Phase I/II study of dasatinib in combination with decitabine in patients with accelerated or blast phase chronic myeloid leukemia
Funding information: Bristol-Myers Squibb; University of Texas MD Anderson Cancer Center, Grant/Award Number: CA016672
Abstract
Treatment of advanced-phase chronic myeloid leukemia (CML) remains unsatisfactory. Single-agent tyrosine kinase inhibitors have modest and short-lived activity in this setting. We conducted a phase I/II study to determine safety and efficacy of the combination of dasatinib and decitabine in patients with advanced CML. Two different dose schedules were investigated with a starting decitabine dose of either 10 mg/m2 or 20 mg/m2 daily for 10 days plus dasatinib 100 mg daily. The target dose level was decitabine 10 mg/m2 or 20 mg/m2 daily for 10 days plus dasatinib 140 mg daily. Thirty patients were enrolled, including seven with accelerated-phase CML, 19 with blast-phase CML, and four with Philadelphia-chromosome positive acute myeloid leukemia. No dose-limiting toxicity was observed at the starting dose level with either schedule. Grade ≥3 treatment emergent hematological adverse events were reported in 28 patients. Thirteen patients (48%) achieved a major hematologic response and six (22%) achieved a minor hematologic response, with 44% of these patients achieving a major cytogenetic response and 33% achieving a major molecular response. Median overall survival (OS) was 13.8 months, with significantly higher OS among patients who achieved a hematologic response compared to non-responders (not reached vs 4.65 months; P < .001). Decitabine plus dasatinib is a safe and active regimen in advanced CML. Further studies using this combination are warranted.
1 INTRODUCTION
Tyrosine kinase inhibitors (TKIs) have significantly improved the outcomes of patients with chronic phase chronic myeloid leukemia (CML-CP) with a significant improvement in overall survival (OS) which now nearly mirrors the normal life expectancy of the general population.1-6 Despite this improvement, 3% to 5% of patients with CML-CP progress to accelerated phase (AP) or blast phase (BP), and an additional 10% to 15% present de novo in AP or BP.7, 8
Single agent TKIs, although to date represent the single most effective agents for advanced phase CML (ie, CML AP and BP), provide only modest and short-lived responses.8-11 Several single-arm phase two studies of single-agent dasatinib in patients with imatinib-resistant or intolerant CML showed modest efficacy in the treatment of advanced-phase CML.12, 13 Major hematologic response (MaHR; ie, the primary endpoint in most series in this setting) in patients with CML-AP, myeloid CML-BP, and lymphoid CML-BP was achieved in 59%, 32%, and 31%, respectively.12 Major cytogenetic response (MCyR) was achieved in 31% and 37% of patients with CML-AP and CML-BP, respectively, compared to 45% in CML-CP.12, 13 Responses were however not durable in advanced-phase patients. In contrast to the estimated 2-year progression-free survival (PFS) rate in phase three trials of 80% in patients with CML-CP, it was only 51% in AP and 11% in myeloid BP CML.10, 14-16 Ponatinib has demonstrated potent single-agent activity in patients with advanced phase CML inducing MaHR in 55% and 31% of patients with CML-AP and CML-BP, respectively, and MCyR in 39% of patients with CML-AP and 23% of patients with CML-BP.17 Of note, this was a heavily pretreated population with more than 90% of patients previously treated with two or more TKIs and had resistance to second generation TKIs. Responses were again not as durable as in CML-CP, with an estimated 1-year PFS rate of 55% and 19% in patients with CML-AP and CML-BP, respectively, compared to 80% in patients with CML-CP.
Hypermethylation has been associated with disease progression in CML.18-20 Decitabine has shown modest single-agent activity in CML.21, 22 In patients with imatinib-refractory or intolerant CML-AP and CML-BP, low dose decitabine induced MCyR in 12% and 17% of the patients, respectively, and responses were short-lived.21 In an attempt to induce deeper and durable responses, imatinib was combined with low-dose decitabine in patients with advanced-phase CML. Note, MCyR was attained in 18% of patients, similar to that achieved by decitabine alone.23 However, upon stratification by BCR-ABL kinase domain mutational status, responses were higher among unmutated patients suggesting that synergy between both agents may be dependent upon residual sensitivity of leukemic cells to imatinib, as had been shown in vitro.23, 24
Given the higher potency of dasatinib against unmutated BCR-ABL, compared to imatinib, and its activity against most imatinib-resistant BCR-ABL mutants,25-27 we conducted a phase I/II study to investigate whether improved responses can be achieved through synergy between dasatinib and decitabine in patients with advanced-phase CML.
