Localized immunoglobulin light chain amyloidosis: Novel insights including prognostic factors for local progression
Abstract
In localized light chain amyloidosis (locAL), amyloidogenic light chains (aLC) are produced and deposited locally by a B-cell clone. We present 293 patients with immunohistochemically confirmed locAL. Lung (nodular pulmonary) with 63 patients was the most involved organ. The aLC was λ in 217 cases (κ:λ ratio 1:3). A local B-cell clone was identified in 30% of cases. Sixty-one (21%) had a concomitant autoimmune disorder (cAD). A monoclonal component (MC) were present in 101 (34%) patients and were more frequent in subjects with cAD (51% vs 34%; P = .03). Cigarette smoking was more prevalent in lung locAL (54% vs 37%; P = .018). After a median follow-up of 44 months, 16 patients died and 5- and 10-years locAL progression-free survival (PFS) were 62% and 44%. Interestingly, locAL-PFS was shorter among patients with an identified clonal infiltrate at amyloid deposition site (40 vs 109 months; P = .02) and multinuclear giant cells and/or an inflammatory infiltrate resulted in longer locAL-PFS in lung involvement (65 vs 42 months; P = .01). However, no differences in locAL PFS were observed in patients with cAD, a MC and involved organ site. Treatment was administered in 163 (54%) patients and was surgical in 135 (46%). Median locAL-PFS after first treatment was 56 months. Responders had longer locAL-PFS (78 vs 17 months; P < .001). Three patients with lung locAL and a MC were diagnosed as systemic AL amyloidosis at follow-up. In summary, locAL pathogenesis seems to be heterogeneous and the clonal infiltrate leads local progression.
1 INTRODUCTION
Amyloidoses are a group of rare diseases caused protein misfolding and deposition in organs and tissues as amyloid fibrils. Thirty-six different amyloidogenic proteins have been identified to date in human.1 In systemic amyloidosis, the amyloidogenic precursor is a plasma protein that might target multiple organs.2 The most frequent type of systemic amyloidosis in the Western countries is systemic immunoglobulin light chain (AL) amyloidosis (sysAL).3 This disease is caused by a light chain (LC) produced by a B-cell clone in the bone marrow.4 However, immunoglobulin LCs are also responsible of local AL amyloidosis (locAL). This is a very rare and less studied disease, accounting for approximately 10% of all amyloidosis cases in referral centers (no other population data available).
In locAL, locally produced LCs deposit at a single anatomic site, forming one or multiple tumor-like amyloid lesions (amyloidomas).5, 6 Our knowledge on locAL has improved in the past 5 years thanks to description of two large case series. The National Amyloidosis Center (NAC) described for the first time the natural history of this disease in 606 patients.7 Bladder, larynx and skin emerged as the most commonly involved organs. Furthermore, patients with locAL may also present concomitant autoimmune disorders (cAD), especially Sjögren syndrome (SjöSy), lymphoproliferative diseases, mainly marginal zone lymphoma (MZL), and a monoclonal gammopathy of undetermined significance (MGUS).8-11 Recently, a report from the Mayo Clinic's group added valuable information about response to therapy and local progression from 413 cases of locAL.12 Although life expectancy in locAL was comparable to the general population, the clinical history was characterized by frequent local progressions requiring further treatment. Importantly, repeated surgical interventions were often cause of consistent morbidity and quality of life impairment.
Currently, it is not clear if some peculiar characteristics of this complex and heterogeneous disease may affect the prognosis in locAL, particularly local progression. Moreover, few data are available on local cellular infiltrate and B-cell clone at amyloid deposition site and its role in natural history of locAL has not been studied so far.13
We present the results of a comprehensive study conducted on a large series of 293 consecutive patients with verified locAL evaluated at the Heidelberg Amyloidosis Center. We coupled an extensive characterization of clinical features with detailed pathology data from tissue biopsy. Finally, we described treatment and outcome and studied local and systemic factors that may affect local progression for the first time.
2 MATERIALS AND METHODS
2.1 Patients and inclusion criteria
Study design and patient population are summarized in Figure S1. The prospectively maintained database of the Heidelberg Amyloidosis Center was searched for patients with locAL evaluated between 04/2000-10/2019. Only patients with conclusive immunohistochemical typing and clinical findings for locAL were included in the study. A multifocal involvement was defined as the presence of multiple amyloid lesions in the absence of systemic involvement. SysAL was ruled out in all patients with evaluation of clonal and organ biomarkers. In selected cases, echocardiogram and abdominal fat pad aspirate were performed (see Supplement).
All patients gave written informed consent for their clinical data to be used in retrospective studies in accordance with the Declaration of Helsinki.
2.2 Response to treatment and local progression
Follow-up of amyloidomas was performed by clinical, radiographic and endoscopic examination. Response to treatment and local progression were defined according to changes in symptoms and/or size of the amyloidomas. Particularly, changes in size of the amyloidomas at imaging were evaluated for response and progression in asymptomatic patients. Modifications of imaging and endoscopic findings over time was assessed and documented by local radiologists or physicians. Local progression was evaluated in patients from diagnosis and after first treatment. This was done in order to better evaluate the natural history of locAL and the impact of different therapeutic strategies and response to treatment. Progression to sysAL was defined by detection of the amyloidogenic light chain (aLC) in the serum and/or urine and onset of another involved organ site with detection of the aLC in abdominal fat pad or organ biopsy.
