Individual red blood cell fetal hemoglobin quantification allows to determine protective thresholds in sickle cell disease
Nicolas Hebert and Marie Georgine Rakotoson contributed equally to this work.
Abstract
Polymerization of the sickle hemoglobin (HbS) is a key determinant of sickle cell disease (SCD), an inherited blood disorder. Fetal hemoglobin (HbF) is a major modulator of the disease severity by both decreasing HbS intracellular concentration and inhibiting its polymerization. However, heterocellular distribution of HbF is common in SCD. For HbS polymerization inhibition, the hypothesis of an “HbF per red blood cell (HbF/RBC) threshold” requires accurate measurement of HbF in individual RBC. To date, HbF detection methods are limited to a qualitative measurement of RBC populations containing HbF - the F cells, which are variable. We developed an accurate method for HbF quantification in individual RBC. A linear association between mean HbF content and mean RBC fluorescence by flow cytometry, using an anti-Human-HbF antibody, was obtained from non-SCD subjects presenting homogeneous HbF distribution. This correlation was then used to measure HbF/RBC. Hydroxyurea (HU) improves SCD clinical manifestations, mainly through its ability to induce HbF synthesis. The HbF distribution was analyzed in 14 SCD patients before and during HU treatment. A significant decrease in RBC population containing less than 2 pg of HbF/RBC was observed. Therefore, we tested associations for %RBC above different HbF/RBC thresholds and showed a decrease in the pathognomonic vaso-occlusive crisis incidence from the threshold of 4 pg. This quantity was also correlated with the level of sickle RBC after in vitro deoxygenation. This new method allows the comparison of HbF/RBC distributions and could be a useful tool to characterize baseline patients HbF distribution and therapeutic response to HbF inducers.
1 INTRODUCTION
Sickle cell disease (SCD) is one of the most common monogenic disorder in the world and is due to a single amino acid substitution in the beta-globin chain of the hemoglobin (Glu6Val). Sickle hemoglobin (HbS) has the unique property of forming polymers within red blood cells (RBCs) under a deoxygenated state, distorting their normal shape and altering their properties and ability to carry oxygen throughout the body. Acute chest syndromes (ACS), vaso-occlusive crisis (VOC) and chronic organ damages are the major causes of death in SCD patients.
Fetal hemoglobin (HbF) is known to be the most protective independent factor in SCD by inhibiting HbS polymerization, decreasing clinical manifestation and mortality.1, 2 The HbS content and the percentage of HbF are the two main modulators of clinical severity. However, the association between the HbF percentage of total Hb (%HbF) and chronic SCD complications is variable; conversely a low incidence of VOC has been observed in patients with low %HbF, not explained by other protective factors.3, 4 These observations suggested that rather than the %HbF, a threshold of HbF content in individual RBCs could be a critical determinant of the HbF effect.5 The protective effects of HbF can be easily rationalized when there is a homogeneous HbF distribution, as seen in most HbS/beta thalassemia and HbS/HPFH (hereditary persistence of fetal hemoglobin) patients, while the heterocellular distribution of HbF observed in homozygous patients presents a challenge.6
In the last decades, several techniques have been developed to detect HbF in RBCs. High performance liquid chromatography (HPLC), routinely used, provides the percentage of HbF in a hemolysate.7 Chemical8 or immunological methods estimate the percentage of RBCs containing variable amounts of HbF, named F cells.9-16 Although widely used in maternal/fetal incompatibility diagnosis or hemoglobinopathies, these methods are limited to a qualitative measurement of HbF. In addition, the lack of a standardized HbF level in F cells does not allow comparison between patients from different centers or over time for a same patient.
Herein, we developed a new and easy to implement method to accurately measure HbF amount per RBC (HbF/RBC) providing a quantitative HbF distribution. This method is based on the correlation between the mean corpuscular HbF (MCHbF) and the fluorescence intensity by flow cytometry using an anti-Human-HbF monoclonal antibody coupled with phycoerythrin (PE). This relationship is linear in patients with homogeneous HbF distribution, as for beta thalassemia and HPFH patients. The HbF fluorescence intensity is normalized from standardized beads allowing the analysis on any flow cytometer.