2 MATERIALS AND METHODS
2.1 Patient population
Patients with CML-AP, CML-BP, or Philadelphia chromosome (Ph)-positive acute myeloid leukemia (AML) were eligible regardless of prior TKI therapy. Definitions of CML phases were as previously described.28-30 Patients had to be 18 years or older, have an Eastern Cooperative Oncology Group (ECOG) performance status of ≤3, and have adequate hepatic and renal function [serum creatinine ≤177 μmol/L or creatinine clearance ≥1.0 mL/s/m2, total bilirubin ≤1.5 × upper limit of normal (ULN) unless due to Gilbert's syndrome or hemolysis, alanine aminotransferase (ALT) ≤3 × ULN unless due to leukemic involvement]. Prior therapy with TKIs including dasatinib was allowed. With the exception of hydroxyurea and TKIs, patients had to be off all prior therapy for ≥2 weeks prior to the start of the trial. Hydroxyurea and TKIs (except dasatinib if patient already receiving it) were discontinued ≥24 hours prior to the start of study therapy. Patients who were receiving dasatinib prior to enrollment were allowed to continue the drug until the start of the study therapy. Major exclusion criteria were severe congestive heart failure (CHF; NYHA class 3-4); uncontrolled medical comorbidities including uncontrolled active systemic infections, unstable angina within 3 months, uncontrolled arrhythmia or hypertension, and QTcF interval > 470 ms; clinically significant pleural or pericardial effusion; history of significant bleeding disorder unrelated to malignancy; planned allogeneic stem cell transplantation (SCT) within 4 weeks.
2.2 Study design and treatment
This was an open label, phase I/II study of two different dose schedules of dasatinib in combination with decitabine in patients with advanced-phase CML. The primary objectives were to determine the maximum tolerated dose (MTD) (up to the target dose) and assess the efficacy of the combination regimen. Secondary objectives included determining the response duration (RD), safety profile, and overall survival (OS).
Patients were randomly assigned to one of the two schedules and dose escalation proceeded using a 3 + 3 design. The starting schedule was decitabine 10 mg/m2 intravenously (IV) daily for 10 days, plus dasatinib 100 mg orally (PO) daily (schedule A), and decitabine 20 mg/m2 IV daily for 10 days, plus dasatinib 100 mg PO daily in a 28-day cycle (schedule B). The next dose level (target dose) was decitabine 10 mg/m2 IV daily for 10 days (schedule A) or 20 mg/m2 IV daily for 10 days (schedule B) with dasatinib 140 mg PO daily. Once the MTD or target dose (if MTD not exceeded) was determined for both arms, the phase 2 portion of the study was to start, and all subsequent patients were to be randomized to the two schedules. Patients who achieved a MaHR were allowed to receive maintenance therapy in the form of dasatinib 100 mg PO daily (or maximum dose tolerated) and decitabine for 5 days at the maximal dose tolerated. Continuation with only one of the two drugs was allowed if required for tolerance or convenience, as clinically indicated, as determined by the treating physician. The study was terminated early during the phase II portion of the study due to slow enrollment.
The study was approved by the Institutional Review Board and all patients signed informed consent according to the Declaration of Helsinki (clinicaltrials.gov study number NCT01498445).
2.3 Safety assessment
Adverse effects (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. The MTD was defined as the highest dose at which zero of three or ≤1 of six patients experienced a first cycle dose-limiting toxicity (DLT). The DLTs were defined as AEs occurring during the first cycle of therapy and fulfilling one of the following criteria: grade ≥3 non-hematologic treatment-related adverse event (TRAE) including grade ≥3 nausea, vomiting, and diarrhea uncontrolled with optimal therapy; grade ≥3 laboratory abnormalities accompanied with clinical consequences; grade ≥3 electrolyte abnormalities not corrected by optimal replacement therapy; grade ≥3 neutropenia and/or thrombocytopenia in the presence of a hypocellular bone marrow (BM) with <5% BM blasts lasting for ≥6 weeks from the start of therapy (in case of a normocellular BM with <5% blasts, 8 weeks of pancytopenia was considered a DLT) and confirmed by a second hypocellular BM evaluation performed seven to 14 days after the discontinuation of dasatinib.