2.3 Amyloid typing and cellular infiltrate characterization
A sample of tissue biopsy or a surgical resection from the involved organ site were sent to a Pathology Unit for amyloid identification and typing. Two-hundred forty-six (84%) samples were sent to the Department of Pathology at the University Hospital Kiel, that has proven its expertise on immunohistochemical typing of amyloid and identification of amyloidogenic clones on tissue biopsies with novel sensitive techniques. In 180 cases, abdominal fat pad aspirate was also obtained and evaluated. Immunohistochemical typing of amyloid was performed on tissue biopsy with custom made antibodies in all patients, as previously described.14 Reports from the Pathology Unit were systematically reviewed for data collection. Data on biopsy size were collected from 189 patients and information about cellular infiltrate at amyloid deposition site was available in 154 cases. B-cell clonality was assessed by immunohistochemistry, in situ-hybridization or PCR-based immunoglobulin heavy chain (IGH) and light chain (IGK) gene rearrangement analyses, as described.15
2.4 Statistical analyses
Overall survival (OS) and local progression free survival (locAL-PFS) curves were plotted according to Kaplan Meier, and differences in survival were tested for significance with the log-rank test. OS was calculated from diagnosis of locAL. LocAL-PFS was calculated in the overall population from diagnosis and in treated patients from time of first treatment. Fisher's exact χ2 test and Mann-Whitney test were used to assess differences between nominal and continuous variables, respectively. MedCalc Statistical Software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014) was used for computation.
3 RESULT
3.1 Patients characteristics
Patients characteristics are reported in Table 1 and detailed information on organ involvement are summarized in Table 2. Lung (nodular pulmonary) was the most commonly involved organ. Patients with lung locAL were older at diagnosis (68 vs 55-year-old; P < .001), had more frequently multifocal involvement (62% vs 40%; P = .026) and presented a high prevalence of smokers (54% vs 37%; P = .014). A female preponderance was observed among patients with skin, soft tissues (ST), eye and central nervous system (CNS) locAL.
| Characteristics | N (%) or median (range) |
|---|---|
| Sex, male | 145 (49) |
| Age, years | 58 (18-83) |
| Organ involvement | |
| Lung (nodular pulmonary) / Larynx / Urinary tract | 63 (22) / 51 (17) / 37 (13) |
| GI / Skin and ST / Lower airways (tracheobronchial) | 35 (12) / 31 (11) / 31 (11) |
| Eye / Nasopharynx / CNS / Lymphatic tissue / Othera | 12 (4) / 12 (4) / 9 (3) / 8 (3) / 4 (1) |
| Multifocal involvement | 130 (44) |
| Smokers | 116 (40) |
| First evaluation in Heidelberg within 12 months from diagnosis | 210 (73) |
| NT-proBNP, ng/L (n = 284) | 83 (18-9004) |
| NT-proBNP >332 ng/L | 40 (14) |
| Proteinuria g/24 hoursb (n = 232) | 0.1 (0.04-1.00) |
| Proteinuria >0.5 g/24 hours | 5 (2) |
| eGFR, mL/min x 1.73 m2 (n = 292) | 89 (141-17) |
| eGFR <30 mL/min x 1.73 m2 | 1 (<1) |
| Alkaline phosphatase (concentration/u.r.l. ratio) (n = 290) | 0.6 (0.3-1.8) |
| Alkaline phosphatase concentration/u.r.l. ratio > 1.5 | 2 (1) |
| Monoclonal proteinc | 63 (22) |
| IgG / IgA / IgM / LC | 47 (16) / 3 (2) / 15 (5) / 5 (2) |
| MC, g/L (n = 9) | 10.6 (4.2-25) |
| LC isotype of MC matching with aLC | 39 (13) |
| FLCR (n = 288)d | 1.10 (0.85-1.46) |
| AbFLCR (n = 288) | 63 (22) |
| AbFLCR matching with aLC (n = 288) | 28 (10) |
| MC and/or abFLCR | 101 (34) |
| LC isotype of MC and/or abFLCR matching with aLC | 53 (18) |
| Clonal infiltrate at amyloid deposition site (n = 154) | 46 (30) |
| aLC, isotype Kappa: lambda | 76 (28): 217 (74) |
| Concomitant lymphomae | 7 (2) |
| Concomitant multiple myelomaf | 5 (2) |
| Autoimmune disorders | 61 (21) |
| Sjögren syndrome / Autoimmune thyroiditis | 18 (7) / 17 (6) |
| Rheumatoid arthritis / Psoriasis / SLE / CREST | 7 (2) / 7 (2) / 2 (1) / 2 (1) |
| ITP / Otherg | 2 (1) / 6 (2) |
| ANA titer ≥1:640 (n = 195) | 40 (21) |
- Abbreviations: abFLCR, abnormal free light chain ratio; aLC, amyloidogenic light chain; ANA, antinuclear antibody; CNS, central nervous system; eGFR, estimate glomerular filtration rate; FLC, free light chain; GI, gastrointestinal; IQR, interquartile range; ITP, idiopathic thrombocytopenic purpura; LC, light chain; MC, monoclonal component; NOS; not otherwise specified; NT-proBNP, N-terminal pro-brain natriuretic peptide; SLE, systemic lupus erythematosus; ST, soft tissues; u.r.l., upper reference limit.
- a Other: 2 bone involvement (cervical and thoracic vertebra), 1 parotis, 1 perineural amyloidoma.
- b All the patients without a 24-proteinuria had a urinary albumin to creatinine ratio, that was normal (u.r.l. 30 mg/mmol).
- c Three patients had biclonal and 1 a triclonal gammopathy.
- d For FLCR, we reported IQR instead of the range.
- e Ann Arbor staging: 3 patients in stage I (1 in stage IE), 1 in stage IIIE and 1 in stage IV.
- f Durie-Salmon staging: 3 patients in stage IA and 1 in stage IIIA.
