Sickle cell microvascular paradox—oxygen supply-demand mismatch
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: 2 K12 HD 52954-6 A1; National Heart, Lung, and Blood Institute, Grant/Award Numbers: 1 U54 HL090511-01, 1K23HL119627-01A1, 1R03HL138321-01, 1U01HL117718-01; National Institute of Biomedical Imaging and Bioengineering, Grant/Award Number: P41 EB001978; Southern California Clinical and Translational Science Institute, Grant/Award Number: RR00043-43; National Institute of Biomedical Imaging and Bioengineering; Sickle Cell Scholar Award, Grant/Award Number: 5 RC1 HL099412-01; National Heart Lung and Blood Institute; National Institutes of Health
Abstract
We have previously demonstrated that sickle cell disease (SCD) patients maintain normal global systemic and cerebral oxygen delivery by increasing cardiac output. However, ischemic end-organ injury remains common suggesting that tissue oxygen delivery may be impaired by microvascular dysregulation or damage. To test this hypothesis, we performed fingertip laser Doppler flowmetry measurements at the base of the nailbed and regional oxygen saturation (rSO2) on the dorsal surface of the same hand. This was done during flow mediated dilation (FMD) studies in 26 chronically transfused SCD, 75 non-transfused SCD, and 18 control subjects. Chronically transfused SCD patients were studied prior to and following a single transfusion and there was no acute change in rSO2 or perfusion. Laser Doppler estimates of resting perfusion were 76% higher in non-transfused and 110% higher in transfused SCD patients, compared to control subjects. In contrast, rSO2 was 12 saturation points lower in non-transfused SCD patients, but normal in the transfused SCD patients. During cuff occlusion, rSO2 declined at the same rate in all subjects suggesting similar intrinsic oxygen consumption rates. Upon cuff release, laser doppler post occlusive hyperemia was blunted in SCD patients in proportion to their resting perfusion values. Transfusion therapy did not improve the hyperemia response. FMD was impaired in SCD subjects but partially ameliorated in transfused SCD subjects. Taken together, non-transfused SCD subjects demonstrate impaired conduit artery FMD, impaired microcirculatory post-occlusive hyperemia, and resting hypoxia in the hand despite compensated oxygen delivery, suggesting impaired oxygen supply-demand matching. Transfusion improves FMD and oxygen supply-demand matching but not microcirculation hyperemic response.
1 INTRODUCTION
Sickle cell disease (SCD) is a common genetic disease resulting in the formation of hemoglobin S (HbS), which polymerizes upon deoxygenation.1-3 HbS polymerization causes decreased red blood cell (RBC) deformability, intravascular hemolysis and increased vascular adhesion, promoting recurrent ischemia reperfusion injury and both large and small vessel disease.3 Clinically, vascular disease manifests as acute vasocclusive pain episodes, stroke, chronic kidney disease, pulmonary hypertension and restrictive cardiomyopathy.4-6 Chronic hemolysis, increased cellular adhesion, hypercoagulability, and increased blood viscosity act in concert, leading to diffuse vascular disease complications.7 However, the SCD research community needs clinical measures of vascular disease that can act as surrogates for clinical outcomes, and be used as metrics for therapeutic trials.
Chronic inflammation and increased RBC adhesiveness in less deformable RBC leads to increased cellular adhesion and microvascular occlusion. This has been demonstrated in vitro, inanimal and in human intravital microscopy studies.8-13 Regional microvascular resistance in rat and mouse cremaster muscle initially increased after a bolus of SS RBC, but returned to levels within 20% of normal, due to recruitment of parallel vascular channels next to occluded capillaries. However, microvascular dysfunction progressed after prolonged exposure to SS RBC.12 Cellular adhesion limits blood flow in SCD microcirculation. This is by adhesion of reticulocytes and less deformable discoid RBC to endothelium in post-capillary venules and, in the precapillary arterioles9, 13, 14; and RBC adherence to endothelium in the microcirculation likely correlates with clinical severity.15
Multiple research groups have demonstrated decreased conduit artery flow-mediated dilation in sickle cell disease.16-18 Our group linked impaired flow-mediated dilation (FMD) in the brachial artery with pulmonary hypertension in patients with sickle cell disease, and in patients with primary pulmonary hypertension. That suggests that endothelial dysfunction is a common precursor to both systemic and pulmonary vascular disease.19, 20 We further demonstrated that cell free hemoglobin was the strongest predictor of FMD, consistent with its known role in nitric oxide (NO) scavenging and endothelial toxicity.7, 21 However, the potential impact of hemolysis on more distal microvascular beds remains understudied.
