A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD)
Funding information: This project was funded by the National Heart, Lung and Blood Institute, Grant/Award Number: R34 RHL121224A
Abstract
Limited evidence guides opioid dosing strategies for acute Sickle Cell (SCD) pain. We compared two National Heart, Lung and Blood (NHBLI) recommended opioid dosing strategies (weight-based vs. patient-specific) for ED treatment of acute vaso-occlusive episodes (VOE).
A prospective randomized controlled trial (RCT) was conducted in two ED's. Adults ≥ 21 years of age with SCD disease were eligible. Among the 155 eligible patients, 106 consented and 52 had eligible visits. Patients were pre-enrolled in the outpatient setting and randomized to one of two opioid dosing strategies for a future ED visit. ED providers accessed protocols through the electronic medical record. Change in pain score (0-100 mm VAS) from arrival to ED disposition, as well as side effects were assessed. 52 patients (median age was 27 years, 42% were female, and 89% black) had one or more ED visits for a VOE (total of 126 ED study visits, up to 5 visits/patient were included). Participants randomized to the patient-specific protocol experienced a mean reduction in pain score that was 16.6 points greater than patients randomized to the weight-based group (mean difference 95% CI = 11.3 to 21.9, P = 0.03). Naloxone was not required for either protocol and nausea and/or vomiting was observed less often in the patient-specific protocol (25.8% vs 59.4%, P = 0.0001). The hospital admission rate for VOE was lower for patients in the patient-specific protocol (40.3% vs 57.8% P = 0.05). NHLBI guideline-based analgesia with patient-specific opioid dosing resulted in greater improvements in the pain experience compared to a weight-based strategy, without increased side effects.
1 INTRODUCTION
SCD is the most common genetic disorder in the United States and occurs primarily among African Americans. The cardinal clinical manifestation of SCD and most common reason for emergency department (ED) visits is the painful, vaso-occlusive episode (VOE).1-3 Treatment in the (ED) with high doses of opioids is often required to manage these painful events.1 There is no standard approach to managing VOE pain in the ED that is widely accepted and used.4
In 2014, the National Heart, Lung, and Blood Institute released evidence based recommendations for the “Management of Sickle Cell Disease” including treatment of VOE.5 Based largely on expert consensus, these guidelines recommend two opioid-dosing approaches for the management of VOE; a patient-specific protocol (agents and doses tailored to an individual patient) or, if patient-specific recommendations are not available, a standard protocol for the specific treatment of VOE. Patient-specific protocols are logistically burdensome to implement and maintain; moreover, there are no extant studies that demonstrate their superiority over standard weight-based VOE protocols.
To address this knowledge gap, we conducted a randomized controlled trial (RCT) in two EDs to: (1) compare changes in pain scores from arrival to discharge between patients with VOE randomized to a patient-specific or standard (weight-based) analgesic protocol and (2) explore protocol differences in secondary outcomes (pain experience, hospital utilization, side effect, and safety) between protocols. We hypothesized that patients treated with a patient-specific protocol would achieve a greater reduction in pain scores from arrival to discharge when compared with those treated with a standard weight-based protocol.
2 METHODS
2.1 Design
A two-site, Phase III, open-label, prospective randomized controlled trial (RCT) was conducted. The Institutional Review board at both study sites and Duke University approved the project and subjects provided informed written consent. The trial was registered in ClinTrials.gov (NCT02222246).
2.2 Setting
Emergency department (ED) study sites (Midwest and Northeast) were urban academic medical centers with emergency medicine residency programs. The hematologist or SCD expert at each site wrote analgesic protocols using the criteria developed for the trial to determine an individual dose, or the weight-based dose. Both protocols were based upon the NHLBI recommendations for the treatment of VOE.
2.3 Population and recruitment
Adults 21 years of age or older with SCD [hemoglobin SS, SC, SB+, SB-, verified by electronic medical record (EMR) review] were eligible. Participants were excluded if they had sickle cell trait, were allergic to both morphine sulfate and hydromorphone, had an explicit care plan stating no admittance to the hospital for only pain control, were non-English speaking, had greater than 24 ED visits in the prior 12 months, or presented with acute organ dysfunction that could affect their opioid tolerance. Study staff enrolled patients either at the end of an ED visit, during an in-patient hospitalization, or during a SCD clinic visit for participation in a future ED visit for treatment of VOE. To avoid potential bias from higher utilizing participants, a maximum of five ED visits/patient were eligible. This recruitment protocol was used to avoid previous difficulty in obtaining sample size targets experienced with other clinical trials for VOE.6, 7 For each ED study visit, patients were compensated $10-$20, depending upon the site.