- g Other: contact urticaria, rheumatic polymyalgia, Reinke edema, primary biliar cholangitis, collagenosis NOS, vasculitis NOS and rheumatic disease NOS in 1 case each.
| Type of organ involvement | Sex male (N - %) | Age (years) | Main symptoms and clinical manifestations | Asymptomatic (N - %) | Multifocal involvement (N - %)a | aLC kappa: lambda (N - %) | MC and/or abFLCR (N - %) | LC matched aLC (N - %) | Autoimmune disorders (N - %) | Local progression (N - %) | LocAL PFS 1 year / 5 years (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory tract, 157 pts | 81 (52) | 60 (18-82) | Dyspnoea, hoarseness | 51 (32) | 79 (50) | 50 (32): 107 (68) | 59 (37) | 32 (20) | 35 (22) | 45 (29) | 89 / 66 |
| Lung, 63 pts. | 37 (59) | 68 (35-80) | Dyspnoea | 45 (71) | 39 (62) | 16 (25): 47 (75) | 29 (46) | 17 (27) | 21 (33) | 15 (23) | 95 / 64 |
| Larynx, 51 pts. | 23 (45) | 51 (19-80) | Hoarseness | 1 (2) | 16 (31) | 20 (39): 31 (61) | 14 (27) | 7 (14) | 9 (18) | 19 (37) | 78 / 52 |
| Lower airways, 31 pts | 14 (45) | 55 (26-78) | Dyspnoea, infections | 3 (10) | 24 (77) | 8 (26): 23 (74) | 13 (42) | 7 (23) | 4 (13) | 8 (26) | 94 / 80 |
| Nasopharynx, 12 pts | 7 (58) | 49 (18-82) | Infections | 2 (17) | - | 6 (50): 6 (50) | 3 (25) | 1 (8) | 1 (8) | 3 (25) | 87 / 73 |
| Urinary tract, 37 pts | 21 (58) | 58 (25-83) | Haematuria | 2 (5) | 12 (32) | 7 (19): 30 (81) | 10 (27) | 5 (13) | 6 (16) | 11 (30) | 70 / 59 |
| Bladder, 28 pts | 14 (50) | 58 (38-83) | Haematuria | 1 (3) | 10 (36) | 5 (18): 23 (82) | 6 (21) | 4 (13) | 6 (21) | 6 (21) | - |
| Ureter, 5 pts | 4 (80) | 65 (56-74) | Haematuria | - | 1 (20) | 1 (20): 4 (80) | 2 (20) | - | - | 4 (80) | - |
| Urethra, 3 pts | 3 (100) | 47 (25-57) | Haematuria | 1 (33) | 1 (33) | 1 (33): 2 (67) | 2 (67) | 1 (33) | - | 1 (33) | - |
| Kidney interstitium, 1 pt | Female | 72 | Pain | - | - | Lambda | - | - | - | - | - |
| GI tract, 35 pts | 24 (69) | 59 (38-80) | Bleeding | 12 (34) | 16 (46) | 6 (17): 29 (83) | 8 (23) | 2 (6) | 3 (9) | 7 (20) | 89 / 71 |
| Bowel, 25 pts | 17 (68) | 62 (43-78) | Bleeding | 9 (36) | 11 (44) | 3 (12): 22 (88) | 5 (20) | 1 (4) | 1 (4) | 4 (16) | - |
| Stomach, 5 pts | 3 (60) | 51 (38-68) | Bleeding | - | 4 (80) | 2 (40): 3 (60) | 2 (20) | 1 (20) | 1 (20) | - | - |
| Oral mucosa, 3 pts | 2 (67) | 55 (49-80) | Lump | All | 1 (33) | All lambda | 1 (25) | - | 1 (33) | 2 (50) | - |
| Tongue, 2 pts | 2 (100) | 52 (40-63) | Dysphagia | - | - | 1 (50): 1 (50) | - | - | - | 1 (50) | - |
| Skin and ST, 31 pts | 11 (35) | 57 (28-82) | Skin changes, lump | 26 (84) | 15 (48) | 5 (16): 26 (84) | 13 (42) | 8 (26) | 14 (45) | 14 (45) | 82 / 52 |
| Skin, 23 pts | 8 (35) | 58 (28-82) | Skin changes, lump | 21 (91) | 12 (52) | 3 (13): 20 (87) | 9 (29) | 5 (22) | 11 (48) | 10 (43) | - |
| Soft tissues, 6 pts | 3 (50) | 45 (38-80) | Pain | 3 (50) | 2 (33) | 1 (17): 5 (83) | 4 (67) | 3 (50) | 2 (33) | 3 (50) | - |
| Breast, 2 pts | All females | 52 (39-65) | Lump | All | 1 (50) | 1 (50): 1 (50) | - | - | 1 (50) | 1 (50) | - |
| Eye, 12 pts | 2 (17) | 51 (27-64) | Tissue swelling | 2 (17) | 1 (8) | 1 (8): 11 (92) | 3 (25) | - | 2 (17) | 4 (33) | 90 / 52 |
| Conjunctiva, 9 pts | 1 (11) | 51 (27-59) | Conjunctivitis | 2 (22) | 1 (11) | 1 (11): 8 (89) | 2 (17) | - | 1 (11) | 2 (22) | - |
| Eyelid, 2 pts | 1 (50) | 57 (51-64) | Tissue swelling | - | - | All lambda | 1 (50) | - | 1 (50) | 1 (50) | - |
| Orbit, 1 pt | Female | 36 | Diplopia | - | - | Lambda | - | - | - | Progressed | - |
| CNS, 9 pts | All females | 48 (37-61) | Seizures | - | - | 1 (1): 8 (99) | 3 (33) | 3 (33) | - | 6 (67) | 89 / 41 |
| Brain, 7 pts | All females | 48 (37-61) | Seizures | - | - | All lambda | 3 (42) | 3 (42) | - | 5 (71) | - |
| Gasser ganglion, 2 pts | All females | 46 (44-48) | Neuralgia | - | - | 1 (50): 1 (50) | - | - | - | 1 (50) | - |
| Lymphatic tissue, 8 pts | 4 (50) | 56 (23-72) | Tissue swelling | 5 (63) | 6 (75) | 5 (62): 3 (38) | 3 (38) | 2 (25) | 1 (13) | 2 (25) | 100 / 76 |
| Lymph nodes, 6 pts | 3 (50) | 65 (33-72) | Tissue swelling | 5 (83) | All | 4 (67): 2 (33) | 2 (33) | 1 (17) | 1 (17) | 1 (20) | - |
| Tonsils, 1 pt | Male | 43 | Infections | - | - | Kappa | 1 | 1 | - | - | - |
| Adenoids, 1 pt | Female | 23 | Hearing loss | - | - | lambda | - | - | - | Progressed | - |
| Bone, 2 pts | 1 (50) | 62 (61-62) | Pain | 1 (50) | - | 1 (50): 1 (50) | All | 1 (50) | - | 1 (50) | - |
| Parotid gland, 1 pt | Male | 65 | Infections | - | Multifocal | Lambda | - | - | - | - | - |
| Perineural amyloidoma, 1 pt | Female | 43 | Neuralgia | - | - | Lambda | - | - | - | Progressed | - |
- Note: Bold values highlight the characteristics of localized AL amyloidosis at each anatomic system (e.g. respiratory tract, urinary tract, GI tract).
