Daratumumab monotherapy compared with historical control data in heavily pretreated and highly refractory patients with multiple myeloma: An adjusted treatment comparison
Funding information: Janssen Global Services, LLC.
Abstract
Daratumumab is a human CD38-directed monoclonal antibody approved in the United States as monotherapy for patients with multiple myeloma (MM) who have received ≥3 prior lines of therapy (LOTs), including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD) or who are double refractory to a PI and an IMiD, and in combination with lenalidomide/dexamethasone or bortezomib/dexamethasone for patients with MM who have received ≥1 prior LOT. This study compared the efficacy of daratumumab monotherapy versus historical controls through adjusted treatment comparison. Patient-level data were pooled from two daratumumab monotherapy studies (16 mg/kg; GEN501 and SIRIUS) and two independent US databases (IMS LifeLink and OPTUM), which reflect treatments used in real-world patients with MM who received ≥3 prior LOTs or were double refractory to a PI and an IMiD. Using a multivariate proportional hazards regression model, the relative treatment effect of daratumumab versus historical controls was estimated, adjusting for imbalances in characteristics between cohorts. Baseline characteristics that differed between patients treated with daratumumab (N = 148) and historical control (N = 658) were prior treatment with pomalidomide (55% vs 15%) or carfilzomib (41% vs 28%) and triple/quadruple refractory status (64% vs 14%). The adjusted overall survival–hazard ratio (OS-HR) for daratumumab versus historical control was 0.33 (95% confidence interval, 0.24-0.46) compared with 0.46 (0.35-0.59) for unadjusted HR. Impact of adjustment was mainly driven by refractory status and prior pomalidomide/carfilzomib exposure. This adjusted treatment comparison suggests that daratumumab demonstrates improved OS compared with historical control data in heavily pretreated and highly refractory MM patients.
1 INTRODUCTION
Comparative assessments of new agents for the treatment of heavily pretreated patients with relapsed and refractory multiple myeloma (MM) who have exhausted approved treatment options are challenging, as active-controlled studies are not feasible because there are no generally accepted standard regimens to use for comparison. In the absence of head-to-head data, it is important to understand treatment outcomes based on current real-world experience in a routine clinical setting to fully recognize the potential benefits of novel agents. These outcomes data may be used as a benchmark and can provide evidence beyond that collected during clinical development in randomized controlled trials. Historical controls may provide useful information for both clinicians and reimbursement decision makers and may serve as a reference point against which newer agents can be evaluated.1
Daratumumab is a first-in-class human IgG1 monoclonal antibody that binds CD38, which is highly and ubiquitously expressed on myeloma cells.2-4 Daratumumab-induced on-tumor activity occurs through several CD38 immune-mediated actions (eg, complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and antibody-dependent cellular phagocytosis), apoptosis, and modulation of CD38 enzymatic activity.5-8 Daratumumab induces an immunomodulatory effect that minimizes the immune-suppressive functions of CD38+ myeloid-derived suppressor cells, regulatory T cells, and regulatory B cells, and increases T-cell clonality.9
In a pooled analysis of two single-arm studies of daratumumab 16 mg/kg monotherapy in patients with heavily pretreated and/or highly refractory MM (the phase 1/2 GEN501 study and the phase 2 SIRIUS study), an overall response rate of 31% and a median overall survival (OS) of 20.1 months was reported.10 Based on the results from these two clinical trials, daratumumab monotherapy (16 mg/kg) was approved by both the US Food and Drug Administration11 and the European Medicines Agency12 for the treatment of patients with MM. More specifically, daratumumab monotherapy (16 mg/kg) is approved in the United States for the treatment of patients with MM who have received at least 3 prior treatments, including a PI and an IMiD, or who are double refractory to a PI and an IMiD.13 More recently, daratumumab in combination with lenalidomide and dexamethasone or bortezomib and dexamethasone was approved in the United States for the treatment of patients with MM who have received at least 1 prior line of therapy.13
To fully contextualize the benefits of daratumumab monotherapy, it is important to compare survival results from clinical trials with outcomes observed in a similar patient population in clinical practice. Recently, real-world historical data from two independent, US patient databases were analyzed to characterize the outcomes of patients with MM who became refractory to at least 1 PI and at least 1 IMiD (double refractory) or who were heavily pretreated (at least three prior lines of therapy [LOTs] and progressed on their most recent regimen).1 Results from these analyzes indicated that median OS remains poor (approximately 8 months) in this patient population despite the availability of more recently approved PIs and IMiDs, such as carfilzomib and pomalidomide. These data provided a historical control set that could be used as a reference point for patients with heavily pretreated and/or highly refractory MM. The objective of this study was to perform an adjusted comparison of patients’ clinical outcomes from daratumumab monotherapy clinical studies versus the historical US data to establish the comparative efficacy of daratumumab versus real-word historical controls (physician's choice).
