Refining amyloid complete hematological response: Quantitative serum free light chains superior to ratio
Funding information: Jabbs Foundation; National Cancer Institute, Grant/Award Number: P50 CA186781; Predolin Foundation
Abstract
Response assessment in light chain (AL) amyloidosis is based on serum and urine monoclonal protein studies. Newly diagnosed patients (n = 373) who achieved very good partial response or complete response (CR) to first line therapy were assessed for the survival impact of each of the monoclonal protein studies. At end of therapy (EOT), negative serum/urine immunofixation (IFE) was achieved in 61% of patients, 72% achieved normal serum free light chain ratio (sFLCR), and the median involved free light chain (iFLC) and difference between involved to uninvolved light chain (dFLC) were 17 mg/L and 5 mg/L, respectively. Overall, 46% of patients achieved a CR at EOT. At EOT, iFLC ≤20 mg/L and dFLC ≤10 mg/L were additive in survival discrimination to negative serum/urine IFE and were independent predictors of overall survival. In contrast, normalization of sFLCR did not add survival discrimination to serum/urine IFE and was not independent predictor of survival. We propose a new definition for hematological CR to include serum/urine IFE negativity plus iFLC ≤20 mg/L or dFLC ≤10 mg/L, instead of the current definition of serum/urine IFE negativity and normal sFLCR. Complete response using dFLC ≤10 mg/L had the best performance in those with significant renal dysfunction and by light chain isotype, making it the preferred partner to IFE. Validation of these results in a multicenter cohort is warranted.
1 INTRODUCTION
Immunoglobulin light chain (AL) amyloidosis is a common type of systemic amyloidosis. It is caused by an underlying clonal plasma cell proliferative disorder (or rarely by B-cell lymphoproliferative disorders), which secrete immunoglobulin light chains that aggregate into amyloid fibrils, which deposit in tissues and organs leading to significant, life-threatening organ impairment.1 Treatment is aimed at eradication of the underlying plasma cell clone to eliminate further production and availability of amyloidogenic light chains to form amyloid. Response criteria in AL amyloidosis are therefore based on measurement of the monoclonal protein secretory compartment, which includes the serum free light chain assay, protein electrophoresis (PEL) and immunofixation (IFE) of serum and urine.2 These criteria are able to discriminate survival outcome and allow setting treatment goals. However, the size of the plasma cell clone and its associated monoclonal protein size is usually small, leading to challenges in response assessment among patients who achieved a profound response. This prompted several groups to propose alternative or additional response targets, including flow-based response,3-7 a low difference between involved to uninvolved light chains (dFLC, <10 mg/L)8 or low involved free light chain (iFLC, ≤20 mg/L)9. In addition, we previously reported that normalization of the serum free light chain ratio (sFLCR), which is a part of complete response (CR) definition, has no impact on survival discrimination when assessed at end of therapy (EOT).9 In the present work we systematically assessed the contribution of each of the monoclonal protein studies to the composite response assessment among patients who achieved a deep response to therapy.
2 METHODS
The inclusion criteria for this study were: (a) newly diagnosed AL amyloidosis patients seen within 90 days of diagnosis between 1 September 2001 and 31 August 2015; (b) patients had achieved very good partial response (VGPR) or CR to first line therapy based on consensus criteria2; and (c) all monoclonal protein studies were performed at EOT, including serum and urine PEL/IFE and serum free light chain assay. All patients had confirmed light type on tissue sample using mass spectrometry in 165 patients (44%), immunofluorescence (IF) in 114 patients (31%), immunohistochemistry (IHC) in 80 patients (21%) and both IF/IHC in the remaining 14 patients (4%). The Mayo Foundation Institutional Review Board (IRB) approved the study. All patients gave written informed consent to have their medical records reviewed according to IRB requirements and Minnesota state law.
First line treatment was defined as the first regimen patients had received, regardless of subsequent modifications. For patients receiving autologous stem cell transplantation (ASCT) after induction therapy and/or followed by consolidation/maintenance, the primary therapy was considered to be ASCT. The EOT was considered the time after which no further therapy was given. Day +100 from autologous stem cell transplant was considered the EOT for ASCT. Seven patients who received consolidation/maintenance therapy after ASCT were included upon completion of that additional therapy.