- Abbreviations: abFLCR, abnormal free light chain ratio; aLC, amyloidogenic light chain; GI, gastrointestinal; LC, light chain; locAL PFS, local progression free survival; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; pts, patients; ST, soft tissues.
- a In 9 (3%) patients, multiple amyloid deposits extended through more than one organ. In 7 cases, amyloid was disseminated through the respiratory tract. In one case locAL presented as nodular amyloidomas in the skin (skin and ST involvement) and in the oral mucosa (GI involvement). In another patient, amyloid deposits were found in nasopharynx (respiratory involvement) and in a lateral cervical lymph node (lymphatic tissue involvement).
3.2 Clinical manifestations and symptoms
One hundred ninety-four (66%) patients complained of amyloid-related symptoms. In 119 (41%) cases, symptoms were caused directly by mass-effect of the growing amyloidoma due to tissue swelling, compression of adjacent structures or obstruction. Recurrent bleeding and infections were present in 53 (18%) and 25 (9%) patients, respectively. Pain was reported in 22 (8%) cases. Clinical manifestations were largely dependent on type of organ involvement. Mass-effect dependent symptoms were more frequent in patients with larynx locAL (92% vs 30%; P < .001). Bleeding and recurrent infections were more prevalent in urinary tract (78% vs 9%; P < .001) and lower airways (tracheobronchial) involvement (29% vs 6%; P < .001), respectively. Patients with lung locAL were more frequently asymptomatic (71% vs 23%; P < .001). Lung involvement was particularly frequent in 65 asymptomatic patients (72%) in which locAL was diagnosed incidentally during regular medical evaluation for a concomitant comorbidity or, in a few cases, during the radiological assessment of an acute trauma.
3.3 Identification of the amyloidogenic light chain and cellular infiltrate
Median length of the histological material from tissue biopsy or surgical resection was 1.2 cm (range 0.1-14.5 cm). Larger samples were obtained from patients with lung locAL (median length 2.5 cm vs 1 cm; P < .001), while smaller specimens came from patients with lower airways locAL (median length 0.6 cm vs 1.5 cm; P = .001). λ aLC was identified by amyloid typing on tissue in most cases with a κ:λ ratio of 1:3. Interestingly, we observed an organ site specific variation of κ:λ ratio (Table 2). A particular predominance of λ LC (ie, >75%) was observed in urinary tract, GI, skin and ST locAL. The prevalence of λ LC was even higher in eye and CNS locAL (92% and 99% respectively). Information about the cellular infiltrate was available in 154 (53%) samples, of which 30% were obtained from patients with lung locAL. An inflammatory infiltrate was present in 123 (80%) cases and multinucleated giant cells (MGC) were identified in 91 (59%) samples. A lymphoplasmacytic infiltrate was found in 76 (49%), and clonality was identified in 46 (30%, composed by plasma cells in 27 and by B-cells in 19). In patients with an identified local clone, the LC restriction of the clone always matched the aLC. A significant correlation was found between biopsy length and identification of a clonal infiltrate (Odds ratio 1.21, 95% CI 1.02-1.45; P = .02). In 5 biopsies an MZL was diagnosed. In 8 cases the clonal infiltrate was characterized as plasma cellular differentiated B-cell neoplasia. A clonal infiltrate was more frequently identified in samples from patients with skin and ST involvement (64% vs 26%; P = .01). In the nine patients with a skin and ST locAL and a documented clonal infiltrate at site of amyloid deposition, median biopsy length was 3 cm (range 0.7-9 cm). Five of them presented with a cAD (SjöSy in 2 cases), 6 had an ANA titer ≥1:640 and 3 had a concomitant monoclonal component (MC) and/or abnormal free LC (FLC) ratio (abFLCR) in the serum or urine matching the aLC.
3.4 Coexisting clonal diseases
Overall, 101 (34%) patients had a MC and/or an abFLCR, matching the LC isotype of LC of the aLC identified in the tissue specimen in 53 (18%) cases (Table 2).
Five patients had a concomitant multiple myeloma (MM) and three received chemotherapy for MM.
Seven (2%) patients had a of B-cell lymphoma, that was a MZL in four and mucosa-associated lymphoid tissue (MALT) lymphoma in three cases, respectively. A systemic lymphoma disease was present in four patients. The presence of the lymphomatous infiltrate at the site of amyloid deposition was demonstrated in 5 of 7 cases.
3.5 Concomitant autoimmune disorders
A cAD was present in 61 (21%) patients and was more frequent in skin locAL (45% vs 18%; P = .001). Moreover, 30 (10%) subjects presented with hypergammaglobulinemia. Among patients with cADs, 31 had a MC and/or abFLCR. A MC and/or an abFLCR were more frequent in patients with a cAD (51% vs 34%; P = .03). SjöSy was most common and present in 18 (6%) patients (8 with lung and 6 with skin and ST locAL), followed by autoimmune thyroiditis in 17 (6%).