We show that this quantitative approach has the potential of being a novel tool in the treatment of SCD patients and could provide an in-depth assessment of any HbF inducers. As a proof of concept, we used this new method to study and analyze changes in the cellular HbF distribution in SCD patients, following 6 months of treatment by the well-known HbF inducer hydroxyurea (HU).17 Multiple studies have shown that HU increases %HbF mainly by increasing the fraction of both F cells and F reticulocytes,18-20 but none of them analyzed the cellular HbF content distribution. Our results strengthen the hypothesis that the number of RBCs above a threshold of HbF is likely more protective than a heterocellular distribution,21 both from a biologic and a clinical point of view, and suggest that this threshold might be low.
2 METHODS
2.1 Patients
Eligible patients were selected from the SICLOPEDIE cohort monitored in our referral center (Henri Mondor Hospital, Creteil, France). This protocol was approved by the local ethics committee (CPP-Île-de-France IV Saint-Louis Hospital, IRB 00003835). In accordance with the Declaration of Helsinki, all patients gave their signed informed consent. All data were rendered anonymous to protect patients' privacy and confidentiality.
Two groups of patients, all ≥18 years, were recruited: group A was used for method development and group B was used to assess changes produced by HU treatment.
2.1.1 Inclusion criteria in group A
Patients carrying HPFH, β or δβ Thalassemia or genetic hemochromatosis (as controls with very low HbF). Homogeneous HbF distribution was confirmed by flow cytometry. Exclusion criteria were SCD (HbSS, Sβ-thalassemia or SC) or sickle cell trait (AS), blood transfusion within 3 months preceding the enrolment and pregnancy.
2.1.2 Inclusion criteria in group B
Patients SS or S-β0 thalassemia at steady state (>1 month from a VOC and > 3 months from a transfusion) and undergoing a treatment with hydroxyurea (HU). Exclusion criteria were SC or S-β+ thalassemia genotype, chronic transfusion program, erythropoietin treatment and pregnancy. We collected samples and biologic parameter values before treatment (D0), between 15 days and 1 month (D15-M1), 3 and 4 months (M3-4) and ≥ 6 months (≥M6) on HU.
2.2 HbF assays
The HbF level was determined in RBCs using three techniques: HPLC of total hemoglobin, complete blood count and flow cytometry.
2.2.1 Percentage HbF determination on HPLC
The HbF dosage was performed through ion exclusion liquid chromatography using a Variant II Hemoglobin Testing System (Bio-Rad). The different Hbs were eluted from column based on ionic interaction with carboxyl group, and then introduced in a photometer detector using two different wavelengths of 690 nm and 415 nm for baseline and sample detection, respectively. The chromatography system run time was 6 minutes and HbF was eluted up to 0.5 minute. Results were acquired with CDM 5.2 software.
2.2.2 Complete blood count
The RBC count, mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) were determined using a Horiba ABX Micros ES 60 counter (HORIBA Medical).

2.2.3 HbF detection by flow cytometry
The HbF fluorescence in RBCs was measured using a eight-color BD FACSCanto II flow cytometer (BD Biosciences). Acquired data on flow cytometer were analyzed using FlowJo v10 software (BD Biosciences).
2.2.4 RBC fixation and permeabilization
Prior to intracellular staining, the RBC membrane was fixed and permeabilized using Fetal Cell Count kit reagents (Cat IQP-363, IQ Products) according to the manufacturer's instructions using 5 μL of packed RBC.
2.2.5 HbF immunofluorescent staining
Mouse monoclonal anti-human HbF antibody conjugated with R-phycoerythrin (R-PE) (reagent F, Fetal Cell Count kit, IQ Products) was used for immunologically based HbF detection, and 20 μL of a PE mouse IgG1 Kappa (BD Pharmingen) used as a negative isotypic control. The RBCs were incubated for 15 minutes, shielded from light at room temperature. Thereafter, RBCs were washed with phosphate buffer saline (PBS) and centrifuged at 300g for 3 minutes. Stained RBCs were immediately analyzed on a flow cytometer. The anti-carbonic anhydrase (CA), presents in the fetal cell count kit, was never used, first, to avoid fluorescence interference on the measured signal from the anti-HbF because of spectral overlap of FITC and R-PE emission spectra and second, because all the quantifications were performed on adult RBCs (not from a fetus).