2 METHODS
2.1 Historical control dataset
Medical records from two independent databases were evaluated, each composed of US patients: the IMS LifeLink, IMS Oncology Electronic Medical Records database (IMS Health Incorporated, Danbury CT) and the OPTUM database (OPTUM, Inc., Eden Prairie, MN). The indexing periods for the IMS LifeLink and OPTUM databases were from 2000 to 2014 and 2007 to 2014, respectively. These indexing periods were chosen based on availability and robustness of data in each database.
Patients with a diagnosis of MM from 2000 to 2011 in the IMS LifeLink database or from 2007 to 2014 in the OPTUM database were eligible for inclusion in the study. ICD-9 codes for MM included 203X, 203.0X, 203.00X, 203.01X, and 203.02X. No other cancer diagnosis prior to the diagnosis of MM, with the exception of benign and in situ neoplasms, basal cell carcinoma, and squamous cell carcinoma, was permitted. Patients were also required to have received at least 3 prior LOTs that included a PI and an IMiD and to have progression of disease within 60 days of completion of the most recent regimen OR be refractory to both a PI and an IMiD, as defined in Supporting Information Table S1.
2.2 Patients treated with daratumumab
2.2.1 Inclusion criteria
This pooled outcomes analysis included patients from 2 open-label studies of daratumumab 16 mg/kg as monotherapy: GEN50114 (ClinicalTrials.gov Identifier: NCT00574288) and SIRIUS15 (NCT01985126). Key inclusion criteria for both studies were patients aged ≥18 years, an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and heavily pretreated and/or refractory MM. In GEN501, patients were relapsed from or refractory to at least 2 prior LOTs that included a PI and an IMiD. In SIRIUS, patients were relapsed from or refractory to at least 3 prior LOTs that included a PI or an IMiD or were double refractory to a PI and an IMiD.
2.2.2 Study designs
Study methodology and primary results from the GEN501 and SIRIUS studies have been described in detail elsewhere.14, 15 Briefly, GEN501 was an open-label, phase 1/2, dose-escalation and dose-expansion study,14 and SIRIUS was an open-label, multicenter, phase 2 study.15
2.3 Endpoints
For patients identified in the IMS LifeLink or OPTUM databases, OS from the start of the last LOT was defined based on death or loss to follow-up more than 30 days prior to the study end date. For patients in the GEN501 and SIRIUS studies, OS was defined as the number of days from the first dose of daratumumab to death.
2.4 Adjusted treatment comparison
The relative treatment effect of daratumumab was estimated using patient-level data from the historical controls (US claims databases) and clinical studies (pooled analysis of patients receiving daratumumab 16 mg/kg in GEN501 Part 2 and SIRIUS).
Analysis of OS was conducted on the intention-to-treat population from both cohorts using Kaplan-Meier analysis and Cox proportional hazards regression. Statistical adjustments were made using patient-level data, assuming no unobserved confounders. To avoid confounding bias, multivariate proportional hazards regression modeling was used to estimate the hazard ratio (HR) of daratumumab versus physician's choice as a measure of relative efficacy/effectiveness for time to event data to account for the differences in patient characteristics between the daratumumab trials and data from the US claims databases. Covariates in the multivariate model included age, gender, exposure to prior therapies, LOT, albumin and hemoglobin levels, and refractory status. Patients refractory to 3 or 4 prior agents, including both a PI and an IMiD, were considered triple refractory (refractory to 2 different PIs and 1 IMiD or 1 PI and 2 different IMiDs) or quadruple refractory (refractory to any combination of 2 different PIs and 2 different IMiDs).
The baseline values for the covariates for each patient were specific by treatment line. The clustering of observations at treatment-line level within patients was controlled by using the robust sandwich estimate for the covariance matrix, making confidence intervals (CIs) somewhat more conservative.16, 17 Adjusted HRs, including 95% CIs, were calculated for the treatments reflecting physician's choice in the US claims databases cohort relative to daratumumab; HRs for treatment and prognostic covariates were presented graphically as forest plots, representing point estimates and 95% CIs. All statistical analyzes were performed using the statistical software package SAS 9.2.
