prior to the first sFLC measurement in three patients (day −3: two patients; day −1: one patient). The median time from the first sFLC measurement to initiation of anti-myeloma therapy was five (IQR: 2-10) days (Figure S2). The time from first sFLC measurement to initiation of therapy in the two patients with previously-diagnosed MGUS were 1163 and 1432 days, respectively.
3.4 Lines of therapy
Within the first 12 months from diagnosis, 199 patients received one, 44 patients received two, 15 patients received three, four patients received four, two patients received five and one patient received six lines of therapy. High-dose melphalan with autologous hematopoietic stem cell transplantation (HDT-ASCT) was carried out in 51 (25%) patients and was part of the first, second and third line of therapy in 45, four and two patients, respectively. The most frequently used drugs (other than steroids) were bortezomib (n = 220), cyclophosphamide (n = 100), melphalan (n = 38), thalidomide (n = 34) and lenalidomide (n = 31). The most frequently used first-line regimens were bortezomib-dexamethasone (VD; n = 70), cyclophosphamide-bortezomib-dexamethasone (CVD; n = 57), bortezomib-melphalan-prednisolone (VMP; n = 16), bortezomib-thalidomide-dexamethasone (VTD; n = 12) and bortezomib-lenalidomide-dexamethasone (VRD; n = 5). Bortezomib was administered as part of the first-line regimen in 188 (94%) patients. The most frequently used second-line regimens were CVD (n = 8), VMP (n = 7), lenalidomide-dexamethasone (RD; n = 5), thalidomide-cyclophosphamide-dexamethasone (CTD; n = 4), daratumumab-lenalidomide-dexamethasone (DRD; n = 3) and VTD (n = 3). Hematologic response to the first and second lines of therapy is shown in Figure S3. The reduction of involved sFLC concentration at the end of the first cycle of therapy at 3 months, 6 months and 12 months after initiation of therapy is shown in Figure S4. A cause of discontinuation was registered in 88% of the administered lines of therapy. The cause of discontinuation was fixed duration regimen in 58 (25%), toxicity in 35 (15%), death in 34 (15%), plateau phase in 34 (15%), progressive disease in 31 (13%), insufficient response in 12 (5%), poor performance status in 11 (5%), patientʼs choice in 7 (3%), and “other” in 10 (4%) of cases. The causes of discontinuation of the first and second lines of therapy are shown in Figure S5. The median TNT between the first and second line of therapy was 46 (IQR: 24-101) days.
3.5 Dialysis dependency and renal recovery
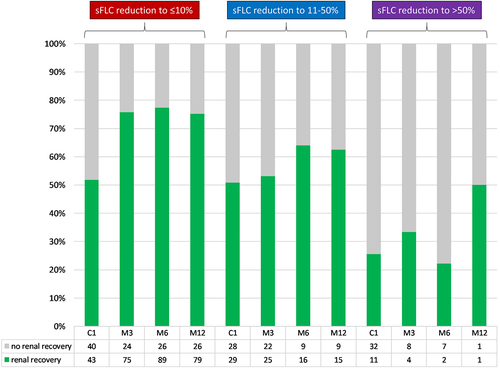
Renal function was available in 188 patients at the end of the first cycle of therapy, 169 patients at 3 months, 157 patients at 6 months and 134 patients at 12 months after initiation of therapy. At these time points, 24%, 17%, 15% and 13% of patients had dialysis-dependent renal failure, respectively (Figure S6A). Renal response according to the IMWG criteria is shown in Figure S6B. Renal recovery according to the simplified criteria defined in this study was achieved in 45% of patients at the end of the first cycle of therapy, in 66% of patients 3 months, in 72% of patients 6 months, and in 73% of patients 12 months after initiation of therapy (Figure S6C). Achievement of renal recovery was associated with deeper reductions of involved sFLC at all time points (P = .009 at the end of the first cycle of therapy, P = .001 at 3 months, and P = 0.002 at 6 months after initiation of therapy; Figure 2).
3.6 Overall survival
The median OS in the study population was 3.7 (95% CI: 2.6-4.7) years (Figure S7A). One-year mortality was 33%. Of the 204 patients with accessible records, 190 (93%), 170 (83%), 158 (77%) and 136 (67%) patients were alive after the first cycle of therapy, at 3 months, 6 months and 12 months after initiation of therapy, respectively. There were 1927 patients in the DMMR who did not fulfill the inclusion criteria for this study. The median OS of these patients was 6.1 (95% CI: 5.2; not reached) years. One-year mortality was 13%. Of the 1927 patients, 1393 had symptomatic MM. Compared with the rest of the patients in the DMMR with symptomatic MM, patients with clinically-suspected CN had worse OS irrespective of HDT-ASCT-eligibility (P < .001; Figure S7B). One-year mortality was higher in both HDT-ASCT-eligible (6% vs 2%) and HDT-ASCT-ineligible (43% vs 20%) patients with clinically-suspected CN.