2.2.6 Flow cytometry acquisition
For each experiment, a negative isotypic control and a positive control (patient two from group A with HPFH and presenting 100% HbF - as confirmed by HPLC - Figure S1) were processed. Light scatter thresholds were used to select RBC population and exclude cellular debris. Every PE fluorescence intensity (FL-2) was monitored with a photomultiplier tube (PMT) voltage set at 400 V. 1 × 105 cells were gated on FSC-A vs SSC-A plot and recorded. Doublet exclusion was performed based on selecting single cells on FSC-H (FSC-Height) plotted against FSC-W (FSC-Width) and then on SSC-H vs SSC-W. In parallel, quantitation beads (Becton Dickinson QuantiBRITE PE, BD Biosciences)23 were analyzed on the same flow cytometer using the same settings as used for RBCs. A minimum of 1 × 104 beads was recorded allowing four fluorescence levels corresponding to a number of PE molecules per bead which is batch specific. A linear regression involving bead fluorescence intensity and number of PE molecules per bead was then obtained (Figure S3A).
2.3 HbF determination method in individual RBC
A method for HbF quantification in single RBC was developed from samples collected from patients assigned in group A. Mathematically, we applied a correction factor to the measured PE fluorescence intensities to consider the variability of the degree of coupling of the antibody, which can differ from one batch to another. Fluorescence:protein ratios were 1.2, 1.04 and 0.66 for batches number 1, 2 and 3, respectively. Fluorescence values of each RBC were converted into PE molecules per RBC giving a normalized HbF fluorescence by using the quantitation beads. Normalized HbF fluorescence per RBC was then determined by replacing bead fluorescence intensity by RBC fluorescence. Because MCHbF corresponds to an average HbF content per RBC when homogeneous HbF distribution is observed, a linear regression associating the normalized HbF fluorescence and MCHbF was obtained. This linear regression was used to assess the HbF/RBC for group B patients.
2.4 F cell detection methods
The F cell percentages were assessed by using two different methods as previously described.16, 24 Briefly, cells were analyzed by flow cytometry as above. The F cell percentage was calculated as the fraction of positive cells when incubated with the anti-HbF antibody, compared to a threshold set up according to the fluorescence intensity of unstained cells (method 1) or cells incubated with the isotypic control (method 2). (See F cell detection strategy - Figure S2).
2.5 Analysis of the HbF distribution normality
To assess the log-normal distribution of HbF in RBCs from group A patients D'Agostino & Pearson normality tests were performed. HbF/RBC was measured by flow cytometry as described. The R-PE fluorescence of each RBC was extracted using FlowJo software and 500 events were randomly selected to test the normality. A homogeneous HbF distribution was considered when the P value was > .05.
2.6 Association between HbF/RBC and vaso-occlusive crisis occurrence
Number of vaso-occlusive crisis (VOC) was collected from computerized patient's file from Henri Mondor Hospital for each group B patient. The VOC incidence during the 3 years period before the beginning of HU treatment was compared to HbF/RBC measured at D0, and the VOC incidence during the 3 years period after 6 months of HU treatment at stable dose was compared to HbF/RBC measured at ≥M6. The VOC was defined as pain or tenderness, affecting at least one part of the body, including limbs, ribs, sternum, head (skull), spine, and/or pelvis, that required opioids, was associated with a hospitalization and was not attributable to other causes.
2.7 In vitro sickling assay
Blood samples were collected from 46 SCD patients from the SICLOPEDIE cohort and not included in group B. Total blood was washed as described and RBCs were diluted 400-fold in PBS pH = 7.4 containing 5 mM glucose (Sigma-Aldrich). The oxygen was enzymatically depleted by adding glucose oxidase and catalase (Sigma-Aldrich) to diluted blood to final concentrations of 0.625 mg/mL and 0.01 mg/mL, respectively. Samples were rested for at least 2 minutes at room temperature and then observed under an inverted microscope (Zeiss AxioObserver).25 Pictures were analyzed using ImageJ 1.52a software (NIH). Results were expressed as the ratio of sickle RBCs on total RBCs.