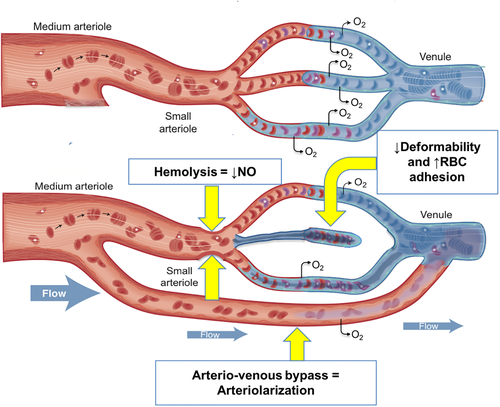
In vivo characterization of the human microcirculation and regional hemoglobin oxygen saturation (%rSO2) can be performed using laser Doppler flowmetry and near infrared spectroscopy.22, 23 Figure 1, upper panel, is a representation of a healthy microcirculation. Blood flow through the capillary network, is regulated by pre-capillary arteriole smooth muscle vasodilation and vasoconstriction. The capillary spacing is designed to minimize oxygen diffusion distance and provide optimal oxygen supply-demand matching. Laser Doppler flowmetry detects small artery and arteriolar flow by directly measuring the concentration of moving RBC and their velocity, while near infrared light quantifies changes in oxygenated and deoxygenated hemoglobin species across the entire perfusion unit.24, 25 The goal of this study was 2fold: 1. To determine the microvascular response to brief, provoked ischemic challenges; 2. to determine the prevalence and predictors of microvascular dysfunction and its interaction with macrovascular disease.26, 27 We postulated that resting microvascular perfusion would be increased because of anemia, but not sufficiently to maintain adequate tissue oxygen delivery and saturation. We further postulated that post occlusive hyperemia would be blunted in SCD patients and would correlate with FMD. Lastly, we postulated that chronic transfusion therapy would reverse these abnormalities compared with non-transfused SCD patients. To explore this, we studied changes in microvascular perfusion and FMD, in response to cuff occlusion and transfusion in subjects with SCD and in normal controls.
2 METHODS
All studies were performed according to Good Clinical Practice and the Declaration of Helsinki. Informed consent was obtained from all patients, and guardians when appropriate, under a protocol approved by the Children's Hospital Los Angeles's Institutional Review Board (CCI-07-01448, CCI-11-00144).
Twenty-six (26) SCD patients on chronic transfusion therapy, 75 non-transfused SCD patients and 18 healthy control subjects were enrolled in a prospective cross-sectional/experimental study. It was designed to evaluate vascular function using a post-occlusive hyperemia protocol; all subjects were eight years of age or older. Patients were recruited from the hematology clinics at CHLA, USC and from the community. The non-transfused and healthy subjects were studied on a single visit (cross-sectional design). The chronically transfused subjects were studied immediately pre-transfusion and 12-h to 5-d post-transfusion. Indications for chronic transfusion included abnormal transcranial Doppler (n = 9), prior stroke (n = 9), chronic pain (n = 2) or recurrent acute chest(n = 6). Chronically transfused patients had been regularly transfused at least 1.4 years (1.4-21.9 years). Any patients who had a history of acute crisis, defined as hospitalization or an emergency department visit with an increase or addition of pain medications above usual dosage, or a history of acute transfusion within the previous 4 wk, were temporarily deferred from study participation. Hydroxyurea use was documented based on patient report and medication effect was assessed by MCV measurements and presence or absence of HbF. Our cutoff to define HU responders was MCV > 100 fL/red cell or presence of HbF on electrophoresis. Some responders may reach an MCV less than 100 fL/red cell, depending on the MCV prior to HU initiation, but that data was not available in all subjects. We provided data on hemoglobin sub-type for the non-transfused subjects in order to be complete in our sample description. The hemoglobin subtype was not associated with any differences in vascular function for our sample, therefore, none of our analyses correct for the various subtypes. For laboratory, demographic, and vascular function data in the transfused group, we calculated a weighted average of the data that was collected pre-transfusion and post-transfusion.
2.1 Flow-mediated dilation of the brachial artery
We used a flow-mediated dilation protocol with a 3-min cuff occlusion of the forearm and 7-min post ischemia monitoring period as previously described.19 All vascular and oxygenation monitoring was performed during this testing procedure (Figure S1). Using the forearm occlusion method, the published mean and SD for healthy subjects was 7.3% ± 0.9%.28 For our combined vascular dysfunction metric we used a cutoff <6.3% based on our control FMD data, mean 7.5%, [95% CI 6.3% to 8.8%].