The serum IFE assessed migration patterns for γ, α, μ, κ, and λ immunoglobulin chains (Hydrasys and Hydragel; Sebia) with a sensitivity of approximately 0.5 g/L.10, 11 Urine IFE assays were performed on the same day as receipt of the sample. Urine samples were concentrated to a maximum of x200 to attempt to achieve final concentrations of urine protein between 20 g/Land 80 g/L. Urine IFE was performed on SPIFE IFE 9/15 gels (Helena Laboratories). All serum and urine IFE gels were reviewed by two technicians as well as by an experienced physician.
The χ2 test and Fisherʼs exact test was used to compare differences between continuous variables, and the Wilcoxon signed-rank test was used for nonparametric group comparisons. Survival analysis was done using the Kaplan-Meier method. Progression-free survival (PFS) was defined as the time from diagnosis until hematological progression or death, where patients known to be alive and progression-free at the end of follow-up were censored. Hematological progression was defined according to consensus criteria as dFLC increased by at least 50% to >100 mg/L12 or upon initiation of second line therapy, whichever came first. Overall survival (OS) was defined as the time from diagnosis until death from any cause, whereas patients known to be alive at end of follow-up were censored. Multivariate analysis was performed using the Cox Proportional Hazards model. Statistical analysis was performed on JMP software (SAS, Cary, NC). A C-statistic (concordance) for a Cox proportional hazards regression model was performed, as a measure of the goodness of fit of the model and its predictive performance. Results typically range from 0.5 to 1, with values close to 0.5 suggesting that the modelʼs performance is no better than random chance, and larger values representing stronger model performance.13
3 RESULTS
The baseline characteristics and treatment details of the participants (n = 373) are presented in Table 1. The median age at diagnosis was 59 years. The kidney was the most commonly involved organ at 70%. Sixty-five percent of the patients received ASCT as their primary therapy. Baseline serum and urine IFE were available for 369 patients (99%). A monoclonal protein in the serum and/or urine was present in 92% of patients. The baseline serum free light chain assay was available for 367 patients (98%) and revealed an abnormal sFLCR in 88% of patients. The median baseline iFLC and dFLC were 150 mg/L and 130 mg/L, respectively. There was no significant difference in baseline dFLC between ASCT and non-ASCT patients (120 mg/L vs 150 mg/L, respectively; =.11), while iFLC was slightly higher in the non-ASCT cohort (130 mg/L vs 180 mg/L, respectively, P = .048).
| Characteristic | N = 373 |
|---|---|
| Age (y), median (IQR) | 59 (53-66) |
| Male sex, N (%) | 220 (59%) |
| Involved organs, N (%) | |
| Renal | 260 (70%) |
| Cardiac | 210 (56%) |
| Nerve | 56 (15%) |
| Hepatic | 55 (15%) |
| Gastrointestinal | 49 (13%) |
| >1 organ | 219 (59%) |
| Lambda restricted, N (%) | 283 (76%) |
| BMPCs, % median (IQR) | 8 (5-11) |
| dFLC, mg/L, median (IQR) | 130 (65-410) |
| Serum creatinine, μmol/L, median (IQR) | 97 (80-124) |
| eGFR, mL/min/1.7 m2, median (IQR) | 71 (52-91) |
| 2004 Mayo AL amyloidosis stage, % I/II/IIIA/IIIB | 34/43/19/4 |
| 2012 Mayo AL amyloidosis stage, % I/II/III/IV | 40/27/19/14 |
| First line treatment | |
| ASCT | 241 (65%) |
| MDex | 81 (21.5%) |
| Bortezomib-based | 37 (10%) |
| IMiD-based | 8 (2%) |
| MP/dexamethasone alone | 6 (1.5%) |
- Abbreviations: AL, light chain amyloidosis; ASCT, autologous stem cell transplantation; BMPCs, bone marrow plasma cells; dFLC, difference between involved-to-uninvolved light chains; eGFR, estimated glomerular filtration rate; IMiD, immunomodulatory drug; IQR, interquartile range; MDex, melphalan-dexamethasone; MP, melphalan-prednisone.