2.8 Statistical analysis
For group A patients, association between MCHbF and normalized HbF fluorescence was assessed by a Pearson correlation. The log-normal distribution of HbF was assessed by the D'Agostino & Pearson normality test.
For group B patients, comparisons of biologic parameters between D0 and ≥ M6 were performed using Wilcoxon matched-pairs signed rank tests. Comparisons of HbF/RBC at D0, D15-M1, M3-M4 and ≥ M6 were performed using Friedman tests. Correlations between %RBC above HbF thresholds and biologic parameters or number of VOC before and after HU treatment were performed using Spearman correlation tests. The ROC (receiver operating characteristics) analysis were performed by applying a cut-off for VOC incidence of ≤1 over 3 years.
Statistical analyeses were performed using Prism 8 software (GraphPad) or STATA 15.1 software (Statacorp). All tests were 2-tailed using a significance level set at .05.
3 RESULTS
3.1 Determination of HbF per RBC
The distribution of HbF content per RBC was assessed by flow cytometry (Figure 1A) for the 18 group A patients (Table S1). A log-normal distribution, as verified by D'Agostino & Pearson normality tests, indicates a homogeneous HbF content among the RBC population (Table S2). Patients A-1 to A-6 and A-8 to A-12 were selected for further study while A-7 and A-13 to A-18 were excluded because of a non-log-normal distribution. To consider a variability introduced by the fetal cell count kit batch, linear regressions were independently determined as the relationship between the mean corpuscular HbF (MCHbF) and the mean normalized HbF fluorescence for each patient, using three different batches (n°1; 2 and 3) (Figure 1B). The linear regressions obtained were similar for the three batches (r = 0.9911 and P = .001; r = 0.9930 and P < .001; r = 0.9978 and P < .001 for batches n°1, 2 and 3 respectively). We thus calculated a mean linear regression including all the data measured with the three batches (r = 0.9984; P < .001). This mean linear regression which functions as a standard curve, allows the determination of the HbF/RBC according to the corrected fluorescence intensity and was used for all the quantifications of the study. The inclusion of the group A patients presenting a heterogeneous HbF distribution (patients A-7 and A-13 to A-18) modified both r parameters (0.9979 and 0.9559) and linearity (R2 = 0.9958 and 0.9137) between mean HbF and fluorescence intensity and was then discarded (Figure S3B).
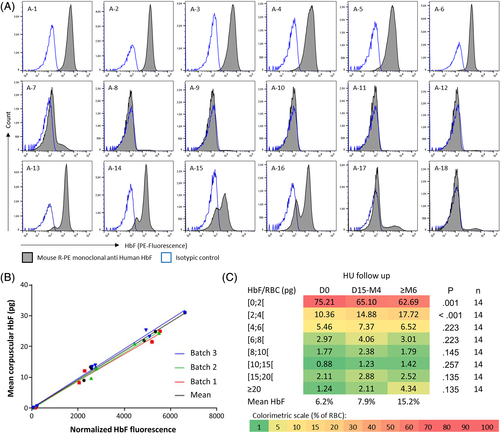
3.2 Repeatability and reproducibility of the HbF/RBC measurement
To further validate the quantification of HbF/RBC using our method, several samples from patients or healthy donors have been analyzed at different times. To simplify, percentages of RBC classified in ranges of more than 10 pg of HbF/RBC were analyzed together. First, freezing of the RBCs does not alter the quantification as we observed similar distributions between fresh and frozen samples (Figure S4). Second, slight differences were obtained when the same samples were acquired using two different flow cytometers with highest coefficients of variation (CV) obtained for the less represented ranges (containing less than 5% of total RBC) (Figure S5). Third, repeatability (Figure S6) and reproducibility (Figure S7) experiments gave good precision results with highest CV (> 20%) only observed for ranges containing less than 1% of total RBC. This is expected to be due to the low mean %RBC in the corresponding ranges (as the coefficient of variation is a ratio of the SD to the mean).