3 RESULTS
3.1 Patients
Separate assessment of the IMS LifeLink and OPTUM databases showed that the 2 cohorts were comparable with respect to patient demographics, and the data were pooled (N = 658).1 Index therapeutic regimens included bortezomib only (no IMiDs/cytotoxic agent; ∼29% of patients), lenalidomide/thalidomide only (∼24%), bortezomib + lenalidomide/thalidomide (± cytotoxic agent; ∼21%), bortezomib + cytotoxic agent (∼7%), any cytotoxic agent (∼5%), any carfilzomib (∼6%), steroid only (∼4%), any pomalidomide (∼3%), lenalidomide/thalidomide + cytotoxic agent (∼1%), and bendamustine (<1%). Demographics from GEN501 and SIRIUS were generally similar and were pooled (N = 148).10 Demographics for these pooled daratumumab-treated and US claims datasets are summarized in Table 1. Median age (64 years vs 69 years) was lower in the daratumumab-treated patients compared to historical controls, and median number of prior LOTs (5 vs 4) was slightly higher. Daratumumab-treated patients were more likely than historical controls to have received carfilzomib (41% vs 28%) or pomalidomide (55% vs 15%), or to be triple/quadruple refractory (64% vs 14%), respectively. Age, LOT, exposure to prior therapies, and refractory status were all adjusted for in the multivariate analysis. Median follow-up in the daratumumab-treated and US cohorts was 20.7 months and 18.3 months, respectively.
| Characteristic |
Daratumumab (N = 148) |
US claims (N = 658) |
|---|---|---|
| Median (range) age (years) | 64 (31–84) | 69 (31–83) |
| Gender (%) | ||
| Male | 53 | 53 |
| Female | 47 | 47 |
| Median (range) number of prior LOTs | 5 (2–14) | 4 (1–28) |
| Hemoglobin (%) | ||
| <80 g/L | 5 | 10 |
| 80–100 g/L | 42 | 20 |
| >100 g/L | 53 | 52 |
| Data missing | 0 | 19 |
| Beta-2 microglobulin (%) | ||
| <3.5 mg/L | 39 | 30 |
| ≥3.5 mg/L | 61 | 47 |
| Missing | 0 | 22 |
| Prior exposure to (%) | ||
| Carfilzomib | 41 | 28 |
| Pomalidomide | 55 | 15 |
| Refractory status (%) | ||
| Not double refractory | 13 | 33 |
| Double refractory | 23 | 53 |
| Triple/quadruple refractory | 64 | 14 |
- LOT, line of therapy.
3.2 Daratumumab relative treatment effects
Median OS in the daratumumab-treated cohort was 20.1 months and 7.9 months in the US cohort. The unadjusted HR for OS for the comparison of daratumumab-treated patients with historical controls was 0.46 (95% CI, 0.35–0.59; P < .001; Figure 1A), representing a 54% reduction in the risk of death. Figure 1B represents the predicted survival for the US cohort as treated (median OS = 8.1 months) versus under daratumumab treatment (median OS = 26.8 months), based on the multivariate Cox proportional hazards regression model (HR = 0.33; 95% CI, 0.24–0.46; P < .001), representing a 67% reduction in the risk of death versus the historical control.
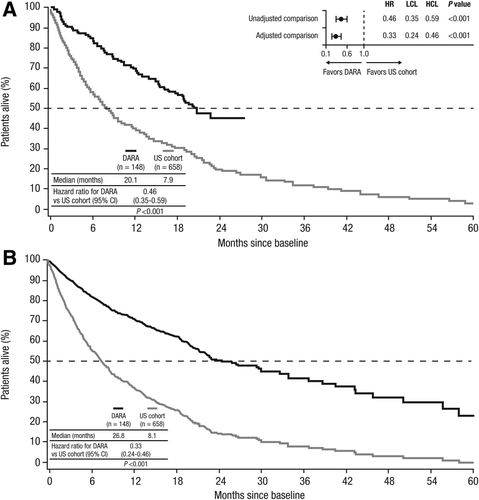
Unadjusted (A) and adjusted (B) overall survival in daratumumab-treated patients versus historical controls from US claims databases. Adjusted and unadjusted HRs are also shown in the forest plot (A). HR, hazard ratio; LCL, lower confidence level; HCL, higher confidence level; DARA, daratumumab; CI, confidence interval
Refractory status and prior pomalidomide or carfilzomib exposure had the greatest impact on adjustment. However, similar to comparison of the total cohorts, OS was longer in daratumumab-treated patients irrespective of prior carfilzomib or pomalidomide treatment compared with the historical control (Supporting Information Figure S1).