3.1 Monoclonal protein studies at end of therapy and response assessment
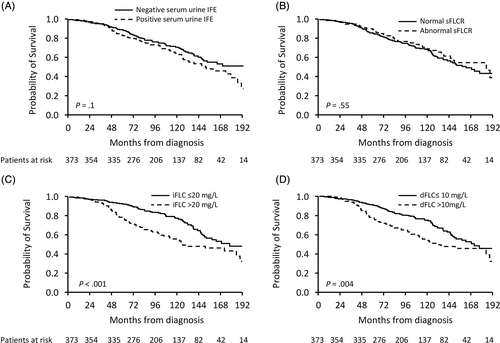
The median time from EOT to monoclonal protein testing was 3 months (IQR 0-3). Negative serum and urine IFE was achieved in 61% of patients (n = 229), while normal sFLCR was observed in 72% of patients (n = 269). Of the 144 positive IFE results, 48 patients had positive serum and urine IFE, 56 patients had positive serum IFE alone and 40 patients had positive urine IFE alone. The median iFLC and dFLC at EOT were 17 mg/L (IQR 10-28) and 5 mg/L (IQR 0-13), respectively, and the median eGFR was 64 mL/min/1.73m2 (IQR 44-81). The median EOT iFLC and dFLC were lower in those who attained CR than in those who attained a VGPR (15 mg/L vs 19 mg/L, P = .08; 2 mg/L vs 8 mg/L, P < .001, respectively). The EOT iFLC and dFLC values were similar regardless of light chain isotype.
We used iFLC ≤20 mg/L (59%, n = 220) and dFLC ≤10 mg/L (69%, n = 257) as threshold values because these figures have been previously studied.8, 9, 14-16 Figure S1 depicts the overlap of negative serum/urine IFE, normal sFLCR, iFLC ≤20 mg/L and dFLC ≤10 mg/L. The intersection between negative serum and urine IFE and each of the three tested light chain measures were: 148/229 (64.6%) for iFLC ≤20 mg/L; 177/229 (77.2%) for dFLC ≤10 mg/L; and 172/229 (75.1%) for normal sFLCR. There was agreement between iFLC ≤20 mg/L and dFLC ≤10 mg/L among 202 patients and disagreement where one was favorable and the other not among 73 patients. More often, the disagreement was due to a favorable dFLC and an unfavorable iFLC (55/73, 75%).
3.2 The effect of monoclonal protein studies at EOT on survival
With a median follow-up of 8.7 years, 51% (n = 191) had hematological progression requiring second line therapy and 36% of patients (n = 134) died. Negative serum/urine IFE at EOT demonstrated marginally improved OS over patients who remained with positive serum/urine IFE (median OS not reached vs 151 months, P = .1; Figure 1A). In contrast, normal vs abnormal sFLCR at EOT was not predictive (median OS 163 vs 186 months, P = .55; Figure 1B). Both iFLC ≤20 mg/L and dFLC ≤10 mg/L at EOT were associated with longer OS (median 178 vs 132 months, P < .001; median 172 vs 132 months, P = .004; respectively, Figure 1C,D). Similar patterns were seen in terms of PFS (Figure S2).
Subgroup survival analysis by light chain isotype demonstrated a survival discrimination for iFLC20 for lambda light chain isotype (P < .001), but not for kappa light chain isotype (P = .82). In contrast, the performance of dFLC10 in survival discrimination based on light chain isotype was maintained for the lambda light chain isotype (P = .02) and had borderline significance for kappa light chain isotype (P = .07) (Figure S3). Normalization of sFLCR did not impact survival by subgroup analysis based on light chain isotype.
3.3 Survival discrimination by combining serum/urine IFE and sFLC variables
We then plotted survival curves of the four possible combinations of integrating serum/urine IFE (positive/negative) with each of the following sFLC assay variables: iFLC at 20 mg/L, dFLC at 10 mg/L or sFLCR (normal/abnormal). These analyses reveal that while sFLCR status does not add further PFS discrimination to that exhibited by the IFE status, iFLC20 and dFLC10 demonstrate an added PFS discrimination to serum/urine IFE status (Figure S4A-C). For overall survival, iFLC20 and dFCL10 were more important than serum/urine IFE, while sFLCR added little to nothing to survival discrimination (Figure S4D,F). A C-statistics analysis for each combination, without multivariable correction, was performed favoring the combination of serum/urine IFE with iFLC20 (OS 0.619, 95% CI 0.571-0.671; PFS 0.629, 95% CI 0.592-0.668), over the combination of serum/urine IFE with dFLC10 (OS 0.585, 95% CI 0.534, 0.642; PFS 0.608, 95% CI 0.578, 0.651), or the combination of serum/urine IFE with sFLCR (OS 0.541, 95% CI 0.509, 0.608; PFS 0.585, 95% CI 0.555, 0.624).