3.3 Quantitative measurements of HbF/RBC upon HU treatment
Fourteen adult SCD patients (11 women and 3 men; mean age [± SD] = 34.9 ± 8 years) were included in group B. Hydroxyurea was administered at an average and stable dose of 15 mg/kg/day. Table 1 provides the biologic parameters assessed before and after ≥6 months of HU.
| D0 | ≥M6 | P | mv | |
|---|---|---|---|---|
| %HbF | 6.2 | 14.2 | .001 | 2 |
| [2.8-8.4] | [7.7-21.1] | |||
| Total hemoglobin (g/L) | 84 | 95 | .006 | 3 |
| [73-94] | [83-105] | |||
| Red blood cells (1012/L) | 2.6 | 2.8 | .865 | 3 |
| [2.2-3.4] | [2.4-3.4] | |||
| Reticulocytes (109/L) | 245.5 | 120 | .008 | 4 |
| [190.5-260.0] | [78.5-211.0] | |||
| MCV (fL) | 89.5 | 100.5 | .002 | 2 |
| [83.0-94.5] | [91.2-115.2] | |||
| MCHC (g/L) | 340 | 330 | .016 | 3 |
| [330-350] | [320-340] | |||
| MCH (pg) | 30 | 34.5 | .001 | 2 |
| [28.0-32.9] | [32-40.7] | |||
| Leucocytes (109/L) | 10.3 | 6.5 | .002 | 3 |
| [9.7-12.1] | [5.4-8.5] | |||
| Platelets (109/L) | 432.5 | 354.5 | .005 | 2 |
| [329.0-529.2] | [269.0-468.5] | |||
| *LDH (μkat/L) | 6.0 | 5.5 | .067 | 3 |
| [3.7-8.5] | [3.6-6.4] | |||
| Total bilirubin (μmol/L) | 42 | 33.5 | .005 | 2 |
| [34.2-53.7] | [24.2-46.2] | |||
| ASAT (μkat/L) | 0.6 | 0.5 | .060 | 2 |
| [0.4-0.9] | [0.4-0.8] | |||
| F cells (%) method 1 | 40.3 | 54.6 | .001 | 0 |
| [20.6-48.2] | [41.1-73.3] | |||
| F cells (%) method 2 | 35.6 | 52.8 | .001 | 0 |
| [18.3-42.7] | [31.8-70.2] |
- Note: All statistically significant values (P < .05) are provided in bold.
- Abbreviations: ASAT, aspartate aminotransferase; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; mv, missing value.
The HbF distributions were assessed by flow cytometry before and during HU treatment showing different types of response (Figure S8). The R-PE fluorescence intensity was collected for each RBC, normalized and referred to the mean linear regression to calculate the corresponding amount of HbF/RBC in picograms. Percentages of RBC were classified according different ranges of HbF/RBC which were compared during HU treatment for the 14 group B patients.
Statistically significant variations were only found in low ranges of HbF/RBC (HbF <2 pg, P = .001 and 2 ≤ HbF <4 pg, P < .001; Friedman test) (Figure 1C) with a 16.6% decrease between D0 and ≥ M6 in RBCs containing less than 2 pg, and a 1.7-fold increase in RBCs containing between 2 and 4 pg. Interestingly, the increase in cells containing 2-4 pg HbF (+7.36%) accounted for almost 40% of the sum of the increase of cells containing more than 2 pg HbF (+15.69%). Concomitantly, RBC population with HbF/RBC >20 pg increased up to 3.5-fold but this change was not statistically significant (P = .135; Friedman test). Supplemental Figure 9 displays HbF quantification obtained for patients B-1 and B-8 presenting a similar response to HU in term of mean %HbF increasement but with different HbF distributions at ≥ 6 months (Figure S9).
3.4 Correlations between biologic parameters and HbF/RBC thresholds
As we observed that HU mainly decreases RBCs with low HbF content (Figure 1C), the thresholds of HbF/RBC ≥2, 4, 6, 8, 10 and 20 pg were analyzed (Figure S10). Upon HU treatment, an increase in mean %RBC for every threshold was observed, statistically significant for the amount of RBCs containing at least 2 pg of HbF (P = .001; Friedman test).
