Low-dose ponatinib is a good option in chronic myeloid leukemia patients intolerant to previous TKIs
Funding information:
The only funds used were provided by the authorsʼ institutions.
To the Editor:
Ponatinib is a potent, orally available, third-generation tyrosine kinase inhibitor (TKI) with proven efficacy in both mutated and unmutated BCR-ABL1-positive chronic myeloid leukemia (CML) patients including the “gatekeeper” T315I mutation.1
While its efficacy has been firstly confirmed in the pivotal phase II PACE trial,2 nevertheless, when used at the recommended starting dose of 45 mg/d ponatinib raised some concerns due to an increased risk of cardiovascular (CV) adverse events (AEs). In fact, when assessed in a hind limb ischemia model, owing to its multikinase inhibitory properties ponatinib may cause endothelial dysfunction. Ponatinib may affect targets such as VEGFR and promote the expression of proatherogenic surface adhesion receptors.3 Since these effects were found to be dose-related, suggesting a direct relationship between CV AEs and ponatinib dosage, a reduction of the daily dose has been advised for patients who already achieved at least a major cytogenetic response (MCyR).
While ponatinib efficacy has been confirmed also in the real-world CML setting, both in an Italian multicenter observational study4 and in the French PEARL observational study,5 so far only limited data have been reported on its use at reduced dosage after TKIs intolerance. This is defined as any combination of hematological or non-hematological toxicities of any grade that persist, despite optimal supportive therapies and affect quality of life (QoL), to such an extent that a change of therapy is justified.
Although successful pharmacologic treatment of CML is nowadays likely to result in near-normal life expectancy, yet at least a quarter of patients will change TKI at least once during their life, because of either inadequate response or intolerance. In such context, retrospective real-world studies showed a favorable outcome of ponatinib therapy also after intolerance to a second-generation TKI.6-8
The primary objective of the present study was then to evaluate in the real-life setting the safety and efficacy profile of ponatinib at reduced dosage in CML patients previously intolerant to TKIs, treated between May 2012 and December 2019 in 14 Italian hematological centers.
At diagnosis sociodemographic variables and type and number of concomitant diseases defined and scored according to the Charlson comorbidity index (CCI) were reported. In order to calculate the Systematic Coronary Risk Evaluation (SCORE Chart),9 CV risk factors were recorded stratifying all patients into low to moderate (SCORE <5%) or high to very high (SCORE ≥5%) CV risk.
Concerning CML-specific related data, risk scores (ie, Sokal and ELTS), BCR-ABL1 transcript type, any prior TKI treatment and reasons for TKI discontinuation were registered. With regards to ponatinib, the following variables were collected: initial dose, molecular response at baseline, 3, 6 and 12 months from treatment start, any dose reduction, best achieved molecular response and related AEs.
Monitoring and responses were defined according to the current European LeukemiaNet recommendations: in particular, major molecular response (MMR) as a BCR-ABL1 ratio ≤0.1% with at least 10.000 ABL1 copies; deep molecular response (DMR) as MR4 (BCR-ABL1 ratio ≤0.01% with at least 10.000 ABL1 copies), MR4.5 (BCR-ABL1 ratio ≤0.0032% with at least 32.000 ABL1 copies), or MR5 (BCR-ABL1 ratio ≤0.001% with at least 100.000 ABL1 copies). Hematological and non-hematological toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTC-AE) v4.03.
Overall, 52 consecutive CML patients were treated with ponatinib because of intolerance to previous TKIs, most frequently dasatinib (35 patients, 67.3%), followed by nilotinib (12 patients, 23.1%) and bosutinib (five patients, 9.6%), with a median duration of 1.5 years (range, 0.1-15.4 years). The most common AEs were pleural effusion (24 patients, 46.2%), followed by pancreatic (seven patients, 13.5%), hematological (five patients, 9.6%) and gastrointestinal toxicity (four patients, 7.7%). Main clinical variables of our series at diagnosis are summarized in Table S1. Median time from diagnosis to ponatinib treatment was 5.3 years (range, 0.3-23.4 years). Median starting dose of ponatinib was 22.5 mg/d (range, 15-45 mg/d) (Table S2); the initial dosage, either 15 mg/d or 30 mg/d, was decided according to patientsʼ frailty and type of best molecular response. As reported in Table 1, ponatinib was administered as second line in 20 (38.5%), third line in 23 (44.2%) and fourth line in nine (17.3%) cases.
| Second-line (N = 20) | Third-line (N = 23) | Fourth-line (N = 9) | Overall (N = 52) | |
|---|---|---|---|---|
| TKI pre-ponatinib, n (%) | ||||
| Nilotinib | 2 (10.0) | 5 (21.7) | 5 (55.6) | 12 (23.1) |
| Dasatinib | 17 (85.0) | 16 (69.6) | 2 (22.2) | 35 (67.3) |
| Bosutinib | 1 (5.0) | 2 (8.7) | 2 (22.2) | 5 (9.6) |
| Adverse events, n (%) | ||||
| Hypertension | 2 (10.0) | 4 (13.0) | / | 6 (11.5) |
| Cardiovascular | / | 1 (8.7) | 3 (33.3) | 4 (7.7) |
| Pancreatitis | 3 (15.0) | 1 (4.3) | 3 (33.3) | 7 (13.5) |
| Others | 4 (20.0) | 2 (8.7) | 1 (11.2) | 7 (13.5) |
| MR before ponatinib start, n (%) | ||||
| <MMR | 12 (60.0) | 15 (65.2) | 7 (77.8) | 34 (65.4) |
| MMR | 4 (20.0) | 4 (17.4) | 1 (11.1) | 9 (17.3) |
| DMR | 4 (20.0) | 4 (17.4) | 1 (11.1) | 9 (17.3) |
| MR at last follow-up, n (%) | ||||
| <MMR | 2 (10.0) | 7 (30.4) | 4 (44.4) | 13 (25.0) |
| MMR | 8 (40.0) | 6 (26.1) | 1 (11.2) | 15 (28.8) |
| DMR | 10 (50.0) | 10 (43.5) | 4 (44.4) | 24 (46.2) |
- Abbreviations: DMR, deep molecular response; MR, molecular response; MMR, major molecular response.