Among the 195 patients tested for ANA, 40 (21%) presented an ANA titer ≥1:640. In 17 of these patients a cAD was not present. Organ involvement was skin and ST in 16 (40%) and lung in 9 (23%) cases, respectively. A concomitant MC and/or an abFLCR was observed in 19 (48%) patients with ANA titer ≥1:640.
3.6 Ruling out systemic AL amyloidosis
Overall, 25 (9%) patients with a MC and/or an abFLCR presented with increased organ biomarkers at first evaluation. SysAL was ruled out in all these cases (see Supplement). Particularly, three patients with suspected locAL were correctly diagnosed as sysAL during the first evaluation (Figure S1).
Abdominal fat pad aspirate was negative in 179 of 180 (99%) patients. The only case with amyloid deposits in the abdominal fat was an insulin-dependent diabetic patient with GI involvement: amyloid typing identified λ LC-derived amyloid in the duodenal biopsy and insulin-derived amyloid in the abdominal fat, the latter being a well-known local complication of repeat insulin injections.
3.7 Progression to systemic AL amyloidosis
Progression to sysAL was observed in 3 patients (2 with lung and 1 with nasopharynx locAL) after 24, 46 and 241 months from diagnosis of locAL. In these subjects, onset of MC matching the aLC isotype preceded the new involved organ site. New involved organ site was thyroid and GI in one case each of lung locAL and lymph nodes in a patient with nasopharynx locAL. However, no patient had a progression to sysAL with heart, kidney or liver involvement.
3.8 Response to treatment
Details on treatment and outcome according to organ involvement are summarized in Table 3 and Figure S1C. The treatment approach was decided by local physicians in most cases. A “watch and wait” strategy was adopted in 130 cases. One hundred sixty-three (56%) patients received a treatment, which was surgical in 135 (83%). Treated patients were younger (62 vs 67-year-old; P = .013), with more urinary tract (17% vs 8%; P = .034) and less GI (5% vs 21%; P < .001) and lower airways locAL (8% vs 15%; P < .001) (see Table S1).
| Type of organ involvement | Surgical Treatment (N - %)a | Radiotherapy (N - %)b | Surgery and radiotherapy (N - %) c | Steroids (N - %)d | Chemotherapy (N - %)e | Improved / stable disease f (N - %) | Local progression after treatment (N - %) | LocAL PFS 1 year / 5 years (%) |
|---|---|---|---|---|---|---|---|---|
| Respiratory tract, 86 pts | 76 (88) | 1 (1) | 2 (2) | 4 (5) | 3 (3) MM | 64 (75) / 21 (24) | 32 (37) | 82 / 55 |
| Lung, 32 pts | 30 (94) | - | - | - | 2 (6) MM | 25 (78) / 7 (22) | 8 (25) | 93 / 56 |
| Larynx, 34 pts | 32 (94) | - | - | 2 (6) | - | 25 (74) / 9 (26) | 17 (50) | 72 / 47 |
| Lower airways, 11 pts | 7 (64) | 1 (1) | - | 2 (18) | 1 (1) MM | 5 (45) / 5 (45) | 4 (36) | 81 / 54 |
| Nasopharynx, 9 pts | 7 (78) | - | 2 (22) | - | - | All improved | 3 (33) | 84 / 62 |
| Urinary tract, 27 pts | All surgery | - | - | - | - | 20 (74) / 7 (26) | 10 (34) | 63 / 50 |
| Bladder, 20 pts | All surgery | - | - | - | - | 15 (75) / 5 (25) | 5 (24) | - |
| Ureter, 5 pts | All surgery | - | - | - | - | 3 (60) / 2 (40) | 4 (80) | - |
| Urethra, 2 pts | All surgery | - | - | - | - | All improved | 1 (50) | - |
| GI tract, 8 pts | 6 (75) | - | - | 1 (12) | 1 (12) NHL | 6 (75) / - | 3 (38) | 100 / 60 |
| Bowel, 6 pts | 5 (83) | - | - | 1 (17) | - | 5 (83) / - | 2 (33) | - |
| Stomach, 1 pt | - | - | - | - | Chemotherapy NHL | - | - | - |
| Oral mucosa, 1 pt | Surgery | - | - | - | - | Improved | Progressed | - |
| Skin and ST, 18 pts | 12 (67) | 2 (11) | - | 4 (22) | - | 14 (78) / 4 (22) | 10 (55) | 71 / 38 |
| Skin, 11 pts | 7 (64) | - | - | 4 (36) | - | 7 (64) / 4 (36) | 6 (55) | - |
| Soft tissues, 6 pts | 4 (67) | 2 (33) | - | - | - | All improved | 3 (50) | - |
| Breast, 1 pts | Surgery | - | - | - | - | Improved | Progressed | - |
| Eye, 6 pts | 5 (83) | - | - | 1 (17) | - | 4 (67) / 2 (33) | 4 (67) | 80 / 33 |
| Conjunctiva, 4 pts | 3 (75) | - | - | 1 (25) | - | 2 (50) / 2 (50) | 2 (50) | - |
| Eyelid, 1 pt | Surgery | - | - | - | - | Improved | Progressed | - |
| Orbit, 1 pt | Surgery | - | - | - | - | Improved | Progressed | - |
| CNS, 7 pts | 3 (43) | 1 (14) | 1 (14) | 2 (29) | 4 (57) / 3 (43) | 5 (71) | 96 / 42 | |
| Brain, 5 pts | 1 (20) | 1 (20) | 1 (20) | 2 (40) | - | 3 (60) / 2 (40) | 4 (80) | - |
| Gasser ganglion, 2 pts | All surgery | - | - | - | - | 1 (50) / 1 (50) | 1 (50) | - |
| Lymphatic tissue, 7 pts | 4 (67) | 1 (14) | 1 (14) | 1 (14) | 6 (86) / 1 (16) | 2 (29) | 100 / 74 | |
| Lymph nodes, 5 pts | 2 (40) | 1 (20) | 1 (20) | - | 1 (20) | 4 (80) / 1 (20) | 1 (20) | - |
| Tonsils, 1 pt | Surgery | - | - | - | - | Improved | - | - |
| Adenoids, 1 pt | Surgery | - | - | - | - | Improved | Progressed | - |
| Bone, 2 pts | - | 1 (50) | 1 (50) | - | - | 1 (50) / 1 (50) | 1 (50) | - |
| Parotid gland, 1 pt | Surgery | - | - | - | - | Stable disease | - | - |
| Perineural amyloidoma, 1 pt | - | - | Surgery and radiotherapy | - | - | Stable disease | Progressed | - |
- Note: Bold values highlight the characteristics of localized AL amyloidosis at each anatomic system (e.g. respiratory tract, urinary tract, GI tract).