Patients who experienced grade ≥3 non-hematologic TRAEs (including uncontrolled nausea, vomiting, diarrhea, and electrolyte abnormalities despite optimal therapy) were considered for treatment interruption until resolution of the toxicity after which treatment was re-initiated at the next lower dose. One dose level reduction was also considered for patients with response to therapy and pre-course absolute neutrophil count (ANC) >1 × 109/L and platelets >50 × 109/L who had sustained low ANC (<0.5 × 109/L) and/or platelet counts (<25 × 109/L) for more than two consecutive weeks with <5% BM blasts.
2.4 Efficacy assessment
Response criteria were as previously published.29 Overall hematologic response (OHR) included complete hematologic response (CHR), no evidence of leukemia (NEL), and minor hematologic response (MiHR). Complete hematologic response was defined as normal white blood cell count (WBC), no blasts or promyelocytes in peripheral blood (PB), ANC ≥1 × 109/L, platelets >100 × 109/L, PB basophils <5%, ≤5% BM blasts, and no extra-medullary disease (EM). So, NEL had the same criteria as CHR except for platelets between ≥20 × 109/L and <100 × 109/L and/or ANC between ≥0.5 × 109/L and <1 × 109/L. Note, MaHR was defined as CHR and NEL. So, MiHR was defined as <15% blasts in PB and BM, <30% blasts plus promyelocytes in BM and PB, <20% basophils in PB, and no EM disease. Cytogenetic response was classified as complete (CCyR; 0% Ph-positive), partial (PCyR; 1-35% Ph-positive), and minor (36-65% Ph-positive). A major cytogenetic response (MCyR) included CCyR and PCyR (ie, Ph-positive ≤35%). Molecular response was classified into MR4.5 [BCR-ABL/ABL ratio ≤ 0.0032% international scale (IS)] and major molecular response (MMR; BCR-ABL/ABL ratio ≤ 0.1% IS). BCR-ABL kinase domain mutational analysis was performed by Sanger sequencing.23
2.5 Statistical analysis
Due to the slow response to decitabine repeatedly reported in previous studies, patients had to have completed at least two full cycles of therapy to be included in the efficacy population, unless they developed disease progression compelling early drug discontinuation.22, 31 The safety population included all patients who received at least one dose of decitabine. The randomized phase two portion of this trial was conducted using the Gehan's two-stage design to evaluate the activity of both treatment arms. The OS was calculated from start of therapy to date of death or last follow-up. The response duration and OS were estimated using Kaplan-Meier method and P values were calculated by log-rank test. Safety was assessed by analyzing the reported incidence of treatment-emergent adverse effects (TEAEs).
3 RESULTS
3.1 Patient characteristics
From June 2012 to December 2017, a total of 30 patients were enrolled, 23 in phase one and seven in phase two. Patient characteristics are summarized in Table 1. Median age was 51 years (range, 18-89 years) with male predominance. Nineteen patients had CML-BP (18 myeloid and one lymphoid BP), seven had CML-AP, and four had Ph + AML. The BCR-ABL kinase domain mutational status was determined in 20 patients, of whom four had mutations [two patients with E255K (p-loop),32, 33 one with V299L (active site),34 and one patient with both T315I and L298V (active site)32, 33, 35]; one additional patient had a 162 base pair deletion. Cytogenetic clonal evolution was identified in 17 patients (57%).
| Patient characteristics (N = 30) | Number (%) | Median [range] |
|---|---|---|
| Age (years) | 51 [18-89] | |
| ≥60 years | 5 (17) | |
| Male gender | 19 (63) | |
| Race | ||
| White | 16 (53) | |
| Black | 7 (23) | |
| Hispanic | 6 (20) | |
| Asian | 1 (3) | |
| Diagnosis | ||
| Blast phase | 19 (63) | |
| Myeloid | 18 (60) | |
| Lymphoid | 1 (3) | |
| Accelerated Phase | 7 (23) | |
| Ph-positive AML | 4 (13) | |
| Prior CML-CP | 14 (47) | |
| Extramedullary disease | 3 (10) | |
| Prior TKI therapy | 18 (60) | 2 [1–4] |
| >1 prior TKI | 10 (33) | |
| Imatinib | 12 (40) | |
| Dasatinib | 11 (37) | |
| Nilotinib | 8 (27) | |
| Ponatinib | 7 (23) | |
| Bosutinib | 1 (3) | |
| Prior SCT | 6 (20) | |
| BCR-ABL kinase domain mutation | ||
| No mutation | 15 (50) | |
| Not available | 10 (33) | |
| ABL kinase domain mutations | 4 (13) | |
| E255K (p-loop) | 2 (7) | |
| V299L (active site) | 1 (3) | |
| T315I/L298V (active site) | 1 (3) | |
| 162 base pair deletion | 1 (3) |
- Abbreviations: AML, acute myeloid leukemia; CML-CP, chronic phase chronic myeloid leukemia; Ph, Philadelphia chromosome; SCT, stem cell transplantation; TKI, tyrosine kinase inhibitor.