2.2 Regional tissue hemoglobin oximetry (%rSO2)
We utilized a clinical near infrared spectroscopy cerebral/somatic regional oximetry monitor, Invos (Somanetics Corporation Troy, MI), for all regional oximetry monitoring. This measures the balance of oxygenated and deoxygenated hemoglobin in the tissue which includes both pre-capillary and post-capillary vasculature. A near infrared patch was placed on the dorsal surface of both hands. Regional tissue oximetry was sampled every 4 s for the duration of the study. The cuff occlusion protocol includes baseline monitoring for at least 5-min, following a rest period of 10 min. Then cuff occlusion to >50 mmHg above systolic blood pressure was done for 3 min, and then release of the cuff with monitoring for 7-min post occlusion.19
2.3 Microcirculatory flowmetry; a measure of RBC perfusion
We utilized a laser Doppler flowmetry system, PeriFlux 5000 (Perimed AB Stockholm, Sweden) to monitor microcirculatory blood flow at a level ~0.5-1 mm below the skin surface. The laser Doppler measures the concentration of moving red blood cells and the velocity of flow, then calculates a perfusion unit based on the product of those two components. We placed laser Doppler probes at the base of the nailbed of the third finger on both hands due to the robust blood supply. Laser Doppler flowmetry (LDF) measurements were sampled at 32 Hz throughout the cuff occlusion protocol, simultaneously with the %rSO2 measurements. The laser Doppler signal from the nailbed is pulsatile, matching the heart rate, with a morphology similar to arterial tonometry, and consistent with flow in pre-capillary arterial vessels. Normal microcirculatory function was based on the percent change by LDF after cuff release in our healthy cohort, using <234% as the cutoff [mean 357%, (95% CI: 234% to 480%)]. Perfusion curves for a sample of healthy control subjects, non-transfused patients with SCD and chronically transfused patients with SCD are shown in Figure S2. This demonstrates a wide variation in resting perfusion and morphology of the post-occlusive hyperemia.
2.4 Venous blood samples for rheology and laboratory markers
Venous blood samples of ~15 cc were drawn into EDTA tubes to assess markers of hemolysis, blood counts, hemoglobin electrophoresis, markers of inflammation, and blood rheology. Rheologic markers included blood viscosity measured over shear rates of 1 s−1 to 1000s−1, and red blood cell deformability measured over shear stresses of 0.3 to 50 Pascals (Pa). and Red cell aggregation was measured at stasis and low shear (3 s−1) in autologous plasma, and red cell aggregability was measured at stasis and low shear (3 s−1), in a pro-aggregating dextran suspension (3% dextran 70 kDa in isotonic pH = 7.4 PBS buffer). Aggregation in plasma and in the dextran medium at 40% hematocrit was measured using the Myrenne aggregometer, (Myrenne GmbH, Roetgen, Germany). Blood viscosity was measured using an automatic tube viscometer (Rheolog, Health Vector Co., Pennsauken, NJ) as described previously.29 Red blood cell deformability was measured using a laser diffraction ektacytometer LORRCA (Mechatronix, Inc., Amsterdam, NL) with a standardized diffraction pattern, consistent with our prior studies of deformability measurements in sickle cell disease.30, 31
2.5 Statistical analysis
Data normality was determined by the Shapiro-Wilk test. We used parametric tests (Analysis of Variance) for normally distributed data and non-parametric tests (Kruskal-Wallis) for non-normally distributed data. Post hoc group comparisons were performed using the methods by Tukey and Steel with control for parametric and non-parametric data respectively. Due to controversy over metrics concerning hydroxyurea use and response, post hoc analysis of hydroxyurea data was performed using dichotomized variables: hydroxyurea (y/n) and hydroxyurea+MCV > 100 (y/n). We also used continuous variables for regression analysis: HbF% vs all vascular parameters. Paired statistics pre-transfusion and post-transfusion were performed using paired t test for parametric data and Wilcoxon Signed Rank Test for non-parametric data. Univariate and multivariate stepwise analyses were used to determine candidate variables for multivariate linear regression models. Area under the ROC curve was used to determine optimal cutoffs when determining continuous predictors of categorical variables. We combined all three groups and controlled for diagnosis/groupwise differences in the final models. All statistics were performed using JMP Pro version 13.0.0 (Copyright © 2016 SAS Institute Inc., Cary NC).
3 RESULTS
3.1 Patient characteristics
We enrolled 75 non-transfused patients, 26 chronically transfused (all homozygous SS) and 18 healthy subjects. Patient demographics are summarized in Table 1. The transfused SCD group was younger than the non-transfused SCD and control groups (P = .002). The sex distribution was similar across the three groups. The SCD groups had elevated markers of inflammation and hemolysis compared to the control (P < .0001), independent of transfusion status. Chronic transfusion therapy improved but did not fully correct the anemia (P < .0001 comparing non-transfused and transfused by Tukey post hoc analysis, or Bonferroni correction for non-normal data). Laboratory markers of hemolysis, inflammation and anemia, and markers of vascular function (FMD, post-occlusive hyperemia and %rSO2) in non-transfused patients were independent of hydroxyurea use. This was true whether the comparison included all patients prescribed hydroxyurea, or was limited to good responders. Standard fingertip pulse oximetry was similar in all three groups, although there was a small subset of non-transfused patients with resting saturation in the high 1980s to mid-1990s on pulse oximetry. adjusAments for low baseline pulse oximetry did not change our results.