In daratumumab-treated patients, OS was shorter in triple/quadruple refractory patients compared with those classified as not double refractory (median OS: 17.5 months vs not reached, respectively; HR = 2.12; 95% CI, 0.96–4.67; P = .06; Figure 2A); OS was similar between double refractory patients and patients classified as not double refractory (median not reached in both groups; HR = 1.20; 95% CI, 0.48–3.01; P = .70). A similar finding was observed in the US controls, with patients in the group that were not double refractory displaying a similar OS compared with double refractory patients (median OS: 10.5 months vs 7.7 months, respectively; HR = 1.14; 95% CI, 0.91–1.43; P = .24) and a longer OS compared with triple/quadruple refractory patients (median OS: 10.5 months vs 5.1 months, respectively; HR = 1.79; 95% CI, 1.28–2.49; P = .0006; Figure 2B). Interestingly, triple/quadruple refractory patients treated with daratumumab had a longer median OS than non–double refractory patients from the US claims cohort.
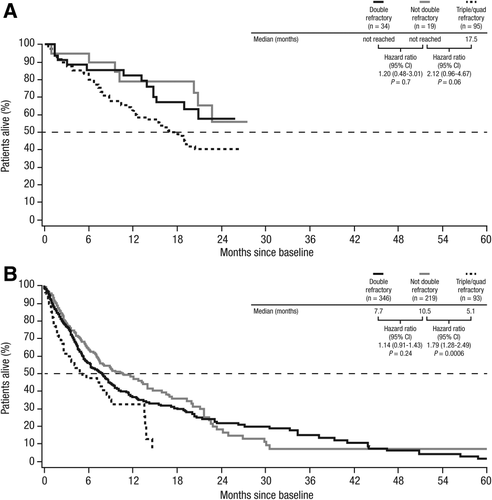
Overall survival of daratumumab-treated patients (A) and historical controls from US claims databases (B) by refractory status. CI, confidence interval; quad, quadruple
Cox proportional HRs were calculated for patient subgroups according to demographics, disease characteristics, and treatment history in a multivariate model (Figure 3). For the analysis of age, 45 years or younger was used as a reference category in comparison with 5-year increments from 50 to 80 years and 80 years or older. Male gender, albumin level <35 g/L, hemoglobin <80 g/L, no prior exposures to pomalidomide and carfilzomib, and not double refractory were also reference categories. Other common markers of disease status, such as ECOG status and comorbidity index, were not captured in great detail within the US claims databases, so these data were not robust enough to be included in this analysis. Of these comparisons, only age categories above 70 and prior carfilzomib or prior pomalidomide HRs had statistically significant HRs versus the reference category.
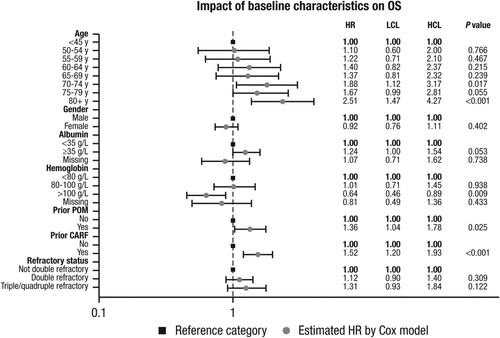
Impact of baseline characteristics on overall survival of daratumumab-treated patients compared with historical controls from US claims databases, based on multivariate Cox model: HR and 95% confidence interval. OS, overall survival; HR, hazard ratio; LCL, lower confidence level; HCL, higher confidence level; POM, pomalidomide; CARF, carfilzomib
4 DISCUSSION
While significant progress has been made in the treatment of MM over the last decade,18-20 novel therapeutic strategies are still urgently needed, particularly for patients with refractory disease. In an International Myeloma Working Group study conducted in 2012, results indicated that outcomes in patients refractory to bortezomib and at least 1 IMiD (thalidomide and/or lenalidomide) were poor, with a median OS of approximately 9 months.21 Since then, several new agents have been approved for MM and are now being used routinely in clinical practice, including pomalidomide and carfilzomib.