Three of the four patients with Ph + AML received one or more prior therapies including cytotoxic chemotherapy (N = 2), azacitidine (N = 2), SCT (N = 2), and dasatinib (N = 1). Fourteen patients (47%) with CML-BP (N = 11) and CML-AP (N = 3) had prior documented CML-CP and received previous therapy including TKIs (N = 14), SCT (N = 4), interferon (N = 2), and omacetaxine mepesuccinate (N = 2). Of these 14 patients, a total of four, two, five, and three patients had received one, two, three, and four TKIs, respectively, with a median of three TKIs per patient (range, 1-4). Eleven patients had de novo CML-BP (N = 7) and CML-AP (N = 4), of which three patients with CML-BP received prior therapy including azacitidine (N = 1), nilotinib (N = 1), and dasatinib (N = 1); one additional patient had CML-BP progressing from CML-AP treated with imatinib, but had no previously documented CML-CP. Three patients with CML-BP (two de novo and one previous CP-CML) had extramedullary disease prior to study enrollment. Median time from diagnosis to study enrollment was 0.7 months (range, 0.1-27.4). For the 15 patients with previously documented CML-CP and CML-AP, the median time from initial CML diagnosis to disease progression was 49.2 months (range, 7.8-221.3).
3.2 Safety and toxicity
The number of patients treated at each dose schedule, stratified by diagnosis, is shown in Table 2. All patients were evaluable for toxicity. The TEAEs are summarized in Table S3. The most common non-hematologic TEAEs were infection (67%), nausea (47%), and pyrexia (43%). Twenty-one patients (70%) experienced at least one grade ≥3 non-hematological TEAE, with infections (30%), bleeding (13%), and electrolyte imbalance (13%) being the most common. Grade ≥3 hematological adverse events were reported in 28 patients (93%). Neutropenia (80%), thrombocytopenia (70%), and anemia (10%) were the most common grade ≥3 hematologic TEAEs.
| Dose level | Schedule | Dasatinib/decitabine (mg/d) | Total (N) | CML-BP (N) | CML-AP (N) | Ph + AML (N) | DLT (N) |
|---|---|---|---|---|---|---|---|
| 0 (Start dose) | A | 100/10 | 4 | 3 | 1 | 0 | 0 |
| B | 100/20 | 3 | 2 | 1 | 0 | 0 | |
| +1 (Target dose) | A | 140/10 | 11 | 8 | 1 | 2 | 1 |
| B | 140/20 | 12 | 6 | 4 | 2 | 1 |
- Abbreviations: AML, acute myeloid leukemia; AP, accelerated phase; BP, blast phase; CML, chronic myeloid leukemia; DLT, dose-limiting toxicity; Ph, Philadelphia chromosome.
No DLT was observed at the starting dose level for either schedule. One DLT was observed at the target dose level of each schedule among 11 and 12 patients treated at each dose schedule, respectively (1A: grade three heart failure; 1B: grade four cardiac arrest), therefore the target dose was considered the dose to be further explored in phase two for each arm. At dose level 1A, one patient with multiple comorbidities, including hypertension, dyslipidemia, and diabetes, developed grade three congestive heart failure (left ventricular ejection fraction of 41%) after one cycle of therapy determined to be possibly related to dasatinib. At dose level 1B, one patient with no significant past medical history developed grade three chest pain and grade four myocardial infarction and cardiac arrest after 2 weeks of therapy.
Patients received a median of two cycles (range, 1-35) of the combination therapy. The AEs leading to a dose-reduction or transient treatment interruption occurred in 27% and 50% of the patients, respectively. Discontinuation of therapy due to an AE occurred in three patients (10%): two for pleural/pericardial effusion and one for cardiac arrest.