3.4 Univariate and multivariate analysis for independent predictors of OS
We performed two multivariable models, one that included response assessment variables only and one that also included additional clinically relevant variables. In the former multivariate model, only quantitative FLC variables (iFLC20 and dFLC10) were independent predictors of OS; negative serum/urine IFE and normal sFLCR were not (Table 2). In a model that also included clinically-relevant variables (age 65 and above, ASCT as primary therapy, Mayo stages III/IV), negative serum/urine IFE at EOT, iFLC ≤20 mg/L and dFLC ≤10 mg/L were independent predictors of survival while normalization of sFLCR was not. Concordance was 0.708 and 0.707 when iFLC or dFLC were included in the model, respectively. Similar patterns were seen in terms of PFS, although serum/urine IFE was also predictive for PFS in addition to iFLC20 or dFLC10 in the response-variable model only (Table S2).
| Response variables only model | Combined clinical and response variables model | |||||||
|---|---|---|---|---|---|---|---|---|
| Model A (iFLC) | Model B (dFLC) | Model A (iFLC) | Model B (dFLC) | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age ≥65 y | Not included | Not included | 2.1 (1.4-3.1) | <.001 | 2.3 (1.5-3.3) | <.001 | ||
| ASCT as primary therapy | Not included | Not included | 0.5 (0.3-0.7) | <.001 | 0.5 (0.3–0.7) | <.001 | ||
| Mayo stage 2012 (III/IV vs I/II) | Not included | Not included | 2.1 (1.3-3.4) | .002 | 2.1 (1.3-3.4) | .002 | ||
| Negative serum/urine IFE at EOT | 0.8 (0.6-1.2) | .25 | 0.8 (0.6-1.2) | .28 | 0.7 (0.4-0.95) | .02 | 0.7 (0.5-1.0) | .058 |
| Normal sFLCR at EOT | 1.2 (0.8-1.7) | .41 | 1.4 (0.9-2.1) | .09 | 1.1 (0.7-1.6) | .71 | 1.3 (0.8-2.0) | .26 |
| iFLC ≤20 mg/L at EOT | 0.5 (0.4-0.8) | <.001 | Not included | 0.7 (0.5-0.98) | .04 | Not included | ||
| dFLC ≤10 mg/L at EOT | Not included | 0.6 (0.4–0.8) | .002 | Not included | 0.6 (0.4–0.9) | .01 | ||
| Model concordance | 0.613 (95% CI 0.573, 0.675) | 0.606 (95% CI 0.552, 0.657) | 0.709 (95% CI 0.664, 0.767) | 0.707 (95% CI 0.661, 0.768) | ||||
- Note: All statistically significant values (P <.05) are provided in bold.
- Abbreviations: ASCT, autologous stem cell transplantation; CI, confidence interval; dFLC, difference between involved-to-uninvolved light chains; EOT, end-of-therapy; iFLC, involved light chain; OS, overall survival; sFLCR, serum free light chain ratio.
3.5 Testing a new proposal for hematological CR definition
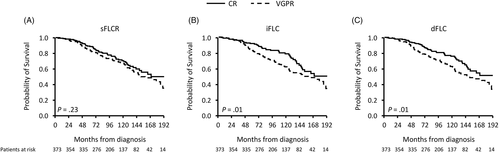
Because serum/urine IFE negativity and either iFLC ≤20 mg/L or dFLC ≤10 mg/L had additive survival discrimination while sFLCR did not, we compared outcomes using the current hematological CR definition to alternative CR definitions, ones that include negative serum/urine IFE and either iFLC ≤20 mg/L or dFLC ≤10 mg/L. As demonstrated in Figure 2 (OS) and Figure S5 (PFS), these alternate definitions for hematological CR had better survival discrimination over the current CR consensus definition.
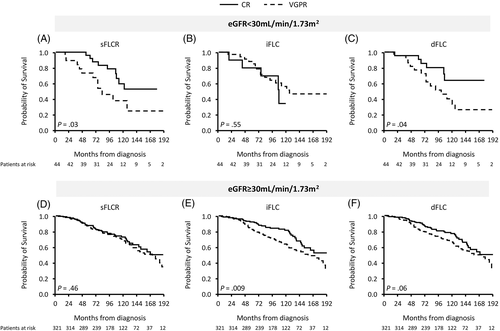
We next tested the current and alternative definitions for hematological CR among those with (n = 44, 12%) and without renal dysfunction (n = 321, 88%), using an estimated glomerular filtration rate (eGFR) cut point of 30 mL/min/1.73 m2 at EOT (available for 365 patients). The results of these comparisons are depicted in Figure 3 (OS) and Figure S6 (PFS). Using the dFLC at 10 mg/L cut point for CR definition demonstrated the best performance for those with severe kidney dysfunction, while CR using iFLC ≤20 mg/L was of no value in these patients.