| Demographics | Non-transfused N = 75 | Transfused N = 26 | Control N = 18 | P-Value |
|---|---|---|---|---|
| Age in years (interquartile range) | 27.1 (19,39) | 19 (15,25) | 24 (17.8,41.4) | .002 |
| Sex (%male) | 39% | 46% | 56% | .380 |
| Race (%African American) | 89% | 85% | 83% | .660 |
| SS | 34 | |||
| SC | 15 | |||
| SF | 17 | |||
| SB | 3 | |||
| Unknown | 6 |
| Labs | ||||
|---|---|---|---|---|
| WBC Count (×103/ul) | 8.8 ± 3.5a | 13.6 ± 4.7a ,b | 4.9 ± 1.2 | <.0001 |
| Hemoglobin (g/dl) | 9.3 ± 1.8a | 10.4 ± 1.7a ,b | 13.4 ± 1.6 | <.0001 |
| Hematocrit (%) | 26.8 ± 5.1a | 30.8 ± 4.7a ,b | 39.7 ± 4.3 | <.0001 |
| Platelet Count (×103/ul) | 348.2 ± 126.4a | 305.9 ± 106.8 | 254.7 ± 52.6 | .004 |
| Reticulocyte Count (×106/ul) | 7.2 (4.2,11.2)a | 8.4 (4.7,15.1)a | 0.9 (0.8,1.4) | <.0001 |
| Hemoglobin S% (%) | 78.7 (59.4,85.9)a | 29 (20,44)a ,b | na | <.0001 |
| hs-CRP (mg/L) | 4 (1.8,8)a | 2.3 (1.2,8.2)a | 0.35 (0.1,2.8) | <.0001 |
| LDH (U/L) | 959 (7591302)a | 1073 (7751520)a | 491 (458539) | <.0001 |
| Plasma Hemoglobin (mg/dl) | 15 (10,23)a | 17.5 (8,34)a | 4.3 (2.9,6.5) | <.0001 |
| Pulse oximetry (%) | 96 (94,98) | 97 (95,98) | 97 (96,98) | .026 |
- Abbreviations: LDH, lactate dehydrogenase; hs-CRP, high sensitivity C-reactive protein; SS, homozygous S form of sickle cell disease; SC, heterozygous for hemoglobin S and hemoglobin C; SF, number of patients with fetal hemoglobin present; SB, hemoglobin S-beta zero thalassemia.
- a P < .05 between patients and control subjects by Tukey post-hoc correction or Steel method with control.
- b P < .05 between transfused and nontransfused subjects by Tukey post-hoc correction or Steel method with control.
3.2 Effect of hydroxyurea on vascular parameters
Post hoc analysis of hydroxyurea use was performed using a dichotomous variable of hydroxyurea yes (y) or no (n) that was defined by two different methods. They accounted for stated hydroxyurea use and the amount of fetal hemoglobin: (1) notation of hydroxyurea use at the time of study inclusion, (2)presence of fetal hemoglobin and MCV > 100. We also used hemoglobin F% as a continuous variable for regression against NIRS, FMD and microcirculatory perfusion. There was no effect of hydroxyurea as a dichotomous variable, or hemoglobin F% as a continuous variable, on the resting microcirculatory perfusion, post-occlusive reactive hyperemia, regional hemoglobin saturation, and flow-mediated dilation of the brachial artery between patients (data not shown).
3.3 Acute effect of transfusion on resting hemoglobin saturation
There was no acute effect of a single transfusion on %rSO2 (P = .11) in the hand, or resting perfusion by laser doppler flowmetry (P = .46), in patients on chronic transfusion therapy. There was no acute effect of a single transfusion on laser doppler flowmetry post occlusive hyperemia, (P = .64) or regional hemoglobin saturation response to post occlusive hyperemia (P = .58). The only vascular function parameter that improved after a single transfusion was flow-mediated dilation of the brachial artery, which we previously published.32
3.4 Resting microcirculatory flow by laser Doppler flowmetry and resting regional hemoglobin oxygen saturation (%rSO2)
Resting microvascular perfusion was 76% higher in non-transfused and 110% higher in transfused SCD patients compared to control subjects (Figure 2A). Increased perfusion was due increased concentration of moving blood cells in transfused (median 69, IQR 53101) and non-transfused SCD (median 53, IQR 40.5,75.8) patients compared to control (median 30, IQR 23,64), P = .006. There was no difference in velocity measured during the resting period. Since Doppler flowmetry is sensitive to cell flux and arterial blood is nearly completely saturated in both groups (Table 1), peripheral oxygen delivery is increased in SCD patients. This implies that the decreased oxygen content (caused by the anemia and small differences in arterial oxygen saturation) is compensated by increased microvascular blood flow in the fingertip and, in some patients, increased above that which is expected for the degree of anemia.
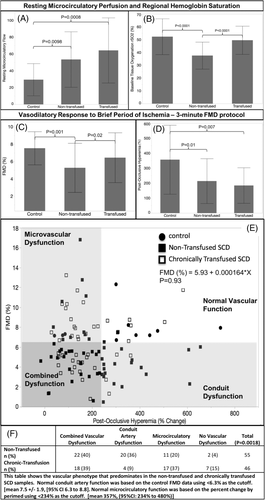
If peripheral oxygen delivery is compensation for anemia, one would expect %rSO2 to be normal. However, nontransfused SCD patients exhibited 12% lower (absolute percentage units) resting %rSO2 value in the hand versuscontrol subjects (Figure 2B). In the chronically transfused group resting %rSO2 was not different from control subjects.