- Abbreviations: GI, gastrointestinal; locAL PFS, local progression free survival; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; pts, patients; ST, soft tissues.
- a Surgical approach comprehended resection of amyloidomas, laser CO2 ablation and stenting. Importantly, in 31 subjects, surgical excision of amyloidomas was done before diagnosis of amyloidosis, suspecting a neoplasia. Surgical approach yielded a response in 106 of 135 (79%) patients.
- b A response to treatment was observed in 3 of 5 patients exposed to upfront radiotherapy.
- c This combined approach resulted in a response in 5 of 6 cases.
- d Six patients received oral steroids and 6 a topic treatment. Response to steroids was observed in 4 of 12 (33%) patients.
- e Among patients that received a chemotherapy 4 were treated for the concomitant clonal disorder (3 MM and 1 NHL). One patient received chemotherapy for lymph nodes locAL. Systemic AL amyloidosis was ruled out in this patient. An improvement of symptoms and/or a reduction of amyloid lesions was observed in only 1 case with MM.
- f Three patients (1 lower airways locAL and 2 GI locAL) were not evaluable for response.
Overall, 119 (73%) patients improved after treatment and 41 (25%) had a stable disease. In symptomatic patients (n = 115), 80 (71%) had an improvement of symptoms and 32 (28%) were stable. Among 48 asymptomatic treated patients, imaging showed an improvement in 39 (81%) cases.
3.9 Overall survival from diagnosis
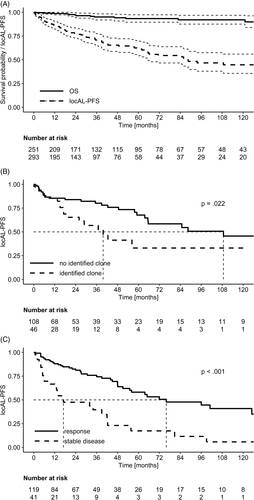
After a median follow-up of 44 months, 16 patients died (9 with lung, 4 with lower airways and 3 with nasopharynx locAL). Ten of these subjects were also smokers. Median OS at 5- and 10-years was 94% and 92% (Figure 1A). OS was poorer in patients with lung locAL (5-years OS 79% vs 97%; P < .001). Death was attributed to locAL in only one case. Not-amyloid related causes of death were neoplasia (2 patients), chronic-obstructive pulmonary disease exacerbation (2 patients), sepsis (1 patient) and cerebral hemorrhage (1 patient). Cause of death was unknown in the remaining nine subjects (see Supplement). One of these patients died 22 months after progression to sysAL.
3.10 Local progression free survival from diagnosis and after treatment
After a median follow-up of 34 months, 91 (31%) patients had a local progression. Among these, 66 (40%) had a local progression after treatment with a median follow-up of 43 months. Organ-site specific disease outcomes from diagnosis and after first treatment are summarized in Tables 2 and 3.
Median locAL-PFS from diagnosis was 88 months and 5 and 10-years locAL-PFS were 62% and 44% (Figure 1A). No difference in locAL-PFS was observed between patients with κ or λ aLC (88 vs 86 months; P = .63, Figure S2A). In patients with lung, larynx, urinary tract, GI and skin LocAL (ie the most frequently involved organs), 5-year locAL-PFS was similar and no differences were seen in locAL-PFS (overall median locAL-PFS 85 months; P = .34). In CNS locAL, 5-years locAL-PFS was particularly low (41%). When this small group was compared with the overall population, significance was slightly missed (40 vs 88, P = .07). No difference in locAL-PFS was found in patients with multifocal involvement (85 vs 138 months; P = .99), cAD (138 vs 88 months; P = .28) or ANA titer ≥1:640 (138 vs 88 months; P = .67), as in those with a MC and/or an abFLCR with the same LC isotype as the aLC (88 vs 65 months; P = .78, Figure S2B). Notably, a shorter locAL-PFS was observed among patients with an identified clonal infiltrate at amyloid deposition site (40 vs 109 months; P = .022, Figure 1B), and was similar in those with a plasma cellular or a lymphoid clone (40 vs 43 months; P = .89). Presence of MGC and/or inflammatory infiltrate showed no significant difference (85 vs 43 months; P = .37). However, a better locAL-PFS was found in lung locAL patients in which MGC and/or an inflammatory infiltrate (65 vs 42 months; P = .01) were identified. Importantly, presence of an identified local plasma cell or B-cell clone again showed a shorter locAL-PFS (42 vs 65 months; P = .02).