2.4 Randomization
Participants were randomized, by research staff after consent, to patient-specific or weight-based protocols during future ED visits for VOE for the study duration without crossover. Allocation was 1:1 with permuted block sizes of four, stratified by site and conducted by the statistician. The study was un-blinded to study staff and patients; however, patients were consented prior to randomization.
2.5 Analgesic protocols
eAppendix 1 summarizes the differences between the two analgesic protocols. Briefly, analgesic agent choice (morphine sulfate or hydromorphone), routes (intravenous or sub-cutaneous), and re-dosing intervals (20–30 minutes) were identical and based upon the NHLBI recommendations. The primary difference between protocols was the initial opioid dose. Patients randomized to the patient-specific analgesic protocol received an opioid dose based upon their current chronic opioid therapy, when applicable, and previously known effective VOE management as determined by their SCD provider. The total maximum dose of short and long acting opioids at home was determined. The maximum home opioid dose was defined as the maximum dose of prescribed opioids taken by the patient at home in any 24-hour period. The maximum dose was calculated by combining all long acting and short acting opioids taken within a 24-hour period and converting that medication to IV morphine equivalents. The SCD team member used the maximum home opioid dose to generate a suggested starting dose of IV opioid for severe sickle cell pain in the ED as follows: preliminary calculation of IV dose for next ED visit (based on home opioid consumption) = 20% of patient's maximum home opioid dose converted to IV morphine or IV hydromorphone. This dose was then compared to doses administered in recent ED visits. Discrepancies of more than 10% were reviewed by the research team and a final dose was determined, erring on the low dose for safety. The NHLBI recommendations for a “standard” SCD VOE protocol were not specific; therefore, in the standard arm, the study team selected a starting opioid dose based only on weight (hydromorphone 0.02 mg/kg, morphine sulfate 0.1 mg/kg). Research staff assessed patients for pain, sedation and vital signs every 30 minutes. If either protocol was ineffective, clinicians had discretion to administer additional doses as needed. There were several doses of fentanyl, oxycodone and ketorolac prescribed by ED physicians for a small number of visits. These were included in the analysis.
2.6 Procedures
Each subject's analgesic protocol (both study arms) was uploaded to the EMR. A decision support alert notified ED physicians that the patient was enrolled in the trial and the research opioid protocol “popped up” when attempting to order analgesics for a study patient.
Socio-demographic and clinical data were collected by research staff at enrollment. During ED visits, patient interviews were conducted every 30 minutes starting from patient placement in a treatment room until either: (1) a decision was made to admit the patient or transfer to an observation unit, (2) the patient was discharged home, or (3) six hours elapsed, whichever occurred first. Medical records were reviewed at 30 days to determine ED recidivism and hospital admissions within 72 hours, 7 and 30 days. Data collection was halted when we achieved 126 ED visits vs. the target 160 ED visits; this was approved by the DSMB. We planned to enroll 77 different patients, and we exceeded planned patient enrollment (n = 106 patients). The trial was halted because the DSMB stated we had enough data to conduct analysis.
2.7 Study measurements
The primary outcome was change in pain score from arrival to discharge as defined above. Pain severity was assessed using a 100 mm visual analogue scale (VAS).7 A 13 mm reduction per person was considered a clinically significant improvement.7, 8
Secondary outcomes included measures of the pain experience, hospital utilization, side effects and safety (Table 1). Hospital utilization was assessed by determining the total number of return ED visits within 72 hours, 7 and 30 days, and the total number of hospital admissions within 7 and 30 days after each ED visit. More specifically, hospital admission was defined as an admission to the hospital associated with the ED study visit. Hospital readmission was defined as an admission that occurred after discharge from a hospital admission associated with an ED visit. Side effect and safety outcomes included baseline and every 30-minute blood pressure (BP), heart rate (HR), saturation of peripheral oxygen (SpO2), respiration rate (RR), sedation score (none, mild, moderate, and severe) and self-reported nausea or vomiting. Protocol adherence was evaluated by comparing analgesics prescribed and administered (drug name, route, dose, and time) for the first dose, and a total intravenous morphine sulfate equivalent (IVMSE) value (mg) administered during the study period was determined.