3.3 Response and survival
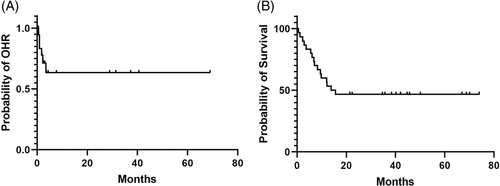
Twenty-seven patients (90%) were evaluable for response (Table S4). Three patients (de novo AP = 1, BP = 1, Ph + AML = 1) were unevaluable for response as they did not complete two cycles of therapy due to drug toxicity (n = 2; one cardiac arrest due to myocardial infarction and one pleural effusion) or insurance denial (n = 1). Thirteen patients (48%) achieved MaHR (CHR = 10 and NEL = 3) and six patients (22%) achieved MiHR. Of these 19 patients with hematologic response, 13 had myeloid CML-BP and six had CML-AP (including three de novo CML-AP). None of the patients with Ph + AML responded to therapy. Median duration of OHR was not reached with the median time to OHR of 1.4 months (range, 0.2-14.7) (Figure 1A).
Twelve patients (44%) achieved MCyR including 10 with CCyR [dose schedules 0A (N = 2); 1A (N = 4); 1B (N = 4)] and two with PCyR [dose schedules 0A (N = 1); 1A (N = 1)]. Four of the twelve patients that achieved MCyR (3 CCyR, 1 PCyR) had baseline cytogenetic clonal evolution. Nine patients (33%) achieved MMR including four that achieved MR4.5.
Six of the 19 patients who achieved OHR relapsed (BP = 5, AP = 1), with a median time to relapse of 1.35 months (range, 0.6-6.7). Two of these patients were assessed for ABL kinase domain mutations and none was detected. The five patients with CML-BP died 0.2, 4.6, 5.9, 6.8, and 13.4 months, respectively, after relapse; three of these patients received subsequent therapy in the form of TKIs (N = 3), chemotherapy (N = 2), and allogeneic SCT (N = 1). The remaining patient with CML-AP proceeded with allogeneic SCT after achieving CHR with decitabine plus dasatinib, relapsed 1 month after SCT, received TKIs after SCT (ponatinib for 5 months discontinued due to intolerance; dasatinib for 10 months, discontinued due to intolerance; imatinib for 33 months, discontinued due to insurance denial), and is alive 71+ months after SCT off TKI maintenance therapy for 20+ months in MR4.5.
Of the remaining 13 patients who achieved OHR, six patients with CML-BP received allogeneic SCT. Five of the patients who received SCT are alive and in MMR 32+, 38+, 42+, 47+, and 66+ months after SCT, and one died in MR4.5 two months after SCT due to transplant-related complications. Five other patients (AP = 3, BP = 2) remain in MMR 35+, 36+, 38+, 42+, and 70+ months from start of therapy including three patients on dasatinib maintenance and one who switched to bosutinib due to dasatinib-induced pleural and pericardial effusions. Finally, one patient with CML-AP achieved CHR and remained on study for 22 months without achieving cytogenetic or molecular response, and one patient with CML-AP achieved MiHR and died 6 months after the start of therapy due to sepsis.
In total, eight patients were successfully bridged to allogeneic SCT with the best response to therapy prior to SCT of CHR in two patients, MCyR in two patients, and MMR in four patients. Six of these patients received TKI after SCT (dasatinib = 5, ponatinib = 2, and imatinib = 1). Two patients relapsed after SCT; one died 7.8 months after SCT due to disease progression and the other remains alive for 71+ months after SCT. Five of the other six patients are alive and in MMR, achieving CHR 1, MCyR 2, and MMR 2 prior to SCT, and one patient died in MR4.5 due to transplant-related complications.