Median locAL-PFS after treatment was 56 months. Local progression was observed in 6 of 11 patients that received radiotherapy as part of the first-line treatment after a median of 42 months (range: 17-56 months), particularly in 2 of the 6 cases in which surgery was coupled with radiotherapy. However, no difference in locAL-PFS was observed according to treatment strategy (overall median locAL 55 months; P = .48). LocAL-PFS was significantly longer in symptomatic patients who responded to treatment (65 vs 32 months; P = .01). LocAL-PFS was better for responders also in asymptomatic patients (96 vs 14 months; P < .001). Overall, the 119 responders had a better locAL-PFS than the 41 with stable disease (76 vs 17 months; P < .001, Figure 1C). This benefit on locAL-PFS was observed also when analysis was performed in patients treated only with a local approach (ie no chemotherapy or systemic steroids, 67 vs 17 months; P < .001). No difference in locAL-PFS was observed between symptomatic and asymptomatic treated patients either (51 vs 96 months; P = .24).
4 DISCUSSION
In this comprehensive work, we present in detail the main features of locAL, starting from precise classification of clinical manifestation and focusing on characterization of the local infiltrate and the amyloidogenic B-cell clone and description of outcome and local progression.
The NAC and Mayo Clinic have already published two studies reporting large series of patients with locAL. Although several similarities, the present study's design differs mainly for two aspects. The first is amyloid typing. In NAC's and Mayo Clinic's cohort, an unequivocal typing was available in 91 (15%) and 170 (41%) patients, respectively. With 293 immunohistochemically confirmed locAL cases, our study represents the largest precisely classified series of locAL. Amyloid typing was performed by immunohistochemistry with custom made antibodies in all patients. This technique has been proven adequate and effective for amyloid typing and was validated in patients with sysAL.14 As a further confirm, in all patients the LC of the local clone was the same as aLC. The second peculiar aspect of our study is the histological and molecular characterization of the cellular infiltrate at amyloid deposition site.
Our findings confirm and extend data from previous studies. Patients with lung locAL are usually older,12 and skin and ST locAL are more common in women.16 A cAD and a MC and/or abFLCR are particularly common in these patients. In general, prognosis of locAL is good, however, a worse survival is observed in patients with lung locAL. However, considering also that the majority was asymptomatic at diagnosis, it seems more likely that in these cases death was related to their older age or other comorbidities. Response to treatment and local progression were evaluated according to changes in clinical manifestation, imaging and endoscopic findings, as reported before by the Mayo Clinic group.12 Most patients responded to treatment but 40% showed local progression. Five-years locAL-PFS was similar among patients with different involved organ sites. As expected, in treated patients a response resulted in longer locAL-PFS. This was confirmed both when response was assessed with clinical and radiological evaluation (ie in symptomatic patients) or with imaging alone (ie in asymptomatic patients). At 5 years from treatment, 41% of responders and 71% with stable disease had a local progression. We confirm the effectiveness of radiotherapy, particularly in combination with surgical debulking, in selected cases.17-19 However, in general we observed no differences in outcome according to different treatment regimens.
Progression to sysAL seems to be a rare event. Similar to NAC's colleagues, we observed a systemic progression in 1% of cases. All these patients had a MC matching the aLC. However, none of the patients with concomitant MM or systemic lymphoma had a systemic progression. Moreover, no patients developed a sysAL with typical organ involvement (ie heart, kidney or liver). As stated by the Mayo Clinic colleagues, “systemic progression” in locAL can be also results of a misclassification of a primarily systemic disease as locAL. Indeed, we had three cases of suspected locAL correctly diagnosed as sysAL during the first evaluation at our center. Ruling out sysAL is mandatory but can be sometimes difficult, especially in patients with lymph node and lung involvement, which can be affected in both, locAL and sysAL.20-22
One difference to past studies relate to the frequency of organ involvement. In our series, the lung is the most commonly involved anatomical site, while in the other two series most patients had urinary tract locAL. These differences could be related to referral bias.
The second difference is the κ:λ ratio. A higher prevalence of LC κ was reported in locAL.5 The κ:λ ratio reported in NAC and Mayo Clinic case series was 3:1 and almost 1:1, respectively. In the present study, we observed a higher prevalence of λ, with a κ:λ ratio of 1:3. This is comparable to sysAL and nicely fits with the evidence for a higher inclination of λ LC to form amyloid fibrils.
Our cohort also showed an organ type-specific variation of the κ:λ ratio for locAL, for example, almost all patients with CNS involvement presented with aLC λ, while in lymph nodes locAL aLC κ was more frequent. These observations confirm and extend previous findings from small case series of CNS and lymph node locAL.11, 22 Thus, variations of κ:λ ratios in different case series may be also related to the variable compositions of anatomical sites. Curiously, the preferential manifestation of either λ or κ in different tissues and organ sites has also been described in sysAL.23, 24 Recently, the Mayo Clinic group showed a higher frequency of LV2-14 in GI locAL, suggesting a link between IGVL usage and organ involvement, similar to sysAL.25-28 In support of these findings, λ was also very common in our series of patients with GI locAL (83%).
For the first time, we evaluate factors that might affect local progression. In the entire cohort, local progression seems to be not affected by anatomical site, aLC isotype and presence of autoimmune system dysregulation. A concomitant MC and/or abFLCR matching the aLC has also no impact on locAL-PFS. Notably, a MC and/or abFLCR were more frequent in patients with a cAD and present in almost 50% of patients with an ANA titer >1:640. We suppose that the higher prevalence of MC than in the general population29 is related to the high frequency of autoimmune system dysregulation observed in these patients. Indeed, a higher risk for developing secretory active B-cell lymphomas has been described.29 Thus, it is reasonable to suppose that the MC and an abFLCR have not a direct correlation with amyloid deposition in most of cases. This may be particularly true in those cases in which the LC of the MC and/or abFLCR does not match the aLC and that represent the 16% of our case series. On the other hand, the presence of a MC with the same LC isotype of aLC requires careful attention, considering the possibility of a misdiagnosed sysAL and the possible risk of systemic progression. The presence of sysAL and of a systemic underlying clone should be systematically ruled out with an extensive evaluation comprehending echocardiogram, abdominal fat pad aspirate, flow cytometry of peripheral blood lymphocytes and bone marrow biopsy.