Correlations were evaluated between percentages of RBC by each HbF/RBC threshold and biologic parameters, independently of the duration of HU treatment (Figure 2). Increase in mean %HbF, MCV and MCH and decrease in RBC count were significantly associated with the number of RBC containing at least 2 pg of HbF (P < .001; < .001; < .001; and < .001, respectively). Interestingly, LDH, total bilirubin and ASAT were statistically not correlated with a HbF/RBC threshold.
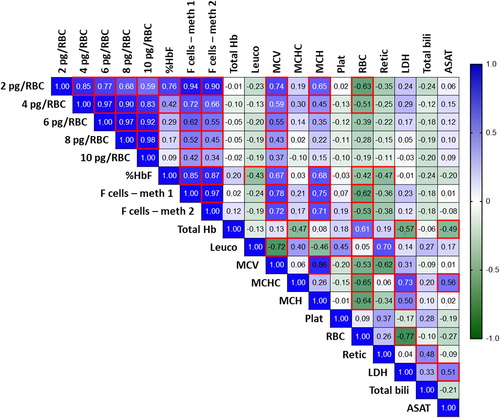
Moreover, when looking at the change between day 0 and ≥ 6 months, the median increase in %RBC with more than 2 pg of HbF/RBC (+12.06% [4.09-22.0]; median [interquartile range]) was correlated with the median increase in %HbF (+7.8% [2.2-14.1]) (r = 0.7426; P = 0.007), and correlated with the median decrease in total bilirubin (−8.0 μmol/L [13.5-0.7]) (r = −0.5860; P = 0.037) and decrease in reticulocytes (−78.0 109/L [159.5-3.5]) (r = −0.7133; P = 0.012) (Spearman correlations).
3.5 Associations between HbF/RBC thresholds and VOC incidence
The VOC incidence for the 14 group B patients was collected for the 3 years preceding D0 and the 3 following years after 6 months of HU at stable dose. The VOC number/3 years before and after HU was not statistically different (P = 0.441; Wilcoxon test). Among the 14 patients, six decreased their VOC incidence after HU treatment (B-2; B-6; B-10; B-11; B-12 and B-14), four did not change (B-1; B-4; B-8 and B-13) and four increased (B-3; B-5; B-7 and B-9). When looking at the HbF distribution, 66.6% of patients with decreased VOC incidence (4/6) had pancellular distribution while 75% of patients with increased VOC (3/4) had heterocellular distribution after HU (Figure S8).
Percentage of HbF determined by HPLC, and F cell frequency assessed by methods 1 and 2, were not significantly related to VOC incidence (r = −0.036; P = .856, r = −0.203; P = .299, r = −0.287; P = .139, respectively - Spearman correlation) (Figure S11A).
Hypothesizing that a threshold of HbF/RBC could impact the VOC incidence, we analyzed hospitalizations for VOC for the two 3-year periods (before and after HU treatment) with corresponding HbF/RBC thresholds. The association of VOC incidence during 3 years was statistically significant with the percentage of RBC above HbF/RBC thresholds of 4, 6, 8 and 10 pg, with a trend toward association with threshold of 2 pg (Figure S11B).
The ROC analyzes were performed to compare %HbF, F cell frequency and HbF/RBC thresholds as a predictive value of the VOC incidence with a threshold of ≤1 VOC over a period of 3 years (Figure 3A). For each threshold area under curve (AUC) were higher than %HbF (AUC = 0.549; P = .659) or F cells frequency (AUC = 0.734; P = .139 and AUC = 0.693; P = .086, for methods 1 and 2, respectively). Moreover, only ROC curves performed with %RBC above the different thresholds of HbF/RBC were statistically significant as compared to global %HbF and F cell frequency.