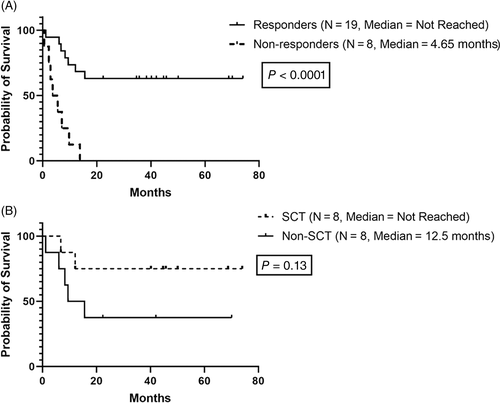
With a median follow-up of 43 months (range, 21.5-74), 14 patients (47%) are alive and 16 patients (53%) died. Causes of death included disease progression (N = 12), sepsis (N = 3), and SCT-related complications (N = 1). Median OS for the entire cohort was 13.8 months (95% CI: not evaluable) (Figure 1B), with a significantly better OS among patients who achieved an OHR compared to non-responders (Figure 2A; median: not reached vs 4.65 months, P < .001). After excluding three patients with de novo CML-AP who achieved CCyR 1, 3, and 9 months after therapy, there was a noted improvement in the median OS among responders who received SCT compared to non-SCT recipients (Figure 2B; median: not reached vs 12.5 months, P = .13).
4 DISCUSSION
To the best of our knowledge, this is the first clinical trial using the combination of decitabine plus dasatinib in advanced-phase CML. The combination regimen was well tolerated with only two DLTs observed including heart failure and cardiac arrest. The MTD was declared to be the target dose in each schedule to which patients were randomized in the phase two portion of the study. Decitabine plus dasatinib was active in patients with CML-AP and BP inducing MaHR, MiHR, MCyR, and MMR in 48%, 22%, 44%, and 33% of the patients, respectively. Most responses are hematologic, which has been the primary endpoint in most trials for advanced phase CML using TKI. This underscores the unfortunate low probability, even with combination therapy, of attaining cytogenetic and molecular responses that are more prominently pursued in CML-CP. However, patients who achieved OHR had a survival benefit compared to those who did not respond to therapy, similar to the results of Oki et al using decitabine and imatinib combination.23 In addition, such patients may have the option to be bridged to SCT, something that was accomplished in eight of the patients reported in this study. Due to poor accrual leading to study termination, only seven patients were enrolled to the randomized phase two portion of the study which was insufficient to determine a difference in outcome by treatment schedule.
Previous studies have shown modest single-agent activity of decitabine in patients with advanced-phase CML with cumulative (AP + BP) OHR and MCyR rates reaching 57% and 13%, respectively.21, 22 Dasatinib has also demonstrated single-agent activity in advanced-phase CML, especially in patients with CML-BP. In the phase one trial conducted by Talpaz and colleagues, the rates OHR and MCyR induced by single-agent dasatinib in 34 patients with CML-AP and myeloid CML-BP resistant or intolerant to imatinib were 79% and 32%, respectively.30 Subsequent trials conducted specifically in patients with imatinib-resistant or imatinib-intolerant CML-BP proved its efficacy in this subset of patients, with OHR and MCyR rates in patients with myeloid CML-BP and lymphoid CML-BP of 53% vs 36%, and 31% vs 50%, respectively.10, 13 In our study, among the 23 patients with CML-AP and myeloid CML-BP who were evaluable for response, 83%, 56%, and 52% of the patients achieved OHR, MaHR, and MCyR, respectively. Although these response rates do not appear significantly higher than what was reported with dasatinib alone,30 our patients were more heavily pretreated; 70% received prior TKI therapy, 39% received more than one TKI, and 39% received prior single-agent dasatinib. Of note, the rates of OHR, MaHR, and MCyR in the nine patients with CML-AP (N = 1) and myeloid CML-BP (N = 8) who received prior dasatinib were 55%, 44%, and 22%, respectively, thereby supporting the in vitro and in vivo data suggesting potential synergy between HMAs and TKIs.
Excluding patients with de novo CML-AP who have excellent response to TKIs, outcomes of patients who progress to advanced-phase CML are dismal if not receiving SCT.8-11 The TKI therapy, with or without chemotherapy, followed by allogeneic SCT has been shown to improve outcomes of patients with advanced-phase CML.36-41 A nonrandomized prospective study conducted in patients with CML-AP treated with either imatinib alone vs imatinib followed by allogeneic SCT showed superiority of the latter therapy with a 6-year OS of 51.4% vs 83.3% (P = .023), respectively.42 In a subsequent retrospective study conducted by Jiang et al, there was a significant improvement in the 4-year OS (46.7% vs 9.7%, P < .001) and event free survival (47.1% vs 10%, P < .001) rates among CML-BP patients treated with TKIs plus allo-SCT compared to TKIs alone, confirming previous results.43 Consistent with these studies, we report an improvement in the median OS among responders who received SCT compared to non-SCT recipients, although the numbers were too small to achieve statistical significance. This may suggest the potential use of this combination regimen as a safe and effective bridge to SCT in patients with advanced CML.