| Outcome |
Standard weight-based protocol (N = 64 ED visits) |
Patient-specific analgesic protocol (N = 62 ED visits) |
P-value |
|---|---|---|---|
| Primary outcome | |||
| Primary analysis | |||
| Change in pain (arrival to discharge, adjusted scores) | 26.4 ± 10.6 | 43.0 ± 18.6 | .03 |
| Change in pain (arrival to discharge, unadjusted scores) | 26.6 ± 23.4 | 42.4 ± 30.7 | — |
| Supplemental analyses | |||
| At least 13 mm change in pain (arrival to discharge) | 47 (73.4%) | 52 (83.9%) | .13 |
| Secondary outcomes | |||
| Pain experience | |||
| Time to discharge (placement to discharge, in minutes) | 291.1 ± 5.9 | 269.8 ± 10.1 | .14 |
| Patient's self-identified goal for pain score met | 16 (25.0%) | 22 (35.5%) | .21 |
| Patient's report need for more pain medicine | 43 (67.2%) | 34 (55.8%) | .12 |
| Patient's report of pain change as “a lot better” | 13 (20.6%) | 24 (38.7%) | .03 |
| Patient's satisfaction with pain medicine | .17 | ||
| Not at all | 19 (29.7%) | 10 (16.1%) | |
| Somewhat satisfied (happy) | 30 (46.9%) | 32 (51.6%) | |
| Very satisfied | 15 (23.4%) | 20 (32.3%) | |
| Patient's satisfaction with ED pain management at discharge | .13 | ||
| Very/somewhat dissatisfied | 19 (33.3%) | 14 (23.0%) | |
| Somewhat/very satisfied | 38 (66.7%) | 47 (77.0%) | |
| Hospitalizations | |||
| Hospital admission for VOE pain control* | 37 (57.8%) | 25 (40.3%) | .05 |
| Hospital re-admission for VOE in 72 hours** | 6 (9.5%) | 8 (12.9%) | .55 |
| Hospital re-admission for VOE in 7 days** | 9 (14.3%) | 10 (16.1%) | .77 |
| Hospital re-admission for VOE in 30 days** | 19 (30.2%) | 19 (30.6%) | .95 |
| ED re-visit for VOE in 72 hours | 4 (6.4%) | 9 (14.5%) | .13 |
| ED re-visit for VOE in 7 days | 10 (15.9%) | 17 (27.4%) | .12 |
| ED re-visit for VOE in 30 days | 25 (39.7%) | 31 (50.0%) | .25 |
| Outcome |
Standard weight-based protocol (N = 64 ED visits) |
Patient-specific analgesic protocol (N = 62 ED visits) |
P-value |
|---|---|---|---|
| SECONDARY OUTCOMES | |||
| SIDE EFFECTS AND SAFETY | |||
| Nausea and/or vomiting | 38 (59.4%) | 16 (25.8%) | <.001 |
| Administration of naloxone | 0 (0%) | 0 (0%) | — |
| Respiratory distress | 0 (0%) | 0 (0%) | — |
| Intubation or other assistive ventilation techniques - including bag, valve, or mask | 0 (0%) | 0 (0%) | — |
| Supplemental use of oxygen via nasal cannula after opioid therapy initiation | 15 (23.4%) | 7 (11.3%) | .07 |
| Severe sedation | 6 (9.4%) | 4 (6.5%) | .74 |
| No sedation | 32 (50.0%) | 29 (46.8%) | |
| Mild sedation | 19 (29.7%) | 24 (38.7%) | |
| Moderate | 7 (10.9%) | 5 (8.1%) | |
| Saturation of peripheral capillary oxygen (SpO2) | |||
| SpO2 < 95% | 37 (57.8%) | 30 (48.4%) | .29 |
| SpO2 lowest value, median (25th, 75th) | 94.0 (91, 97) | 95 (93, 96) | .52 |
| Respiration rate (RR) | |||
| RR < 10 | 2 (3.1)% | 4 (6.5%) | .38 |
| RR lowest value, median (25th, 75th) | 14.5 (12.0, 17.0) | 15.0 (12.0, 17.0) | .77 |
| RR highest value, median (25th, 75th) | 22.0 (18.5, 24.0) | 22.0 (20.0, 24.0) | .44 |
| RR highest-lowest difference, median (25th, 75th) | 7.5 (5.0, 10.5) | 8.0 (6.0, 12.0) | .38 |
| Heart Rate (HR) | |||
| HR lowest value, median (25th, 75th) | 73.5 (61.0, 84.0) | 75.0 (70.0, 83.0) | .14 |
| HR highest value, median (25th, 75th) | 95.5 (82.5, 102.0) | 94.5 (88.0, 104.0) | .45 |
| Systolic Blood Pressure (SBP) | |||
| SBP lowest value, median (25th, 75th) | 105.0 (98.0, 114.0) | 110.0 (99.0, 117.0) | .21 |
| SBP highest value, median (25th, 75th) | 127.0 (120.0, 139.0) | 128.0 (122.0, 138.0) | .83 |
| Diastolic Blood Pressure (DBP) | |||
| DBP lowest value, median (25th, 75th) | 55.0 (50.0, 61.0) | 59.0 (59.0, 69.0) | .01 |
| DBP highest value, median (25th, 75th) | 77.0 (69.0, 88.0) | 80.0 (72.0, 89.0) | .56 |
- Analyses of pain change and pain experience were conducted using hierarchical mixed-effects models, adjusting for nested patient and site effects (N = 126); Pain change for each protocol: Adjusted mean ± SD derived from the mixed-effects model (N = 126) and unadjusted mean + SD prior to imputation of 11 missing change scores (N = 115, patient-specific = 4; standard = 7 missing) provided, with higher positive pain change scores indicating greater pain reduction; Binary secondary outcomes related to hospitalizations, side-effects, and safety: n (%), chi-square test or Fisher's Exact Test when observed or expected cell counts are below 5; Continuous vital signs: median, 25th,75 percentile, Wilcoxon Two-Sample Test due to skewness; Protocol difference in sedation (severe vs nonsevere) tested.