At a median follow-up from ponatinib start of 19.2 months (range, 0.4-89.5), 21 (40.4%) patients increased the depth of their molecular response (including six patients with MMR and 15 with DMR) (Figure 1A). A significant improvement in molecular response was achieved also by subjects initially treated at a daily dose of 15 mg (Figure 1B). Interestingly, among patients who obtained a DMR during ponatinib, treatment-free remission was successfully achieved in one case.
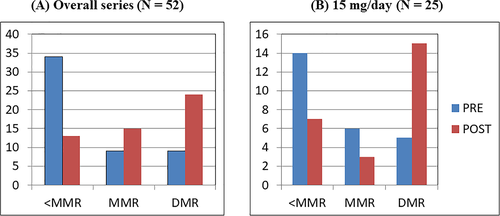
During follow-up, ponatinib dosage was reduced in 21 (40.4%) subjects: in 17 (32.7%) because of an AE and in four (7.7%) after achieving an improvement in molecular response, without significant hematological and non-hematological toxicity.
Adverse events were reported in 24 (46.2%) patients, including pancreatitis in seven (13.5%), hypertension in six (11.5%) and CV AEs in four (7.7%) cases, including one acute myocardial infarction (AMI) and one ischemic stroke, the latter both registered in patients with pre-existing CV risk factors and who were initially treated with ponatinib at a daily dose of 30 mg. Additionally, the patient who experienced AMI received also nilotinib as a previous treatment.
Even though due to concern on its CV safety profile ponatinib has been used so far mostly in advanced lines of therapy in case of failure to prior TKIs, still a careful benefit-risk balance evaluation is mandatory particularly in intolerant cases.
Owing to the occurrence of CV events and venous thromboembolisms reported in the PACE trial, since October 2013 ponatinib dose reduction has been suggested when at least an MCyR was obtained.2 This strategy offers new opportunities to mitigate the risk of CV events, not only for patients resistant to other TKIs but also for those with clinically documented or perceived intolerance. In this context it should be emphasized that since all TKIs can lead to long-term toxicities which may not only have a negative impact on QoL but also bring to a premature change or cessation of therapy, the management of their side effects stands as a new challenge.
With the aim of investigating the feasibility of low-dose ponatinib in the real-life setting, we retrospectively analyzed 52 consecutive CML patients intolerant to second-generation TKIs treated with ponatinib in 14 Italian hematological centers.
The most frequently daily dosage was 15 mg or 30 mg, while only a few subjects due to a suboptimal response to previous TKIs were treated at 45 mg/d; all the latter cases subsequently reduced the dose.
Most patients (82.7%) received ponatinib as second or third line of therapy, being dasatinib the most frequent TKI previously used. Interestingly, this observation is in line with that recently reported in a retrospective analysis from the monitoring registries of the Italian Medicines Agency (AIFA).10
In order to minimize the risk of CV AEs, before starting ponatinib the SCORE Chart was applied to all patients. Indeed, even though almost half of the evaluated cases were aged >65 years, most of them (71.2%) were classified at low to intermediate risk and 28.8% at high to very high risk. In details, at least one CV risk factor was reported in more than half of the cases (63.5%), more frequently represented by hypertension (36.5%). Since the latter was the most important risk factor also in the PACE trial, it should be aggressively treated in order to prevent possible life-threatening complications.2
Furthermore, at a median follow-up of 19.2 months, arterial CV AEs were recorded in only four patients (7.7%), without venous complications, a significantly lower frequency than reported in the PACE study.2 Therefore, the possible occurrence of CV events should not discourage “a priori” the use of ponatinib, provided that identification of pre-existing concomitant risk factors and appropriate management are thoroughly performed in order to reduce the related AEs.
Alongside the low rate of AEs, ponatinib at reduced dosage has also proven to be a fully effective therapeutic option in patients intolerant to other TKIs, as suggested by the increased depth of molecular response recorded in 21 patients.
This option should not be considered as an empirical choice but rather as a rationally selected tool allowing a greater treatment flexibility; by optimizing AEs prevention strategies and tolerability profile ponatinib at reduced dosage can also improve long-term treatment adherence, a critical prognostic factor.
In our study limitations are represented by its retrospective nature and the relatively low number of enrolled patients; CML however is a rare disease and the subset of patients requiring ponatinib is even smaller, allowing only for limited sample sizes.
In conclusion our results, showing a lower incidence of drug-related AEs without any detrimental effect on therapeutic activity, highlight not only the safety but also the efficacy of ponatinib at reduced dosage in the setting of CML patients previously intolerant to TKIs. Even if further prospective studies are needed to better assess the long-term benefits of dose reduction, this treatment strategy may represent an effective option for TKI intolerant patients.
CONFLICT OF INTERESTS
A.I. and P.P. has received speaker honoraria from Novartis, Pfizer and Incyte. E.A. has received honoraria for advisory board and consultancy from BMS, Novartis, Pfizer and Incyte. M.B. has received honoraria for advisory board and consultancy work from Novartis, Pfizer, Incyte and Celgene. The remaining authors declare they have no potential conflicts of interest.