3.5 Vascular flow reserve - regional desaturation during occlusion and post-occlusive hyperemia
The apparent disconnect between increased resting perfusion and decreased %rSO2 we observed in SCD patients could be explained if their peripheral oxygen utilization were increased. To test this possibility, we assessed microcirculatory flow and %rSO2 during and following a period of total forearm vascular occlusion. During occlusion, the laser Doppler perfusion signal rapidly declined to zero indicating no bulk blood flow in the microcirculation. The %rSO2 decreased slowly as the tissue utilized oxygen in the absence of arterial and venous blood flow. The rate of hemoglobin deoxygenation during occlusion was similar between all three groups, suggesting tissue oxygen consumption was not different between the groups (Figure S3).
After cuff release, there is compensatory peripheral vasodilation caused by local products of tissue metabolism, leading to rebound hyperemia. Post occlusion, there was a trend toward increased absolute tissue perfusion by laser Doppler in both SCD groups compared to control (P = .06, data not shown). However, the percent increase in perfusion (post-occlusive hyperemia) was decreased compared to control in both transfused and non-transfused subjects (Figure 2D).
Figure S3 summarizes %rSO2 changes in response to cuff occlusion and release. In control subjects, the post-occlusive hyperemia caused %rSO2 to rapidly rebound more than 20 saturation points above baseline, as oxygen delivery transiently exceeds tissue utilization. This relative oversupply of oxygen persists for approximately 4 min. Similar behavior was observed in the SCD patients except the return to baseline was more rapid (approximately two and half minutes). Differences in peak %rSO2 mirrored baseline differences.
3.6 Vascular flow reserve - conduit artery function - brachial artery flow-mediated dilation
Post -occlusive hyperemia in the hand causes increased flow velocity and wall shear stress in the brachial artery. In a healthy vasculature, shear-mediated nitric oxide release from the endothelium causes the brachial artery to dilate (flow mediated dilation, or FMD). FMD was blunted in non-transfused SCD patients (Figure 2C). FMD was also decreased in chronically transfused SCD patients, but the differences did not reach statistical significance compared to control using post-hoc Tukey test.
Although post-occlusive hyperemia produces the vascular shear that triggers brachial artery dilation, we observed no association between the two vascular responses (P = .93). To determine whether patterns of vascular dysfunction were different in transfused and non-transfused SCD patients, we categorized vascular dysfunction by conduit artery dysfunction (FMD < 6.3%), microcirculatory dysfunction (post-occlusive hyperemia <234%), combined dysfunction if both microcirculatory and conduit artery dysfunction were present, or normal vascular function if neither were present. The vascular dysfunction cutoffs were defined by 95% confidence interval derived from control subjects. Both groups had a significant number of patients with combined dysfunction (dark gray shaded area in Figure 2 panel E and panel F); however, the transfused group had a predominance of microcirculatory dysfunction, and the non-transfused group had a predominance of conduit artery dysfunction.
3.7 Predictors of peak microcirculatory blood flow, FMD and %rSO2
3.7.1 Microcirculatory flow
Resting microvascular flow by laser doppler flowmetry depended only on the presence or absence of sickle cell disease; it was independent of the hematologic, biochemical and rheologic parameters measured in this study. Peak, post ischemic microvascular flow was predominately associated with resting flow (Figure S4: R2 = 0.50, P < .0001); weak associations were also observed with platelet count (R2 = 0.04, P = .02) and SCD diagnosis (R2 = 0.02, P = .05 regardless of transfusion status) on univariate analysis. Only platelet count (ß = −0.1, P = .02) and higher resting flow (ß = 1.56, P < .0001) were independent predictors of higher peak flow during hyperemia.
3.7.2 FMD and %rSO2
Table S1 summarizes the univariate predictors of %rSO2 and FMD. Hemoglobin/hematocrit and RBC deformability were positively associated with both vascular responses while age, hemoglobin S, inflammation, and markers of hemolysis were negatively associated. Some markers of inflammation, hemolysis and red cell characteristics are interdependent and act as surrogates for SCD and transfusion status. So, we evaluated several multivariate models to determine independent predictors. There were two primary models for both FMD and %rSO2 (Table 2). For %rSO2: younger age (after log transformation), increased resting microcirculatory perfusion and lower plasma free hemoglobin (after natural log transformation) were highly predictive of improved %rSO2. For FMD: lower plasma free hemoglobin, younger age, and being female predicted higher FMD. If we added RBC deformability over a range of physiologic shear stress (0.3-50 Pascals) to either model, it was a strong independent predictor of both %rSO2 (deformability at shear stress >3 Pa) and FMD (deformability at shear stress >9 Pa), despite fewer subjects in each model (Table S2).