- * Hospital admission associated with the ED study visit.
- **Hospital re-admission occurred after discharge from a hospital admission associated with an ED visit.
2.8 Statistical analysis
Nondirectional statistical tests were performed with significance set at 0.05 for each two-sided test. The significance level was not adjusted for multiple outcomes.
The primary analysis applying an intention-to-treat principle was conducted using a hierarchical linear mixed-effects model (HLM) to test for protocol differences in change in pain from arrival to discharge, adjusting for nested patient and site effects. Initial models included site, protocol assignment and site-by-protocol assignment interaction terms. Nonsignificant interaction terms were removed from the final parsimonious model. Supplemental analyses, adjusting for patient and site effects, were conducted to test for protocol differences in measures related to pain change and experience. A Generalized Linear Mixed-Effect Models (GLMM) was used to test for protocol differences in the proportion of visits in which the patient experienced a ≥13 mm change in pain from arrival to discharge. Protocol differences in secondary pain outcomes were also examined using HLMs and GLMMs. Protocol and site differences in hospitalizations, ED re-visits, side-effects, and safety outcomes were evaluated using chi-square/Fisher's Exact Tests or Wilcoxon Two-Sample Tests, and were not adjusted for nested effects.
Each analgesic dose received during the ED visit was converted to the IVMSE and the IVMSE total analgesic dose/visit was calculated.9, 10 A HLM, nesting for patient and site effects, was conducted to test for protocol and site differences in the total analgesic dose. Differences in dose administered relative to dose prescribed in the protocol were calculated. Chi-square/Fisher's Exact and Wilcoxon Two-Sample Tests were used to compare protocol differences per patient and provider adherence to the prescribed protocol per visit.
2.9 Statistical power
For the primary outcome, a sample size of 126 visits (63/arm) provided at least 80% power to detect a 13 mm or greater change in pain score from arrival to discharge between the two protocols using a HLM with a two-sided alpha of 0.05.
3 RESULTS
3.1 Patient enrollment and ED study visits
From April 2014 to May 2015, 106 patients were enrolled and randomized (Figure 1). A total of 49 patients refused participation for the following reasons: (19 not interested, 11 stated they lived far and would not have an ED visit, 10 did not have time to consent, 5 were concerned about not getting enough medication in the protocol assigned, 2 were re-establishing care and wanted more time to think about participating, 1 practiced homeopathic treatments for pain and the final individual did not want to answer questions by research staff during a VOE). Among 106 enrolled, 52 patients had one or more subsequent ED visits for a VOE. The 52 patients had a total of 126 ED study visits over the 13 months (standard: N = 64 visits; patient-specific: N = 62 visits).
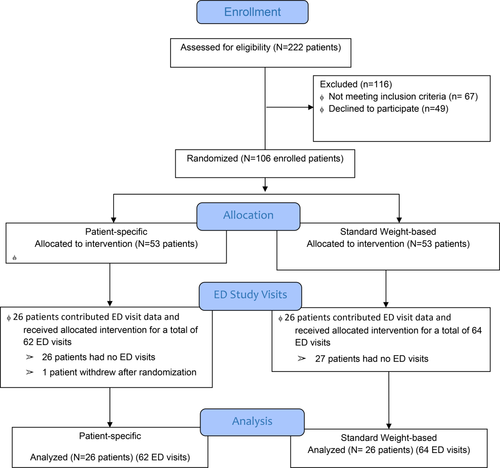
Study sample and randomization [Color figure can be viewed at wileyonlinelibrary.com]
3.2 Patient characteristics
Table 2 presents the characteristics of the 52 patients with visits. With the exception of secondary education, there were no significant differences in patient characteristics between groups. Sites did not differ on patient characteristics, except for race/ethnicity. Site 2 had a significantly higher percent of non-black (P = .01) and Hispanic/Latino (P = .004) patients than site 1. The median visits/patient was 2, with 18 (35%) having one visit, 12 (23%) two visits, 12 (23%) three visits, 2 (4%) four visits, 8 (15%) five visits, respectively. The number of visits/patient did not differ between protocols or sites. For each ED visit, mean pain scores at arrival did not differ significantly between the patient-specific (M = 86.1, SD = 18.3) and standard protocols (M = 82.6, SD = 16.3; P = .28).