3.7 Factors associated with overall survival
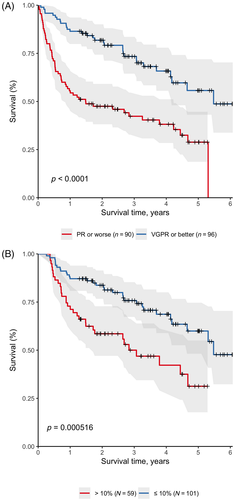
The following baseline variables were associated with OS: age (HR: 1.78; P < .001), performance status 2 (HR: 2.19; P = .006), performance status 3 (HR: 6.20; P < .001), performance status 4 (HR: 6.80; P < .001), serum albumin (HR: 0.95; P < .001), beta-2-microglobulin (HR: 1.03; P = .006), and LDH (HR: 1.00; P = .022). High-dose melphalan with autologous hematopoietic stem cell transplantation performed in any line of therapy was associated with longer OS (HR 0.26; P < .001). There was no difference in OS of HDT-ASCT-eligible nor HDT-ASCT-ineligible patients based on the administered first-line regimens (Figure S8). In multivariate analysis adjusted for the effects of age, performance status, creatinine, albumin, LDH and HDT-ASCT, very good partial response or better in the first line of therapy (HR 0.46; P = .002) and involved sFLC reduction to 10% or lower of baseline at 3 months after initiation of therapy (HR: 0.56; P = .039) were independently associated with longer OS (Figure 3).
4 DISCUSSION
Our large, real-world study provides nationwide data on the characteristics, treatment, renal recovery and OS of patients with newly-diagnosed MM and clinically-suspected CN, defined as serum creatinine higher than 177 μmol/L and involved sFLC higher than 1000 mg/L. This disease presentation was found in 10% of all MM patients. Despite the prompt initiation of effective bortezomib-based therapy in 94% of patients, one third of the patient group died in the first year after diagnosis: a mortality higher than in the rest of the patients registered in the DMMR who were diagnosed in the same period. One-year mortality was especially high in HDT-ASCT-ineligible patients. Patients who survived the crucial first year after diagnosis had comparable survival to the rest of the patients registered in the DMMR. Very good partial response or better in the first line of therapy and deep reduction of involved sFLC at 3 months resulted in superior OS.
A strength of our study is the description of a group of patients who are underrepresented in clinical trials. Besides the inclusion criteria, the study population was unselected and population-based. All treatment decisions were results of real-world clinical scenarios. As expected, we observed higher one-year mortality than reported in the MYRE and EULITE trials, even though these trials were conducted in more severely affected populations, having only included patients with dialysis-dependent renal failure at the time of diagnosis.25, 26
A limitation of this study is the lack of systematic histological verification of CN. When performed, kidney biopsies showed CN in 74% of cases, similar to the findings of previous biopsy studies in MM.17, 18 A kidney biopsy is the only diagnostic method of CN; no threshold sFLC concentration or biomarker is specific for this mechanism of acute kidney injury. It is, though, generally assumed that CN does not develop in patients with an involved sFLC concentration lower than 500 mg/L.14 Our data, based on 2136 patients from the DMMR, supported the use of a sFLC cutoff of 1000 mg/L for renal failure (Figure S1). It is our opinion that the sFLC assay, despite being unspecific for CN, together with the initial diagnostic work-up, provides sufficient clinical information to initiate acute treatment in most patients. We expect that CN remains a clinically-suspected rather than a histologically-verified diagnosis in the future.
The standard frontline treatment in our patient group consisted of CVD induction followed by HDT-ASCT in transplant-eligible patients, and VD in transplant-ineligible patients. Daratumumab is a monoclonal antibody that can be safely administered in patients with renal failure.47, 48 There were only seven patients in our cohort that were treated with daratumumab, which had been reimbursed in Denmark since September 2016. At the time of the writing of this manuscript, daratumumab has not yet been granted reimbursement for the frontline treatment of patients with MM in Denmark. However, it is expected that daratumumab soon will be an available first-line option in MM patients presenting with clinically-suspected CN, improving patient outcomes.
In conclusion, MM patients presenting with clinically-suspected CN, especially transplant-ineligible patients, have an alarmingly high one-year mortality when treated with current standards of care. Early and deep hematologic response and reduction of toxic free light chains are crucial for survival. The expected inclusion of daratumumab in future first-line regimens will likely improve outcomes in this group of MM patients.
ACKNOWLEDGEMENTS
We thank the Department of Internal Medicine and the Hematological Clinical Research Unit at Vejle Hospital for providing the financial and logistical background for this study. Data management for this study was provided by Open Patient Exploratory Network, University of Southern Denmark. The authors received no financial support for the research, authorship or publication of this article.
CONFLICT OF INTEREST
A.G.S.: consulting for Janssen; M.Ø.V.: honoraria from Celgene; T.P.: consulting for Janssen, Celgene, Takeda, Abbvie and Genmab; A.J.V.: honoraria from Janssen and Celgene and consulting for Takeda, Sanofi and Oncopeptides. The remaining authors claim no conflicts.
AUTHOR CONTRIBUTIONS
Agoston G. Szabo designed the study, created the study database, conducted patient chart review, created figures and wrote the manuscript. Jonathan Thorsen carried out data analysis, statistics, created figures and participated in the writing of the manuscript. Annette J. Vangsted entered patients in the study database, supervised the study and participated in the writing of the manuscript. Torben Plesner supervised the design of the study and participated in the writing of the manuscript. The remaining authors all entered patients in the study database and participated in the writing of the manuscript.
Open Research
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to Danish data protection regulations.