| Resting regional hemoglobin oxygen saturation (%rSO2) vs X Model (n = 105, R2 = 0.54, P < .0001) | Parameter Estimate | Std Error | 95% CI | P value |
|---|---|---|---|---|
| Log [age (years)] | −9.3 | 2.2 | −13.6 to −5.0 | <.0001 |
| Microcirculatory Resting Flow | 0.01 | 0.002 | 0.005 to 0.015 | <.0001 |
| Non-Transfused SCD | −7.3 | 1.3 | −10 to −4.6 | <.0001 |
| Ln[Plasma Hgb (g/dl)] | −4.1 | 1.2 | −6.5 to −1.6 | .0015 |
| Healthy subjects | 6.1 | 2.1 | 1.9 to 10.3 | .0047 |
| FMD (%) vs X Model (n = 113, R2 = 0.31, P < .0001) | Parameter estimate | Std error | 95% CI | P value |
|---|---|---|---|---|
| Sex (female) | 0.93 | 0.24 | 0.46 to 1.41 | .0002 |
| Age (years) | −0.07 | 0.02 | −0.11 to −0.02 | .001 |
| ln(plasma free hgb) | −0.97 | 0.32 | −1.60 to −0.33 | .003 |
| Hematocrit | 0.07 | 0.05 | −0.02 to 0.16 | .11 |
- Abbreviations: FMD, flow mediated dilation; hgb, hemoglobin.
- Note. Models including RBC deformability at various physiologic shear stress are included in the supplemental data.
4 DISCUSSION
We have previously demonstrated that while cardiac output and vital organ blood flow increase in patients with chronic anemia, the total oxygen delivery remains normal, consistent with Fick principles of oxygen transport.32-35 We postulated that regional oxygen delivery would be preserved as well in SCD patients, using laser Doppler flowmetry and %rSO2 as surrogate biomarkers. In this study, we observed increased laser Doppler perfusion index, indicating that microvascular flow increases in the fingertip were compensatory and, in some patients, out of proportion to the anemia severity. Despite this, %rSO2 in the hand was either normal (transfused subjects) or markedly decreased (non-transfused patients with an average 12% decrease in absolute hemoglobin saturation), suggesting either inefficient oxygen delivery or increased oxygen utilization. However, cuff occlusion studies did not suggest increased metabolic rate in SCD patients (Figure S1), therefore, inefficient oxygen supply-demand matching is the most likely explanation. This is consistent with a previous finding by Waltz and colleagues that demonstrated normal muscle oxygen utilization and fatigue in patients with sickle cell disease.36
The presence of surplus perfusion and inefficient oxygen delivery in the same limb is evidence of oxygen supply-demand mismatch, consistent with the review by Nath and colleagues.37 Balancing of oxygen delivery to meet changing metabolic requirements is a complicated process. It is modulated by local metabolites, blood rheology, red cell biochemistry, and the autonomic nervous system, all of which are deranged in sickle cell disease. Others have described resting hyperemia in the face of microvascular pruning;37-39 however, our simultaneous measures of conduit artery function, microvascular perfusion and %rSO2 is the most complete assessment in a single study. Figure 1 (lower panel), summarizes our working model of this process: 1) arterialization of the venous system/arterio-venous bypass channels, 2) decreased red blood cell deformability, 3) increased red blood cell adhesion and 4) chronic hemolysis causing decreased NO bioavailability.
Arteriolarization occurs when RBCs traverse the microcirculation via bypass channels that are larger in diameter than capillary vessels. In-vitro vascular preps, animal and human intravital microscopy studies have demonstrated both the presence of bypass channels and increased intermittent flow in capillaries, after exposure to SS RBC and in patients with SCD. Microvascular post-occlusive reactive hyperemia (PORH) visualized by intravital microscopy is decreased in patients with SCD. The mechanism appears to be a combination of increased cellular adhesion leading to pruning of capillary density, increased bypass channels and reactive arterial dilation. These all culminate in increased resting flow, but decreased vasodilatory capacity as measured by PORH, with which our results are consistent.11-13 As depicted in the lower portion of figure 1, the arterio-venous bypass channels would allow RBCs to flow through tissue without traversing the capillary vessel, where oxygen extraction in the tissues is optimized. These bypass channels have flow-limited oxygen diffusion creating functional arteriovenous shunts.40, 41 Arteriolarization results in higher oxy-hemoglobin saturation in the venous collecting system and more pulsatile flow into the post-capillary venules. This study confirms the work of others that have demonstrated arteriolarization of the capillary and post-capillary vasculature in the cerebral and peripheral circulation of patients with sickle cell disease.34, 35, 40-43 Arteriolarization can cause %rSO2 to increase or decrease depending on the relative blood volumes of the high flow and low flow beds as well as their spatial overlap. In the present study, we Do not know if increased oxygen delivery and low %rSO2 were present in the same capillary bed, because the near infrared and laser Doppler probes were in different locations. It is possible that arteriolarization was limited to the digits, causing a “steal” phenomenon that left the more proximal hand relatively underperfused. Regardless, this would still reflect a potentially dangerous derangement of oxygen supply demand matching in SCD patients.
Proper microvascular supply-demand matching is impacted by RBC deformability as demonstrated in Table S2. Thus, RBC deformability is dependent on shear stress and physiologic shear stress in the vasculature ranges from ~0.5 to 15 Pa, with some evidence that up to 30 Pa may exist.44-46 We found that deformability at shear stress >2.8 Pa predicts regional hemoglobin saturation and > 8.89 Pa predicts FMD. This is important when we consider average capillary diameter is 5-6 μm, and RBCs are approximately 8 μm. Therefore, RBCs must physically deform in order to traverse the capillaries, creating complex interactions between the vessel wall, plasma suspending medium and RBCs. It is the only place in the circulation where the RBC membrane contacts endothelial membrane.