| Characteristic | Sample (N) |
Enrolled patients with study visits (N = 52) |
Standard weight-based protocol (Control, N = 26) |
Patient-specific protocol (Individualized, N = 26) |
P-value |
|---|---|---|---|---|---|
| Age, median (25th, 75th) | 52 | 27.0 (23.0, 32.5) | 27.0 (23.0, 33.0) | 28.5 (23.0, 32.0) | .81 |
| Female, n (%) | 52 | 22 (42.3%) | 11 (42.3%) | 11 (42.3%) | 1.00 |
| AA/Black, n (%) | 52 | 46 (88.5%) | 23 (88.5%) | 23 (88.5%) | 1.00 |
| Hispanic/Latino, n (%) | 49 | 7 (14.3%) | 2 (8.7%) | 5 (19.3%) | .42 |
| Secondary education, n (%) | 51 | 27 (52.9%) | 17 (68.0%) | 10 (38.5%) | .03 |
| Full-time employment, n (%) | 52 | 14 (26.9%) | 6 (23.1%) | 8 (30.8%) | .53 |
| Insurance, n (%) | 51 | 48 (94.1%3.1) | 23 (88.5%) | 25 (100.0%) | .23 |
| Sickle cell genotype, n (%) | 52 | 1.00 | |||
| Hemoglobin SS | 37 (71.2%) | 19 (73.1%) | 18 (69.2%) | ||
| Hemoglobin SC | 12 (23.1%) | 6(23.1%) | 6 (23.1%) | ||
| Hemoglobin SB+ | 3. (5.8%) | 1 (3.9%) | 2 (7.7%) | ||
| ED visits, median (25th, 75th) | 52 | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | .93 |
| ED Sites | Enrolled Patients (Study Visits) | Standard Weight-based Protocol | Patient-specific Protocol | ||
| Clinical Site 1 |
25 patients (67 visits) |
12 patients (30 visits) |
13 patients (37 Visits) |
||
| Clinical Site 2 |
27 patients (59 visits) |
14 patients (32 Visits) |
13 patients (27 Visits) |
- Continuous measures: P-value for Wilcoxon Two-Sample Test due to skewness and sample sizes < 30; Categorical measures: P-value for chi-square test or Fisher's Exact Test when the observed or expected cell count was less than 5. Education ranged from “less than high school” (n = 2, 3.9%) to “Bachelor's degree” (n = 3, 5.9%). Secondary education = Post-high school education (Some college, Associate's degree, Bachelor's degree).
3.3 Change in pain scores (Table 1)
The adjusted mean reduction in pain scores from arrival to discharge adjusted for nested patient and site effects was 16.6 mm greater in the patient-specific cohort relative to the standard protocol (95% CI for mean difference = 11.3 to 21.9, P = .03). The adjusted mean pain scores at discharge were 14.8 mm lower in the patient-specific (M = 40.9, SD = 25.4) than in standard protocol (M = 55.7, SD = 21.9; 95% CI for mean difference = 6.5 to 23.2, P = .007). The percent of patients with a drop of 13 mm or higher was similar between the protocols with respect to our willingness to accept the difference is due to chance (83.9% vs 73.4%, OR = 1.9, 95% CI = 0.8 to 4.5, P = .13). Figure 2 presents pain scores per protocol over time. Scores are presented from the first ED interview upon arrival and q 30 minute pain scores for 2 hours, and the final discharge pain score. After 2 hours, patients began to complete the protocol and met discharge criteria.
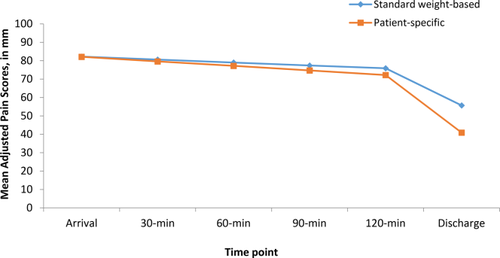
Trajectory of change in pain scores during ED visits [Color figure can be viewed at wileyonlinelibrary.com]
3.4 Pain experience
The two protocols and sites did not differ in time to disposition after placement in the treatment room (Table 1). Protocols did not differ on the pain experience outcomes, with the exception that the patient-specific arm had a higher percent of visits in which the patient reported the pain change as “a lot better” relative to the standard protocol (38.7% vs 20.6%, P = .03).