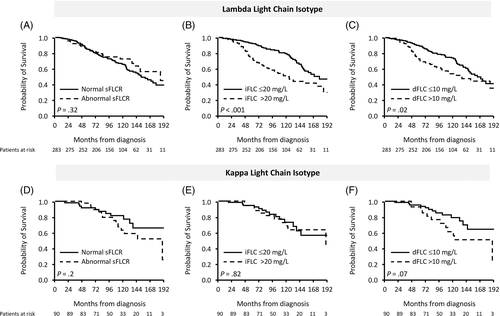
A head-to-head comparison of the current hematological CR definition to the alternative CR definition based on light chain isotype is depicted in Figure 4. While CR based on iFLC-CR provided better survival discrimination over dFLC-CR among lambda light chain isotype, the performance of any CR definition among kappa light chain isotype was poorer, albeit trending to survival discrimination when dFLC-CR was used (P = .14).
3.6 Agreement between bone marrow assessment and response category
Bone marrow aspiration and biopsy was performed in 69% of patients at the time of response assessment (n = 255). Bone marrow assessment at EOT was performed mainly in those who underwent ASCT, with 91% (n = 232) of all marrow assessments done in this treatment group. Eighty-six percent (n = 220) of these patients with a VGPR or better had morphologically negative bone marrows. The agreement between the morphological evaluation of the bone marrow and amyloid response category at EOT was 47%. When the revised definitions of hematological CR were compared to morphological assessment the agreement was 47% for CR using iFLC and 52% for CR using dFLC.
A flow cytometry-based assay was available for 250 patients (98%), of which 76% of patients had low sensitivity flow (sensitivity 10−3 to 3 × 10−4) and 24% of patients had high-sensitivity flow study (sensitivity 1 × 10−4 to 2 × 10−5). An absence of clonal plasma cells was reported in 49% and 71% of patients tested with high-sensitivity flow and low-sensitivity flow, respectively. The agreement between flow cytometry study and amyloid response category at EOT was 58%, 59% and 64% using the current consensus CR, the iFLC-CR, and the dFLC-CR definitions, respectively (Figure S8). When comparing the high sensitivity flow study to the less sensitive flow studies, there was no difference in the agreement between the flow study and response category, for the current CR definition (P = .46) or using the revised CR with iFLC (P = .33) or dFLC (P = .23).
4 DISCUSSION
The serum FLC has made measureable response possible for the majority of patients with AL amyloidosis.17 Prior to the sFLC assay, only CR and absence of CR were the available categories for hematologic response for most AL amyloidosis patients. The sFLC assay made partial response (50% reduction in iFLC and later dFLC) and then very good partial response (dFLC <40 mg/L) tangible endpoints for AL patients.2, 18-20 The enthusiasm for the sFLC assay in AL amyloidosis and in myeloma brought the concept of a normal sFLCR as a feature of stringent complete response in the case of MM and amyloid complete response in the case of AL amyloidosis. However, sFLC assay does not directly measure clonality, and clonality from this assay can only be inferred. Thus, the utility of sFLCR as a criterion for CR has been correctly questioned,9 since extreme ratios are seen in the setting of highly immunosuppressive chemotherapy and a normal ratio does exclude residual disease. In addition, daratumumab, a therapeutic monoclonal antibody which is increasingly being used in patients with AL amyloidosis,21-23 results in substantial immunoparesis and impacts response evaluation using sFLCR despite low iFLC, as already recognized.22, 23 Individual studies have tested the prognostic role of several FLC measures as a marker for very deep responses, including how to deal with those patients with low baseline dFLC.14-16 In these and other works,8, 9, 24 the concepts of normal iFLC, low iFLC, low dFLC have emerged, but often separate from or compared to IFE CR.