Microcirculatory perfusion as measured by laser doppler did not change acutely with transfusion, nor was it associated with RBC deformability. We did not evaluate markers of adhesion, which are likely to predict microcirculatory flow. This is a gap in knowledge we aim to fill in the future. However, our finding is decreased red blood cell deformability independently predicts both %rSO2 and FMD. That may relate to less deformable RBCs tending to adhere and accumulate along the endothelial wall, while more deformable RBCs migrate inward toward the center of flow.47-49 This would lead to less deformable cells accumulating in certain microcirculatory tributaries, creating spatial separation between deformable and non-deformable cells. Second, stiff RBCs are more likely to adhere to vascular endothelial cells in the post-capillary venules, increasing resistance to flow and exacerbating spatial maldistribution of capillary flow.9 The less deformable discoid red cells appear to have the strongest adhesive properties, while irreversibly sickled red cells do not have strong adhesion properties, but they can become physically trapped in the microcirculation.9 Whether the occlusion is transient or produces permanent vascular pruning, other vascular pathways must accommodate higher flow by increasing transit velocity, increasing diameter or both. Increased transit velocity limits the time available for oxygen to diffuse, while increased vessel diameter limits the effective cross-sectional area for oxygen exchange.50
RBCs also directly contribute to supply-demand matching; deoxygenated RBCs release factors that promote local vasodilation (so-called hypoxic vasodilation). The mechanism of RBC dependent hypoxic vasodilation is controversial with proposed roles for circulating ATP/adenosine, nitrite and S-nitrosylated proteins.51-53 Hemoglobin acts as a sink that is able to store nitric oxide equivalents, whether it is nitrite or S-nitrosylated proteins, for use in remote areas of the vasculature and can convert nitrite to NO under low oxygen conditions.54 Regional nitric oxide release inhibits platelet activation and aggregation, and could impact resting and hyperemic blood flow.55 Some data implicate mechanical stress and RBC deformation as stimuli for local NO.56 Our observation that RBC deformability is a powerful predictor of %rSO2 is consistent with the findings of Charlot and colleagues57 but cannot distinguish among these possible mechanisms.
Lastly, hemolysis products also disrupt oxygen supply-demand matching. Plasma free hemoglobin is a strong independent predictor of impaired FMD and %rSO2, suggesting that hemolysis impacts both conduit artery function and %rSO2, but not microcirculatory hyperperfusion at rest or post occlusion. Some of the FMD data in this study was previously published, but accrual of more patients strengthened the findings of that previous publication. Our in vivo, human vascular study protocol cannot demonstrate the mechanism of free hemoglobin scavenging of NO; however, the association of free hemoglobin with decreased FMD, in the setting of numerous in vitro, in-animal and in-human vascular models is strong evidence for NO dysregulation. Plasma free hemoglobin has been used in clinical trials as volume support in trauma patients. However, it had multiple side effects, including raising mean arterial blood pressure, attributed to diffuse NO scavenging causing vasoconstriction.58 Free hemoglobin decreases nitric oxide bioavailability through scavenging in the lumen and scavenging at the basement membrane.7, 59, 60 Lastly, FMD is a specific test of shear mediated endothelial nitric oxide production and release when placing the cuff distal to the artery measured.61 Thus, our finding of decreased FMD in patients with higher free hemoglobin points to impaired shear-mediated nitric oxide delivery to vascular smooth muscle. Decreased NO bioavailability could also potentially impair supply demand matching, by influencing vascular tone of pre-capillary arteriole sphincters.
Our data suggests that being female is protective, which is consistent with prior data examining sex specific changes in endothelial function and the nitric oxide metabolic pathways, particularly with regard to estrogen effects on endothelial nitric oxide synthase.19, 32, 37, 62 We can postulate that this is one mechanism for improved female survival in sickle cell disease, especially with regard to the adult population. Since our study was focused on sickle cell disease and its associated vasculopathy, the ethnic makeup of our control group was similar to our patient population, 80-90% African-American and 10-20% Hispanic. It is known that nitric oxide dependent vascular function measures in conduit arteries (FMD) and the microcirculation (resting perfusion and PORH) are decreased in African-American subjects without sickle cell disease63. Our data is consistent with that finding. Therefore, we do not believe differences in vascular function due to ethnicity affected our interpretation of the data.