3.5 Hospitalizations, ED re-visits, side-effects, and safety
The hospital admission rate for VOE visits was lower in the patient-specific group compared to the weight-based protocol (40.3% vs 57.8% P = .05, Table 1). Nausea and/or vomiting was observed less often in the patient-specific protocol than in the standard (25.8% vs 59.4%, P < .001). The proportion of patients receiving supplemental use of oxygen via nasal cannula was lower for the patient-specific relative to the standard protocol, but not statistically significant (11.3% vs 23.4%, P = .07). The median lowest diastolic blood pressure was 0.04 mm/hg higher in the patient-specific protocol (median = 59) than the standard protocol (median = 55, P = .01).
3.6 Total ED analgesic dose (Table 3)
The total dose calculation included doses of morphine sulfate (MS) hydromorphone, fentanyl, oxycodone and ketorolac prescribed. The adjusted mean total dose per visit (IVMSE) was 59.1 mg (SD = 42.5; range = 5.0 to 265.3) for the patient-specific and 49.1 mg (SD = 18.3, range = 7.7 to 114.3) for the standard protocol.
Protocol assignment was not associated with a significant difference in total dose. A significant protocol-by-site interaction was observed (P = .02, Table 3), with the adjusted mean total dose for the patient-specific protocol 47.2 mg greater at site 2 compared to site 1 (P = .005). Site 1 patients received slightly higher doses in the weight based vs. patient-specific protocol. Site 2 patients received a higher dose in the patient specific vs. weight based protocol. Overall, patients in the patient specific protocol at both sites had a significantly greater decrease in the pain score. For the patient specific protocol only, patients at Site 2 received significantly total higher doses than patients at Site 1 (P < .001). The total range of doses administered at Site 1 was between 58–109 IVMSE, and at Site 2 all doses except five doses were between 52–109 IVME. Five patients received doses exceeding 109 IVMSE (110, 162, 253, 260, and 265 IVMSE). These three high patient doses skewed the mean. To address whether total analgesic dose influenced the primary outcome results (pain change), we conducted a supplemental analysis with total analgesic dose as a covariate in the final analytic model. The results indicated that total dose did not significantly influence pain change (P = .07), and did not diminish the protocol effects (P = .02)
| Analgesic protocol measures | Standard weight-based protocol | Patient-specific analgesic protocol | P-value |
|---|---|---|---|
| Actual total ed analgesic dose delivered in IVMSE (per visit) | N = 64 | N = 62 | |
| Protocol effect, mean ± SD | 49.1 ± 18.3 | 59.1 ± 42.5 | 0.55 |
| Protocol-by-site interaction | 0.02 | ||
| Site 1, mean ± SD | 49.9 ± 20.3 | 34.7 ± 13.0 | |
| Site 2, mean ± SD | 48.2 ± 15.6 | 81.9 ± 47.7 | |
| Dose 1 administered (per visit) | N = 64 | N = 62 | |
| Dose in IVMSE, median (25th, 75th) | 8.5 (6.7, 11.5) | 12 (8.0, 13.3) | 0.001 |
| Dose 2 administered (per visit) | N = 63 | N = 57 | |
| Dose in IVMSE, median (25th, 75th) | 10.5 (7.5, 13.3) | 13.3 (7.0, 14.7) | 0.37 |
| Dose 3 administered (per visit) | N = 55 | N = 52 | |
| Dose in IVMSE, median (25th, 75th) | 10.0 (7.3, 13.3) | 12.7 (6.7, 14.7) | 0.46 |
| Study assigned analgesic protocol (per patient) | N = 26 | N = 26 | |
| Medication | 0.08 | ||
| Morphine sulfate | 2 (7.7%) | 8 (30.7%) | |
| Hydromorphone | 24 (92.3%) | 18 (69.2%) | |
| Dose in IVMSE, median (25th, 75th) | 7.9 (6.0, 10.7) | 10.3 (6.7, 13.3) | 0.06 |
| Initial Dose unit in mg | 26 (100.0%) | 26 (100.0%) | 1.00 |
| Dose route | 1.00 | ||
| IV-P | 26 (100.0%) | 25 (96.2%) | |
| IM | 0 (0.0%) | 1 (3.8%) | |
| Dosing frequency | 0.7814 | ||
| q20 minutes | 13 (50.0%) | 14 (53.9%) | |
| q30 minutes | 13 (50.0%) | 12 (46.2%) | |
| Dose escalation in IVMSE, median (25th, 75th) | |||
| Standard protocol: Dose for 25% escalation (n=13) | 8.0 (7.3, 8.7) | NA | — |
| Patient-specific protocol: Dose for 1st Escalation (n=25) | NA | 13.3 (6.7, 13.3) | — |
| Patient-specific protocol: Dose for 2nd Escalation (n=13) | NA | 6.7 (2.5, 6.7) | — |
| Patient-specific protocol: Dose for 3rd Escalation (n=12) | NA | 3.3 (2.5, 6.7) | — |
| Adherence to analgesic protocol (per visit) | N = 64 | N = 62 | |
| Dose 1 adherance | |||
| Adherence: Medication | 61 (95.3%) | 55 (88.7%) | 0.20 |
| Adherence: Dose in IVMSE difference, median, 25th, 75th | 0.0 (–0.1, 0.7) | 0.0 (0.0, 0.0) | 0.53 |
| Adherence: Dose in IVMSE difference, minimum, maximum | −5.3, 20 | −9.3, 8.3 | – |
| Adherence: Dose unit in mg | 64 (100%) | 62 (100%) | 1.00 |
| Adherence: Dose route | 62 (96.9%) | 56 (90.3%) | 0.16 |
| Adherence: Time between dose 1 and dose 2 | |||
| Q20 minutes specific in protocol, median (25th, 75th) | 21.0 (16.0, 27.0) | 30.0 (20.0, 45.0) | 0.03 |
| Q30 minutes specific in protocol, median (25th, 75th) | 48 (26.5, 110.0) | 37 (20.0, 76.0) | 0.13 |