In imatinib-resistant cell lines, in vitro studies have shown additive growth inhibition upon combining decitabine with imatinib provided the cell lines have residual sensitivity to imatinib monotherapy.24 Synergism was achieved only at doses of imatinib capable of overcoming resistance to apoptosis through increasing the degree of BCR-ABL kinase inhibition.24 In the phase two study conducted by Oki et al, imatinib plus decitabine was only active in imatinib-resistant advanced-phase CML patients who did not harbor a kinase domain mutation, confirming the abovementioned preclinical findings.23 The results reported in this study compare favorably both in OHR (70% vs 43%) and MCyR (44% vs 18%) rates to those reported with decitabine plus imatinib.23 Furthermore, in our study the median OS for patients with CML-AP and BP were not reached and 59 weeks, respectively (P = .2), which compares favorably to that of CML-AP and BP patients treated with decitabine plus imatinib who had a median OS of 56 and 15 weeks, respectively.23 The higher potency of dasatinib and the broader coverage of mutations inhibited by dasatinib may have contributed to a possible better outcome. However, it should be acknowledged that the percentage of patients with mutations at the start of therapy in the report from Oki et al was somewhat higher than what we observed in the present study (27% vs 13%).23, 25, 26 Collectively, these results support the potential clinical benefit of combining hypomethylating agents (HMAs) with dasatinib. Additional studies are warranted to confirm this observation. Ponatinib may also be a more effective TKI for combination studies considering the higher response rate achieved with single agent in all stages of the disease in heavily previously treated patients, and activity against all tested mutations.44
Interestingly, despite this clinically noted synergy between HMAs and TKIs, correlative studies have failed to show a relationship between response and induction of global and p15 specific hypomethylation.21, 23 Investigators in China have shown that the synergy may be due to decitabine-induced demethylation and re-expression of Src homology region two domain-containing tyrosine phosphatase-1 (SHP-1), which in turn enhances the anti-leukemic effect of TKIs.45 The clinical relevance of using SHP-1 expression as a marker of response to decitabine in advanced-phase CML patients is yet to be discovered.
Myelosuppression has been the main AE noted with the use of either decitabine, dasatinib, or decitabine plus imatinib. Although hematologic toxicities were difficult to assess due to underlying leukemia, myelosuppression was seen in our study at an acceptable rate for this patient population.21-23, 30 Neutropenia, thrombocytopenia and febrile neutropenia were reported in 83%, 73%, and 27% of our patients, respectively, including patients with baseline myelosuppression secondary to leukemia. To put these results in context, the reported rates of these events with decitabine plus imatinib in patients with adequate pretreatment counts (absolute neutrophil count ≥1.0 × 109/L and platelet count ≥100 × 109/L at baseline) were 76%, 75%, and 32%, respectively,23 As expected, we had a higher percentage of grade ≥3 dyspnea (9%) than that reported by Oki et al (4%) which may be attributed to the use of dasatinib.23
In conclusion, decitabine plus dasatinib is a safe and novel approach for managing patients with advanced CML with a survival probability among responders that appears better than what would be expected with either agent alone. Further studies are warranted to confirm the efficacy of this combination, and determine whether there is any difference in outcome by treatment schedule. Studies using ponatinib instead of dasatinib and/or adding other agents to this combination are also warranted.
CONFLICT OF INTEREST
Dr. Jorge Cortes has received research support (to his institution) from BMS, Novartis, Pfizer, Takeda, and Sun Pharma, and is a consultant for Novartis, Pfizer, and Takeda. Dr. Farhad Ravandi has received research funding and advisory board membership from BMS. The remaining authors declare no potential competing financial interests.
AUTHOR CONTRIBUTIONS
Conception and design: J.C., H.M.K.
Provision of study materials or patients: H.K., G.B., R. C., G.G.-M., N.D., F.R., S.V., J.B., Z.E., M.O., M.L., E.J., J.C.
Collection and assembly of data: Y.A., M.L., Y.A.
Data analysis and interpretation: Y.A., J.C
Manuscript writing: Y.A., J.C
Final approval of manuscript: All authors reviewed the provided final approval of this manuscript.