Taken together, the vascular phenotype in SCD patients resembles conditions observed in sepsis or systemic inflammatory response syndrome (SIRS), although not acute or as severe. In these conditions, hypoxic multi-organ system failure occurs despite high organ blood flow.64 Microvascular dysfunction is central to this process leading to functional arteriovenous shunting, impaired oxygen extraction, and vital organ hypoxia.65 A study of trauma patients with sepsis demonstrated that impaired RBC deformability led to impaired oxygen utilization and a higher mixed venous oxygen saturation, which would be consistent with an increase in tissue shunting of blood flow.66 Recent work has even implicated free heme in this process, as it increases pro-inflammatory macrophages leading to vascular/cellular damage.67 Based on these observations, a mechanistic link involving plasma free hemoglobin and free heme is being drawn in sickle cell disease. Free heme induced vascular inflammation due to pro-inflammatory macrophage phenotypic change is ameliorated by hemopexin in a sickle mouse model.68 Although SCD patients are not in distributive shock under normal conditions, they do exhibit high inflammatory tone, mild activation of coagulation pathways, increased sympathetic nervous system activity and increased circulating free hemoglobin and heme levels that mimic changes found in sepsis and SIRS. This demonstrates an important parallel in pathophysiology.
The clinical impact of these and previous findings cannot be understated. There is an acute on chronic vascular disease phenomenon occurring in patients with SCD, and the chronic vasculopathy is becoming more apparent as survival improves. All levels of the vasculature exhibit functional changes in patients with sickle cell disease, including conduit arteries, arterial microcirculation, venous microcirculation and the capillary bed16-19, 26, 40, 57, 69-71 leading to multiple organ dysfunction. Small vessel damage causes silent strokes in the brain,72 pulmonary hypertension in the lungs,73 and focal glomerular sclerosis in the kidney.74 Recently, a cardiac phenotype of diffuse myocardial fibrosis and heart failure with preserved ejection fraction has been identified and linked to microangiopathy as well.6, 75-77 In normal subjects, vital organs tolerate intermittent interruptions in oxygen delivery by increasing oxygen extraction and blood flow as well as redirecting flow from non-vital tissues. Our data suggest that all three of these compensatory mechanisms are impaired in SCD subjects and suggest a role for cell free hemoglobin and red cell deformability in their dysfunction.
Our study has several limitations.Seminal work by multiple SCD research groups have established the importance of vascular adhesion in microvascular dysfunction and we did not explore markers of cellular adhesion, which may play a major role in the LDF parameters that did not associate with free hemoglobin, FMD and %rSO2.11-13, 69 The predictive value of our study is limited by the cross-sectional study design and correlative nature of the findings; however, our large sample size and inclusion of chronically transfused subjects studied pre and post transfusion improves the variability in our sample and predictive value of the results. Furthermore, our multi-modality approach allows us to simultaneously evaluate changes in vascular function at multiple levels of the vascular system while assessing blood and plasma biomarkers. The lack of acute change in regional hemoglobin oxygen saturation after transfusion may be because the oxygen saturation was normal to begin with, the measurement timing did not capture an increase, or the sample size was simply too small (P-value was 0.11). Skin pigmentation may affect the NIRS measurement; however, given the ethnicity matching and occasional use of family members as control subjects, we assume that skin pigmentation would not affect our interpretation of the results. The mechanisms implied by our findings are consistent with previous work in animal and human models, however, these associations cannot prove causation, especially with regard to the pathophysiologic consequences of microcirculatory tissue oxygen supply-demand mismatch. Fortunately, these vascular testing procedures will allow us to directly test mechanisms non-invasively in a prospective fashion to evaluate their role in predicting outcomes, validate them as clinical biomarkers and test therapies.
5 CONCLUSION
Our study addresses the critical problem of translating in-vitro, in-animal and in-vivo microvascular studies into simultaneous vascular assessment of the macrovasculature, microvasculature and resultant microcirculatory oxygen exchange in patients with sickle cell disease. Despite increased resting microcirculatory blood flow that compensates for anemia, tissue oxygen supply-demand mismatch persists in non-transfused patients but is ameliorated by chronic transfusion therapy.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health, National Heart Lung and Blood Institute (NIH# 1 U54 HL090511-01)(T.C.):Sickle Cell Scholar Award; (5 RC1 HL099412-01)(J.D.), K12 scholar award (2 K12 HD 52954-6 A1)(J.D.), K23 mentored career development award (1K23HL119627-01A1)(J.D.) and (1R03HL138321-01)(J.D.); (1U01HL117718-01)(T.D.C., J.C.W., M.C.K. and H.J.M.); National Institute of Biomedical Imaging and Bioengineering (P41 EB001978) (M.C.K.), and by the Children's Hospital Los Angeles General Clinical Research Center (NIH #RR00043-43)(J.D.)
CONFLICT OF INTEREST
The authors have no relevant disclosures pertaining to this research.
AUTHOR CONTRIBUTIONS
J.A.D.: designed/performed the research, collected and analyzed data, performed statistical analysis and wrote the paper. R.M.K., A.D.B., P.S., M.K., and P.C.: performed the research, collected and analyzed data.
M.D.Z. and D.P.: collected and analyzed data.
H.J.M. and M.C.K.: designed/performed the research, collected and analyzed data, and wrote the paper.
T.D.C.: designed the research, analyzed data, and wrote the paper.
J.C.W.: designed the research, analyzed data, performed statistical analysis and wrote the paper.
All authors reviewed and revised the manuscript.