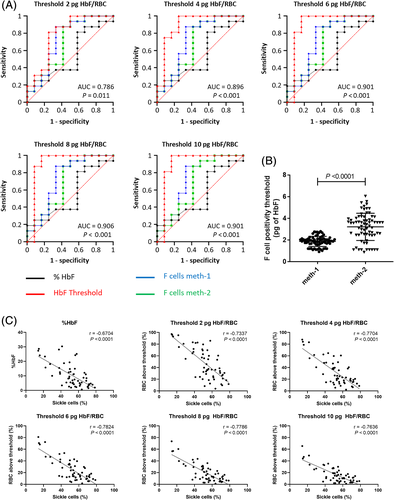
3.6 HbF detection level in F cells is variable
To assess the minimal quantity of HbF/RBC corresponding to the level of detection of F cells by flow cytometry, we performed HbF/RBC quantification on SCD patients and applied the two described methods (Figure S2). The lowest value of fluorescence intensity was extracted and then converted into pg of HbF. We measured a variable HbF positivity threshold of 1.86 pg ± 0.47 (mean ± SD) (ranging from 0.91 to 2.80 pg) and 3.21 pg ± 1.26 (ranging from 0.87 to 6.04 pg) for methods 1 and 2, respectively (n = 71) (Figure 3B), inducing higher values of F cell frequencies measured by method 1 for group B patients (Table 1).
3.7 Associations between HbF/RBC thresholds and in vitro RBC sickling
To confirm our results, in vitro sickling assays by enzymatic deoxygenation were performed on RBCs from 56 SCD patients treated by HU or not (n = 13 and n = 43, respectively) and healthy donors as control (n = 4) (Figure S12). Mean %HbF and mean percentage of sickle cells were 15.3% ± 10.2 and 36.5% ± 18.0 for treated patients and 8.9% ± 6.8 and 57.2% ± 11.6 for untreated patients. Percentage of sickle cells was inversely correlated with global %HbF and HbF/RBC thresholds of 2, 4, 6, 8 and 10 pg (Spearman correlations) (Figure 3C).
4 DISCUSSION
The new method developed here allows the precise and direct measurement of HbF content distribution in RBCs at the single cell level. The development of this technique has been possible by using samples from non-SCD subjects presenting a homogeneous distribution of HbF for whom the mean HbF fluorescence can be directly related to the MCHbF. Despite the choice of an accurate monoclonal antibody, the slight variability we observed came from the batch.26 The integration of the variability of the degree of coupling of PE molecules per antibody, by applying a correction factor to the measured fluorescence intensities, allows greater accuracy of the assay with good reproducibility and repeatability. In a clinical context, it would be necessary to re-perform the linear regression obtained from patients with homogeneous HbF distribution for every new batch. Using flow cytometry allows analysis of thousands of RBCs and subpopulations of interest such as reticulocytes or patients' RBCs in the case of a chronic transfusion program.
To our knowledge, only one attempt to precisely quantify HbF/RBC has been done using a fluorescent antibody.27 The method developed by Horiuchi et al., consisted of HbF content measurement in F cells assessed by fluorescence microscopy. While this method showed a good correlation between total fluorescence intensity and %HbF, it is time-consuming and requires technical expertise for reproducible smear preparation. Contrary to flow cytometry assay, RBCs size, in terms of MCV, could also affect the fluorescence density quantification because of the variation of the inner hemoglobin concentration, making correlation with standards unreliable.
This method could be applied to other hemoglobins with appropriate antibodies. Moreover, combining quantitative measurements of different hemoglobins and the total hemoglobin per RBC, would inform about their relative amount and concentration, which is of interest for an anti-polymerization effect, using the corpuscular Hb concentration (MCHC) per RBC.21, 28 However, the latter requires an individual volume measurement which is difficult to obtain when the shape is not regular as in SCD. To this purpose, imaging flow cytometry, which has already been used to analyze SCD erythrocytes morphology,29 could be coupled to the quantitative Hb measurement.