Herein, we performed a systematic and comprehensive appraisal of the contribution each of the monoclonal protein studies makes in AL amyloidosis response assessment for patients achieving at least a VGPR. We have synthesized those data, clarifying a means by which amyloid hematologic CR could be redefined. We highlighted the inadequacy of sFLC ratio as a response endpoint, corroborated the observations that iFLC ≤20 mg/L and dFLC ≤10 mg/L are prognostic for OS8, 9 and added how these measures synergize with serum/urine IFE. Importantly, among patients with severe renal dysfunction (eGFR <30), the CR definition using dFLC ≤10 mg/L retained its prognostic value, while iFLC ≤20 mg/L threshold did not. In addition, the dFLC-CR definition performed better by light chain isotype when considering both light chain isotypes. Although, the iFLC20 had better survival discrimination for lambda light chain isotype than did the dFLC10, iFLC20 performed poorly for kappa restricted patients. The dFLC-CR performed well in the overall population, but importantly, it performed well in more rare patient populations, making this variable the preferred FLC measure for a new and improved definition of amyloid CR.
The better survival prediction of the quantitative FLC measurements over serum/urine IFE may indicate the low sensitivity of IFE to assess residual disease over the sFLC assay. It will be important in the future as data mature to assess the effect of more sensitive assays, such as blood mass spectrometry (MASS-FIX)25 on survival discrimination alone, and in combination with the sFLC assay. Moreover, since the sFLC assays measure the free light chains, which are the building blocks of the amyloid, circulating light chain measures should be more reliable measure of continuous amyloid deposition.
We also demonstrated a suboptimal agreement between serum/urine response assessment and bone marrow biopsy studies, including flow cytometry-based residual disease assessment. It has been demonstrated that the addition of bone marrow biopsy—including MRD assessments—affects PFS more than OS.3-7, 26, 27 Future consensus response criteria should perhaps also include the following categories of response: stringent amyloid CR which would include a negative bone marrow; amyloid MRD-flow negative; and amyloid MRD-sequencing negative.
In conclusion, we provide systematic assessment of monoclonal protein studies at EOT in AL amyloidosis, demonstrating that normalization of sFLCR is a sub-optimal measurement and that incorporating low iFLC or dFLC is a better complement to IFE in the definition of hematological CR. Since dFLC is part of the current response definition and performs better in those patients with severe renal dysfunction or with kappa light chain amyloid, we suggest that it is the preferred free light chain measure for a revised CR definition. This study has several limitations. It is a single center retrospective study which limits assessment of response at fixed-time endpoint, (eg, 3 months and 6 months from therapy initiation). A fixedtime endpoint assessment overcomes the effect of time on response and would be more applicable for clinical trials purposes. Our study also includes disproportionate number of ASCT patients.
CONFLICT OF INTEREST
Eli Muchtar: None; Morie A. Gertz: Consultancy (Millenium) and honoraria (Celgene, Millenium, Onyx, Novartis, Smith Kline, Prothena, Ionis). Martha Q. Lacy: Research funding (Celgene); Nelson Leung: None; Francis Buadi: None; David Dingli: Research funding (Karyopharm Therapeutics, Amgen, and Millenium Pharmaceuticals); Suzanne R. Hayman: None; Ronald S. Go: None; Prashant Kapoor: Research funding (Takeda, Celgene, and Amgen); Wilson Gonsalves: none; Taxiarchis V. Kourelis: None; Rahma Warsame: None; Yi Lisa Hwa: None; Amie Fonder: None; Miriam Hobbs: None; Stephen Russel: None; John A. Lust: None; Mustaqueem Siddiqui: None; Vincent Rajkumar: None; Robert A. Kyle: None; Shaji Kumar: Consultancy (Celgene, Millennium, Onyx, Janssen, and BMS); and research funding (Celgene, Millennium, Novartis, Onyx AbbVie, Janssen, and BMS). Angela Dispenzieri: Research funding (Celgene, Millennium, Pfizer and Alnylam), Travel grant (Pfizer), Advisory Board (Janssen).
AUTHOR CONTRIBUTIONS
Eli Muchtar designed the study, analyzed the data, wrote the first draft and approved the final version of the manuscript; Morie A. Gertz, Martha Q. Lacy, Nelson Leung, Francis K. Buadi, David Dingli, Suzanne R. Hayman, Ronald S. Go, Prashant Kapoor, Wilson Gonsalves, Taxiarchis V. Kourelis, Rahma Warsame, Yi Lisa Hwa, Amie Fonder, Miriam Hobbs, Stephen Russell, John A. Lust, Mustaqueem Siddiqui, S. Vincent Rajkumar, Shaji K. Kumar, revised the manuscript critically and approved the final version of the manuscript. Robert A. Kyle performed patientsʼ follow-up, revised the manuscript critically and participated in final data analysis and approval of the final version of the manuscript; Angela Dispenzieri designed the study, analyzed the data, wrote the first draft and approved the final version of the manuscript.