In the absence of head-to-head clinical trial results, adjusted treatment comparisons may provide useful insights for clinicians and other health care decision makers on the relative efficacies and potential benefits of novel therapies for MM. In the current study, we compared the efficacy of daratumumab monotherapy, based on a pooled outcomes analysis of data from 2 open-label studies (GEN501 and SIRIUS), with that of real-word historical controls (physician's choice), based on medical records from 2 independent US databases, using patient-level data. Based on a multivariate proportional hazards regression model, adjusted for imbalances in patient characteristics between cohorts, the adjusted OS-HR for daratumumab versus historical control was 0.33 (95% CI, 0.24–0.46; P < .001). Refractory status and prior exposure to pomalidomide/carfilzomib had the greatest effects on adjustment. Daratumumab also showed improved OS compared with historical controls, regardless of refractory status (ie, patients who were double refractory, triple/quadruple refractory, or not double refractory to treatment). These results are consistent with other recent analyzes in which pooled data from GEN501 and SIRIUS were compared with outcomes in heavily pretreated patients from other data sources.22
The results from this study must be considered within the confines of its inherent limitations. Although a range of clinically relevant prognostic factors were available and adjusted for, residual confounding bias cannot be excluded, as in any observational study. Additionally, because the historical control data were extracted from claims databases, certain data were missing or could have been inaccurately coded during data entry, whereas the daratumumab data were derived from registered clinical trials and validated by the study sites, the sponsor, and regulatory bodies. Longer follow-up was available in the claims databases compared with the shorter observation window for the ongoing daratumumab monotherapy trials. Side effects of newly approved therapies are also an important consideration. Adverse events associated with daratumumab monotherapy were recorded during the clinical trials. However, these data are not systematically collected when drugs are used in routine clinical practice and thus were not available in the claims databases to allow comparisons with daratumumab.
This study is also limited by the differences between the claims database and clinical trial populations. Records collected from the US databases described patients treated between 2000 and 2014, whereas daratumumab-treated patients were treated more recently and, thus, are more likely to have had access to different treatment regimens than some of the patients in the historical US cohort. It is possible that the access to newer regimens may have enhanced the apparent survival benefit of daratumumab treatment. However, as the daratumumab cohort included patients who had already received, and relapsed on, these newer regimens, this instead may bias the results toward a lower OS in daratumumab-treated patients. Additionally, daratumumab confers a survival benefit, even to patients with MR and SD,10 suggesting that the survival benefit is due to daratumumab rather than to subsequent therapies. The daratumumab cohort also had a lower median age than the claims database cohort (64 vs 69 years, respectively), which could potentially bias the results toward a benefit to OS for the daratumumab group. However, the daratumumab group had received a greater median number of prior LOTs (5 vs 4, respectively) and had a higher proportion of triple/quadruple refractory patients (64% vs 14%, respectively), both factors that would be expected to have a negative impact on OS. With the exception of access to newer treatment regimens, all of these potentially confounding factors (age, LOTs, and refractory status) were adjusted for in the multivariate model.
In summary, this adjusted treatment comparison suggests that daratumumab monotherapy provides a substantial survival benefit compared with real-world historical controls in patients with heavily pretreated and highly refractory MM. In the absence of head-to-head clinical trials, comparative analyzes adjusting for differences in patient characteristics using patient-level data can provide valuable insights to clinicians and reimbursement decision makers on the relative efficacy of daratumumab versus established standard of care treatments.
ACKNOWLEDGMENTS
This study was sponsored by Janssen Global Services, LLC. Medical writing and editorial support were provided by Christopher Jones, PhD, of MedErgy, and were funded by Janssen Global Services, LLC.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
SZU reports receiving fees for serving on advisory boards and committees from Celgene, Skyline, Sanofi, Janssen, Array BioPharma, and Bristol-Myers Squibb; consulting fees from Amgen and Takeda; lecture fees from Celgene, Amgen, and Takeda; and grant support from Celgene, Amgen, Takeda, Sanofi, Janssen, Array BioPharma, Pharmacyclics, and Bristol-Myers Squibb. IK reports employment with Janssen. JD, TI, MM, and AL report employment with Janssen and hold stock in Johnson & Johnson.