As a proof of concept, we applied this method to measure HbF content in SCD patients during hydroxyurea treatment. Hydroxyurea has been shown to prevent VOC and ACS,30, 31 improve splenic function regeneration,32 cerebrovascular involvement in children,33-35 or to treat early adult glomerular disease,36 reflecting its broad clinical effects. Importantly HU decreases the rate of mortality, mainly, but not exclusively, in a HbF dependent way.2, 31 During the longitudinal follow-up, a significant decrease in %RBC containing a very low HbF level (<2 pg) as well as a significant increase in %RBC containing between 2 and 4 pg of HbF were observed. These results are consistent with published data showing an increase in both %HbF and F cell frequency.18-20
Improvements in biologic parameters were associated with F cell frequency, assessed by the two most commonly used methods. By comparing them, we show that using a positivity threshold corresponding to the fluorescence intensity of unstained cells gave statistically significant fewer variable, but higher results than using an isotypic control. Whatever the method, F cell frequency was not statistically associated with VOC incidence over 3 years in this study. This result might be explained by the fact that F cells corresponds to only one threshold of HbF content and methods used can induce, as we showed, the setting of either a too variable or a too low positivity threshold, resulting in no clinical significance.
Steinberg et al. hypothesized that if HbF content was 10 pg in each RBC, there would be no disease events.21 Here we show that assessing the distribution of HbF/RBC allows analyzing any possible protective threshold against SCD phenotypes. The number of RBC containing at least 2 pg of HbF was significantly associated with an increase in mean %HbF, MCV and MCH and a decrease in RBC count. Moreover, we provide results showing that %RBC above thresholds of HbF/RBC, even from 2 pg of HbF could have a clinical significance according to VOC incidence. As expected, the effect on VOC is more pronounced in case of %RBC reaching higher levels of HbF/RBC. These results were strengthened by performing in vitro sickling assays using samples from other patients, which was also correlated with the threshold of 4, 6, 8 and 10 pg of HbF/RBC in a stronger way than %HbF and F cell frequency as observed by ROC analysis. While studied on a small cohort, the distribution of HbF (ie, pancellular or heterocellular), which is of interest from a biological point of view, seems to be associated with the VOC incidence, with heterocellular distribution having a negative effect, as reported.37
Hemolytic markers LDH, bilirubin and ASAT were not correlated with a HbF threshold, whereas we observed a decrease in bilirubin and reticulocytes count between D0 and ≥ M6, which were correlated with an increase in the %RBC with HbF/RBC higher than 2 pg. These results are consistent with published data showing that hemolytic markers did not correlate with RBC survival, improved by HbF level.38 Interestingly, the HbF threshold could explain the discrepancy between some studies showing a positive effect of the %HbF on survival,2, 31, 39, 40 and those without a close relationship.41, 42 All together our results show that HU effect on hemolysis is probably more complex. A study in larger cohorts of patients including their clinical manifestations, treatments and genetic analysis could be considered to evaluate the best threshold of HbF/RBC and the proportion of cells above it and to study clinical and genetic associations. Indeed, genetic factors have been hypothesized as an explanation of a differential response of patients to HU,43, 44 suggesting that a personalized HU dose according to pharmacokinetic,45 coupled with the study of the thresholds, might attenuate these differences.
Finally, the effect of HU on HbF distribution was undertaken as a proof of concept of a new method for HbF measurement in each RBC. This method opens up interesting prospects for analysis of new therapeutic approaches, including epigenetic and gene therapy HbF inducers.46-50
ACKNOWLEDGEMENTS
The authors would thank Wassim El Nemer and the French institute CNRGS (Centre National de Référence pour les Groupes Sanguins) for providing samples analyzed in this study. They also thank Cécile Fligny for editorial assistance and Michael Marden for manuscript correction.
AUTHOR CONTRIBUTIONS
P. B. designed research; N. H., M. G. R., G. B., L.B., L.K., and G. D. L. performed research; N. H., M. G. R., L.B., G. D. L., F. G., M. C., and P. B. analyzed data; E. A., and N. O. performed statistical analysis; S. P., and F. G. monitored and selected patients for the study; M. S., P. C., B. V., F. P., F. G., and M. C. critically reviewed the manuscript; and N. H., M. G. R., and P. B. wrote the manuscript.
CONFLICT OF INTERESTS
M. G. R., G. D. L., F. P., F. G., M. C., and P. B. are co-inventors of the patent number WO2018083426A1. P. B. declares being member on a standing advisory council or committee consultancy for Addmedica, Roche, Bluebird bio, Emmaus, Agios, Global Blood Therapeutics, Novartis and Hemanext. M.C. and P.B. declare being co-founders of Innovhem. The other authors declare no competing financial interests.




