Frontline antibiotic therapy for early-stage Helicobacter pylori-negative gastric MALT lymphoma
To the Editor:
At least two-thirds of gastric mucosa-associated lymphoid tissue (MALT) lymphomas are associated with a chronic Helicobacter pylori (HP) infection, and HP eradication with triple antibiotic therapy is an effective treatment for patients with early-stage disease. Currently, a definitive involved site radiation, at a dose of 24-30 Gy, is the preferred frontline treatment for patients with HP-negative localized disease. Monotherapy with rituximab or chemoimmunotherapy are an alternative for patients in whom radiotherapy is contraindicated, but they are relatively expensive and can be less effective.1
Successful treatment of HP-negative early stage MALT lymphoma with HP-directed antibiotic therapy has been reported in several small cases series.2 However, larger studies are needed to confirm whether frontline antibiotics may be an effective treatment for patients with early-stage HP-negative gastric MALT lymphoma and, most importantly, whether its initial use may hamper the efficacy of subsequent treatments.
We identified 26 patients with stage IAE HP-negative gastric MALT lymphoma, who received frontline triple antibiotic therapy, consisting of 14 days of amoxicillin or metronidazole, clarithromycin, and a proton-pump inhibitor, at our institution between 01/1991 and 12/2011. A group of 38 HP-positive patients, treated with frontline antibiotic therapy during the same time frame, matched by age, sex, race, and stage, was used as control. Stage was defined according to the Lugano staging system, and included esophagogastroduodenoscopy (EGD) with multiple biopsies and endoscopic ultrasound. HP status was determined by tissue immunohistochemistry (IHC) and serum antibody assay. Response to therapy was assessed by repeated EGD and biopsies at least 2 months after treatment, and complete remission (CR) was defined according to guidelines.3 No repeated imaging was required to confirm CR. Endoscopic surveillance was performed every 12-18 months.
Association with categorical variables was evaluated using χ2 or Fisher exact tests. Progression-free survival (PFS) and overall survival (OS) were defined as the time from the start of therapy to progression of disease, and/or death, or last follow-up, respectively. PFS and OS were calculated using Kaplan-Meier estimates and compared using the log-rank test. A P value of ≤0.05 (two tailed) was considered statistically significant (SPSS v23).
Baseline characteristics are shown in Supporting Information S1. After frontline antibiotic therapy, 6 (23%) HP-negative patients and 18 (47%) HP-positive patients achieved complete response (CR1) (P = 0.07), after a median of 2 months (range, 1-6 months) and 7 months (range, 1-13)(P = 0.009), respectively (Supporting Information S1).
Among 16 HP-negative patients with residual disease after frontline antibiotic therapy, 10 received radiotherapy and 6 were placed on surveillance; among 18 HP-positive patients with residual disease after frontline therapy, 5 received radiotherapy and 13 were observed. All 15 patients treated with consolidative radiotherapy achieved CR (CR2). Overall, combining CR1 and CR2, 16 (62%) HP-negative patients and 23 HP-positive patients (61%) achieved CR (P = 1) (Supporting Information S1). All 19 patients not receiving consolidative radiotherapy progressed during surveillance, after a median of 13 months (range, 1-36 months). Among the 10 HP-negative patients who had residual disease and were surveilled, 9 had a local relapse and 1 had an extra-nodal relapse without transformation; a second course of antibiotic therapy was attempted in 3 patients, and 1 achieved CR. Among the 15 HP-positive patients who had residual disease and were surveilled, 13 had a local relapse, and 2 had an extra-nodal relapse without transformation; a second course of antibiotic therapy was attempted in 7 patients, and 3 achieved CR.
After a median follow-up of 11 years (95% confidence interval [CI], 9-13 years), 13 (50%) HP-negative patients and 21 (55%) HP-positive patients relapsed and/or progressed, and median PFS was 38 months in both groups (P = 0.78). Among 24 patients who achieved CR1, 6 relapsed: of these 6, 2 were HP-negative and 4 were HP-positive (Figure 1A). Median PFS was not reached for patients achieving CR1 or CR2, whereas it was 6 months for patients not achieving CR (Figure 1B). Among HP-negative patients, salvage therapy included: radiotherapy in 4 patients, antibiotics in 3, chemoradiotherapy in 2, rituximab in 2, chemotherapy alone in 1, and radioimmunotherapy in 1 patient. Among HP-positive patients, salvage therapy included: antibiotics in 7 patients, chemotherapy alone in 5, radiotherapy in 4, chemo-radiotherapy in 1, rituximab in 1, radioimmunotherapy in 1, and gastrectomy in 1 patient. Response to salvage therapy was 69% among HP-negative patients and 60% among HP-positive patients (P = 1) (Supporting Information S1). Among patients who received repeated antibiotic therapy, 1 out of 3 HP-negative patients and 4 out of 7 HP-positive patients achieved CR. Of interest, IHC for HP infection was repeated in all 10 cases, and was unchanged as compared to baseline.