Intriguingly, patients with an identified local B-cell clone have a shorter locAL-PFS, without a difference between clonal plasma cells or B lymphocytes. Identification of the amyloidogenic clone on tissue biopsies from patients with locAL is cumbersome. In our series a local B-cell clone was identified in 30% of the cases, whilst a lymphocytic infiltrate was present in 49%. Due to paucicellularity and a concomitant inflammatory response, immunohistochemistry might not be sensitive enough to detect the clone in most cases, while more sophisticated approaches for clonality assessment, for example, in situ hybridization and molecular pathological analyses, are more effective.5, 11, 13 Since these latter techniques were not systematically used in our study, it is possible that we identified only cases with more extended B-cell clones, while cases with more subtle infiltrations could have been missed for example, due to sampling errors (note the correlation with sample size) or technical issue (eg pure DNA quality, below detection limit of IGH-PCR). Indeed, it is reasonable that an amyloidogenic clone is always present at amyloid deposition site, even if it cannot be identified. Larger biopsies may be useful for a better characterization of local cellular infiltrate. However, they are not necessary for the current management of locAL.
Several studies described a clonal lymphoplasmacytic infiltrate within the spectrum of MZL in small case series with locAL.9, 10, 30, 31 Interestingly, in our study five patients with MZL or MALT presented with a B-cell lymphoma infiltrate at the site of amyloid deposition. Thus, a concomitant lymphoma can rarely cause locAL. However, a larger study revealed that the infiltrate is currently best classified as “localized B-cell neoplasia of undermined significance” in the majority of cases.13 Thus, like in sysAL, the amyloidogenic clone is usually small and has not the full characteristics of a well-defined malignant disease.4 The B-cell clones in locAL might have other not yet known biologic characteristics that affect the outcome, as already observed in sysAL.32-36
The etiology of extra-nodal B-cell clones is currently unknown. Chronic antigen exposure and autoimmune stimulation are putative explanations.37 This hypothesis is intriguing and could explain the high prevalence of cAD in locAL, as the presence of hypergammaglobulinemia in some cases. Notably, the local clone and cAD were more frequently detected in skin locAL. Another possible factor of chronic B-cell stimulation could be cigarette smoking in lung locAL, as the prevalence of smokers was higher in these patients. However, this finding could be hampered by a pre-diagnostic bias, as smokers are more likely to get lung imaging with the consequent discovery of suspected focal lesions. The hypothesis of chronic B-cell stimulation seems to be also supported by the IGLV usage in locAL, which is similar to normal B-cell population and by the finding of inflammatory cells, especially MGC, around the amyloid deposits. Curiously, our data from samples of patients with lung locAL showed a possible protective role of the inflammatory infiltrate and MGC against local progression. These cells might interact with amyloid deposits.5
Our study has some limitations. Due to its retrospective nature, a complete rheumatologic assessment was not available in all patients. Data about the cellular infiltrate at amyloid deposition site were present in half of cases. The clonality assessment of the infiltrate was performed with different techniques. Finally, the decision of treatment strategy (ie, watch and wait approach vs treatment) was often taken by local physicians before our evaluation.
In conclusion, our study shows that the local B-cell clone is one main driver of local progression. Response to treatment resulted in longer locAL-PFS. It is noteworthy that effective treatment approaches, as surgery and radiotherapy, result in removal and direct elimination of the B-cell clone. The removal of amyloid deposits would be another interesting therapeutic strategy. However, the effectiveness of the currently tested ant-amyloid therapies has not be confirmed so far. Finally, MGC and a local inflammatory response may be protective. However, our knowledge on the pathophysiology and biology of the B-cell clone in locAL is still poor. It is likely that, as in sysAL, the local B-cell clone harbors peculiar characteristics which might affect clinical manifestation and outcome. We hope that our work will push further basic and clinical research on locAL, in order to first improve our understanding of this mysterious disease and consecutively the management of our patients.
ACKNOWLEDGEMENTS
We would like to thank our patients and their families, local hematologists, general practitioners and our hospital staff for their participation in this study.
AUTHORSHIP CONTRIBUTIONS
Conception and design: Marco Basset, Ute Hegenbart, Stefan O. Schönland. Provision of study materials or patients: Marco Basset, Kamal Hummedah, Christoph R. Kimmich, Kaya Veelken, Tobias Dittrich, Simone Brandelik, Michael Kreuter, Jessica Hassel, Nikolaus Bosch, Christine Stuhlmann-Laeisz, Norbert Blank, Carsten Müller-Tidow, Christoph Röcken, Ute Hegenbart und Stefan O. Schönland. Collection and assembly of data: Marco Basset, Kamal Hummedah, Ute Hegenbart und Stefan O. Schönland. Data analysis and interpretation: Marco Basset, Kamal Hummedah, Christoph R. Kimmich, Kaya Veelken, Tobias Dittrich, Simone Brandelik, Michael Kreuter, Jessica Hassel, Nikolaus Bosch, Christine Stuhlmann-Laeisz, Norbert Blank, Carsten Müller-Tidow, Christoph Röcken, Ute Hegenbart und Stefan O. Schönland. Writing the manuscript or revising it critically for important intellectual content: All authors.
CONFLICT OF INTEREST
U.H. has received travel grants from Janssen, Prothena and Pfizer, served on the advisory boards for Pfizer and Prothena, and has received honoraria from Janssen, Pfizer, Alnylam and Akcea. S.O.S. has received travel grants from Janssen, MSD, Prothena and Takeda, served on the advisory boards for Janssen, Takeda and Prothena, has received honoraria from Janssen, Prothena and Takeda, and he received research funding from Sanofi and Janssen.