- Per visit = ED study visit; IVMSE = IV mg of morphine sulfate equivalent; n (%) for categorical measures; NA = not applicable; Total ED analgesic dose was analyzed using hierarchical mixed-effects models, adjusting for nested patient and site effects; Adjusted mean ± SD provided for total analgesic dose. Components of the analgesic protocol, dose administered, and ED provider adherence to protocol during visits were analyzed using chi-square/Fisher's Exact Tests for binary outcomes and Wilcoxon Two-Sample Tests for continuous outcomes. Dose in IVMSE difference = dose administered minus dose ordered as per study protocol, with higher positive difference scores indicating a higher dose than specified in the analgesic protocol was administered.
3.7 Analgesic protocols and adherence
Table 3 presents the analgesic protocols ordered per study protocol for the 52 patients, first three doses administered for the 126 visits, and adherence to the first dose of the study protocol by the ED providers. The study protocols did not differ with regard to study medication, IVMSE dose, dose unit, dose route, or dosing frequency ordered. Hydromorphone was used more frequently than MS for both protocols. First dose ordered as per protocol ranged from 5.0 to 26.7 for the patient-specific (median = 10.3) and 4.7 to 17.3 mg for the standard protocol (median = 7.9, P = .06). First dose administered was significantly higher in the patient-specific (median = 12.0 mg) compared to the standard protocol (median = 8.5 mg, P = .001).
We assessed how closely the ED providers consistently ordered the patient's protocol during the visit. Medication (drug name) adherence for the first dose was slightly lower for the patient-specific (88.7%) when compared to the standard protocol (95.3%, P = .20). The median difference for the first dose administered and ordered was 0.0 mg for both protocols, with greater variability in the standard protocol. Among those prescribed a q20 minute dosing frequency, the median time between dose 1 and dose 2 at each visit was significantly greater in the patient-specific protocol in comparison to the standard (P = .03).
4 DISCUSSION
Despite the significance of the problem of poorly treated VOE in ED settings, this is the first randomized trial to directly compare the two NHLBI-recommended opioid dosing strategies for acute SCD pain in the ED. As opioids are one of the only tools available to treat VOE, this study fills a large knowledge-gap regarding the best way to use opioids for VOE. Subjects randomized to the patient-specific protocol reported a greater reduction in pain scores from arrival to discharge, had lower hospital admission rates, and had acceptable side effects when compared with patients assigned to a weight-based protocol.
Logistical burdens of establishing individualized protocols are a barrier to implementation,6, 7 and prior to this trial there were no adult data to suggest that the benefits of individualized protocols justified the extra resources needed to implement them. With this trial we demonstrate both a benefit for patient experience (clinically significant improvements in change-in-pain-score from arrival to discharge) and measures of utilization (an 18% absolute reduction in the rate of admission to the hospital). These new data provide the first compelling evidence that the benefits of individualized protocols are indeed worth the logistical investment.
While the total dose administered in the ED did not differ between protocols, the initial dose prescribed was significantly higher for the patient-specific protocol. This demonstrates the importance of defining a patient-specific initial dose that is higher than a weight-based protocol. Over the duration of an ED visit, the total doses may not be different between protocols; however, individualizing the dose was clinically and statistically better. Of note, patients randomized to patient specific doses at Site 2 had a higher variability in doses with three patients exceeding doses of 250 mg IVMSE. Patients at Site 2 received higher total doses and patients at Site 1 randomized to the weight-based protocol received higher doses than the patient specific protocol. Individual doses were written using our protocol by the SCD provider. Doses were based upon out-patient chronic opioid therapy. At Site 2, the SCD provider prescribed higher doses of chronic opioids than the provider at Site 1 resulting in the difference in doses. The difference between sites in protocol dosing did not alter the overall treatment protocol results. Choice of analgesic was determined by the SCD provider when writing the protocols. There was a higher preference for hydromorphone in both groups. This may have been due to the high incidence of renal disease in persons with SCD and anecdotal reports of morphine being associated with negative renal effects more frequently than hydromorphone.