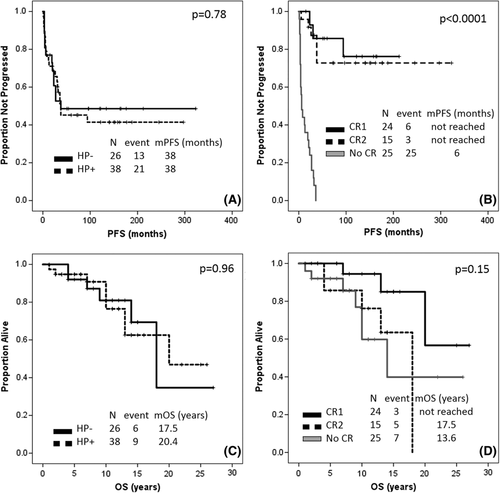
At the most recent follow-up, 6 (33%) HP-negative and 9 (34%) HP-positive patients have died. Median OS was 17.5 and 20.4 years, respectively (P = 0.96) (Figure 1C). The median OS was not reached among patients achieving CR1, was 17.5 years among patients achieving CR2, and was 13.6 years among patients not achieving CR (P = 0.14) (Figure 1D). Second cancers (excluding diffuse large B-cell lymphoma (DLBCL)) were diagnosed in 17 (27%) patients, the most common being non-small cell lung cancer (6 patients). No differences in the incidence of second cancers were observed when comparing patients treated with antibiotic therapy only to patients receiving also other treatment modalities (34% vs. 17%, P = 0.16), or based on radiotherapy exposure (32% vs. 23%, P = 0.56).
Our results show that almost a quarter of patients with early-stage HP-negative gastric MALT lymphoma achieve a CR with antibiotic therapy, encouraging its use as frontline treatment. Hematoxylin and eosin staining has a sensitivity/specificity for HP detection on gastric biopsy of 95%/100%, with false-negative cases being highly unlikely with the addition of IHC and serum antibody assay. However, cases of gastric MALT lymphoma associated with non-HP strains, such as Helicobacter heilmannii, have been reported. Based on encouraging in vivo and in vitro evidence of the antitumoral activity of clarithromycin, this agent has been tested in a randomized trial in association with chemotherapy in patients with newly diagnosed indolent lymphomas, and at high dose in patients with relapsed refractory extranodal MALT lymphoma.4 Independent from the evidence of a concomitant infection, clarithromycin has resulted in clinical benefit, likely as a consequence of direct antitumoral effects or anti-angiogenetic activity. The latter may be responsible for the remissions observed in our study in HP-negative patients.
The CR rate in HP-negative patients with frontline antibiotic therapy in our study is lower than that reported with radiotherapy or rituximab. It must be noted that time to CR with antibiotic therapy can be up to 12 months, and a higher CR rate could have been observed with a more watchful approach. In addition, t(11;18), associated with impaired response to antibiotics, was assessed in only three patients (all negative), as the majority of patients were treated more than 10 years ago. Finally, depth of invasion at baseline, an important predictive factor of response to therapy, was not available for the majority of HP-negative patients. It is reassuring to note that the rate of transformation observed at the time of first response assessment was low, and comparable between the two groups.
Of interest, all patients with residual disease after frontline antibiotic therapy and not treated with consolidative radiotherapy progressed. In this regard, it is important to notice that a large number of international series support instead the use of surveillance in these patients, in light of a relapse rate of 6%-7% and a yearly recurrence rate of 2%.
In our study, PFS and OS were not significantly different between HP-negative patients achieving CR after antibiotic therapy or after radiotherapy, showing that a trial of antibiotic therapy as first-line treatment may spare radiotherapy and/or rituximab in about a quarter of HP-negative patients, without affecting their long-term survival. Recently, low-dose radiotherapy (4 Gy) has been successfully used in patients with extra-nodal MALT lymphoma.5 The latter could be considered, in addition to antibiotic therapy, in an effort to decrease the risk of long-term side effects in these patients.
A higher incidence of gastric cancers and other lymphomas has been reported in the past, likely as a consequence of chronic local antigen stimulation or local therapies.6 However, in our series, non-small cell lung cancer was the most common histology, reflecting aging and environmental exposure rather than a treatment-related complication. Since second cancers represented the most common cause of death, age-appropriate cancer screening is advised in these patients.
Prospective studies aimed at confirming the efficacy of frontline antibiotic therapy in patients with HP-negative gastric MALT lymphoma and clarifying its mechanism of action in HP-negative patients are warranted.
ACKNOWLEDGMENT
This research is supported in part by the MD Anderson Cancer Center Support Grant P30 CA016672.
CONFLICT OF INTERESTS
SSN reports honoraria and research support from Kite, a Gilead Company, Merck, Celgene; research support from Bristol-Myers Sqibb, Poseida, Cellectis, Karus, and Acerta Pharma; and honoraria from Novartis, Pfizer, and Unum Therapeutics.
AUTHOR CONTRIBUTIONS
PS and STL designed the study, analyzed data, and wrote the paper; PT, AK, SA, and RED collected and analyzed the data; FBH, JR, LEF, MAR, FS, NF, JW, MW, HL, CP, JRG, and BD provided clinical care to patients; LF provided statistical support; and SN designed the study, analyzed the data, provided clinical care to patients, and wrote the paper. All authors reviewed and approved the paper.