While several trials have evaluated novel agents for acute SCD pain in the ED (including ketorolac, arginine, magnesium and others)11-17 very few compared different ED opioid administration strategies as was done in the current trial.18 An important pediatric before-and-after study demonstrated reduced time-to-first-analgesic with intranasal opioids in conjunction with a comprehensive quality improvement initiative.19 To our knowledge only one observational pediatric study evaluated the impact of individualized pain plans on ED outcomes by comparing one hospital with individualized pain plans to four others that did not implement individualized plans.20 This study demonstrated improvements in rates of admission and readmission to the hospital but could not compare pain scores or complications across institutions. Our data also support the lower rate of hospitalization for patients randomized to the patient-specific dosing protocol.
We observed very low rates of adverse events and side effects in both groups. Importantly, no administration of naloxone or advanced airway intervention was required for any subject in either protocol. A total of six patients between groups had a respiratory rate <10; however, only oxygen administration was required. There were also unexpected benefits of the patient-specific opioid dosing protocol; subjects in this group experienced less nausea and vomiting and a statistical trend towards less need for supplemental oxygen when compared with the standard protocol group was observed. This is unexpected because patient-specific doses administered were higher at each time point compared to the standard protocol. Confirmation with a larger clinical trial is necessary to validate these initial promising results.
Finally, adherence to the study protocol among the ED physicians was also encouraging. There was excellent adherence to the selection of the drug and route, and very acceptable adherence to the dose. Time between doses exceeded the recommendation; however, the range between doses was shorter than what has been previously reported in other studies.21
There are several limitations of the trial including the small number of sites (n = 2) and participants (n = 52). While the results are compelling, a larger trial is needed to confirm generalizability of the data. Despite this, our data are suggestive of the benefit of individualized protocols recommended by the NHLBI guidelines. An additional limitation is the open-label design. The fact that patients and providers were aware of treatment assignment could bias the results away from the null hypothesis. After much discussion the study team felt the risks of patient refusal were too great to blind the patient to which protocol they would receive. This design was chosen because it is both pragmatic and representative of real-world clinical practice where patients are typically aware of the doses they receive. As stated previously, patients consented prior to randomization therefore they were not aware of which protocol they would receive until after consent; it is also not possible to blind patients to their opioid treatment for VOE. Research staff did not interact with the ED physician after they located the protocols on the EMR. The potential for directional bias away from the null hypothesis is an additional reason to confirm the results on a larger scale prior to widespread implementation efforts. It is possible that the lack of blinding affected results. In particular we do not understand how patients at Site 1 that received a lower patient-specific dose than weight-based protocol doses still had greater reductions in pain scores despite a lower dose. Several explanations are possible. The lack of blinding could account for the difference, or the importance of an individual dose, even if lower, is important. We were unable to determine patient specific characteristics that may have contributed to a more successful reduction in pain for either protocol. Finally, it is possible that patients awareness of which protocol they were randomized to led to a subjective difference in the reporting of pain scores and side effects, in particular nausea which was experienced more frequently in the standard protocol.
5 CONCLUSION
Findings from this two-site RCT indicate improvements in the pain experience, admission rates, and a lack of negative side effects and safety events when patients receive NHLBI guideline-based analgesia with patient-specific opioid dosing protocols compared to a standard weight-based strategy.
ACKNOWLEDGMENTS
The study team at both sites would like to thank the emergency medicine physicians and nurses as well as the hematologist and sickle cell teams that participated in the project. PT and SS had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We would like to thank our project coordinator, Theresa DeMartino, and clinical research associates, Christopher Barczak and Adam Soliman. All authors received funding from NHLBI (R34 RHL121224A) in support of this project. Paula Tanabe also received funding from the Agency for Healthcare Research and Quality for a separate project (R18 HS 19646). Drs. Glassberg and Richardson have other NIH funding not in conflict with this grant. Dr. Bosworth lists the following financial disclosures which are not directly related to the proposed paper. He receives research funds from NIH, VA, Pharma foundation, Johnson and Johnson, Sanofi, Improved Patient Outcomes. He receives consulting funds from Sanofi, Otsuka, and Genentech and received funds as a member of the speaking bureau from Boehringer ingelheim. In the last three years, he has received research funds from MeadWestVaco and Takeda. None of these funds beyond funding form NIH are related to this project. All other co-authors do not have any additional conflicts.




