Management of anemia in patients with congestive heart failure
Author disclosures for conflicts of interest: LTG: Luitpold Pharmaceuticals, Fe3 Medical, Inc.; JCC: Vifor Pharma, FAIR-HF study, CONFIRM_HF study; SRLN: None; SO: None; JAT:AMAG Pharmaceuticals, Inc.; DH: None; MJ: Society for the Advancement of Blood Management (SABM), Gauss Surgical; BH: Vifor Pharma; EB:Vifor Pharma, Maturity Assessment Model for Patient Blood Management (MAPBM project).;IG: Accumen LLC; AS: Vifor Pharma, AMAG Pharmaceuticals, Inc.
Abstract
Anemia is an independent risk factor for adverse patient outcomes. There are no guidelines for management of anemia in patients with congestive heart failure (CHF), despite its high incidence. Four objectives were defined by the International Anemia Management and Clinical Outcomes Expert Panel (AMCO), a multinational group of interdisciplinary experts identified by the Society for the Advancement of Blood Management (SABM) to: determine the prevalence of anemia in outpatients; to determine the prevalence of hospital-acquired anemia; to assess the impact of anemia management on clinical outcomes such as quality of life and functional status; and to provide recommendations for primary care physicians and specialists for the diagnosis, evaluation, and management of anemia in patients with CHF. Anemia and iron deficiency were confirmed to be highly prevalent in patients with CHF. Intravenous iron therapy improves anemia, cardiac function and exercise tolerance, leading to improvement in quality of life. Anemia management has been demonstrated to be cost-effective. Clinical care pathways to manage anemia in patients with CHF are recommended as best practices in order to improve patient outcomes. Am. J. Hematol. 92:88–93, 2017. © 2016 Wiley Periodicals, Inc.
Introduction
Anemia is recognized as an independent risk factor in several clinical settings for a number of adverse outcomes 1, including patients with chronic kidney disease (CKD) and malignancies, for which treatment guidelines have been published by medical societies 2-4. Iron deficiency (ID) is also common in patients with CHF. In 574 community-dwelling adults with CHF in NHANES III 5, ID was present in 61.3% (ferritin level <100 µg l−1 or between 100 and 199 µg l−1 if the transferrin saturation was <20%); was associated with reduced hemoglobin (Hb) levels compared to non-iron deficient adults (13.6 vs. 14.2 g dl−1);and was an independent predictor of cardiovascular mortality. An international analysis of patients with CHF reported ID in 50% of patients 6, and was an independent predictor of clinical outcomes. Despite these observations, guidelines published by the American College of Physicians have not specified that ID or anemia should be treated in patients with heart disease, except for recommending a restrictive blood transfusion strategy 7. In a 2013 report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, the identification and management of anemia is not mentioned in the practice guidelines, in evidence gaps, or in future research directions 8. In this report, we review the prevalence and management of anemia and ID in patients with CHF in order to improve clinical outcomes.
The goal of our anemia management and clinical outcomes panel (AMCO) was to review and summarize the current literature for anemia in patients with CHF; to assess the impact of anemia and anemia management on patient outcomes; and to identify areas for further investigation in order to achieve best practices. The objectives were fourfold: to determine the prevalence of anemia in outpatients; to determine the prevalence of hospital-acquired anemia; to assess the impact of anemia management on clinical outcomes such as quality of life and functional status; and to provide recommendations for primary care physicians and specialists for the diagnosis, evaluation, and management of anemia in these patients.
The literature search (key words: congestive heart failure, anemia, iron deficiency, erythropoietic stimulating agents) initially identified 1,999 publications, of which 590 were selected in the first round by manual search. After exclusion of secondary literature (reviews, editorials) other than meta analyses, a final pool of 327 papers was selected for data extraction and categorization.
Prevalence of Anemia
The prevalence of anemia in a broad range of studies (6–70%) (Table 19-21 reflects heterogeneity in anemia screening; the clinical setting(hospitalized vs. out-patients); and the socio-economic status of study populations. Up to 2/3 of patients have contributing factors, such as CKD and/or anemia of inflammation 28. Longitudinal studies show that patients with CHF who are nonanemic at baseline, may develop anemia over time: from 14.2% at year 1 to 25.5% at year 5 19. Conversely, anemia resolution during follow-up was observed in 43% of those anemic at baseline 29. When patients are prospectively evaluated by a trajectory analysis over a variable period of 3–15 years, 35% had variable rates of anemia 30, 31. Severity of CHF may also influence the prevalence of anemia 15, 16.
| Author/year | Design | Intravenous iron used | Number of patients; iron/control | Age | LVEF (%) | Hb (g dL−1) | Duration of treatment (weeks) | Follow-up (weeks) | Outcomes (compared to control) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bolger 200622 | Open-label Single center Pre-post trial | Iron sucrose | 16 | – | 68 ± 11 | 26 | 11.2 | 1.7 | 14 |
↑ Hb up to 12.6 Improved NYHA ↓ 14 points in MLHFQ ↑ 44 m 6MWT |
| Toblli 200723 | Single center randomized double blind placebo controlled | Iron sucrose | 20 | 20 | 60-94 | 31 | 10.3 | 5 | 24 |
↑ Hb (11.8 vs. 9.8) ↓ 333 pg mL−1 mean NT-ProBNP ↓ CRP (2.3 vs. 6.5 mg dL−1) ↑ LVEF (36 vs. 29%) Improved NYHA ↓ 18 points in MLHFQ ↑ 56 m 6MWT |
| FERRIC-HF 200824 |
Multicenter single blind Randomized controlled trial |
Iron sucrose | 24 | 11 | 64 ± 13 | 30 | 12.5 | 16 | 18 |
↑ Hb Improved NYHA ↑ Peak VO2 |
| Usmanov 200825 | Open-label single center | Iron sucrose | 32 | – | 49 ± 6 | 32 | 10.2 | 26 | – |
↑ Hb by 3 g dL−1 Improved NYHA ↑ LVEF |
| FAIR-HF 200926 | Multicenter randomized double blind placebo controlled | Ferric carboxymaltose | 304 | 155 | 68 ± 10 | 31 | 11.9 | 24 | – |
↑ Hb (13.0 vs. 12.5 g dL−1) ↑ 7 points in KCCQ OSS Improved NYHA and PGA ↑ 35 m 6MWT |
| CONFIRM-HF 201527 | Multicenter randomized double blind placebo controlled | Ferric carboxymaltose | 152 | 152 | 69 ± 9 | 36 | 12.4 | 24 |
↑ 33 m 6MWT ↓0.6 fatigue severity scale ↑ 1.3 en el KCCQ ↑ 2.8 en EQ-5D |
|
| 52 |
↑ 36 m 6MWT ↓0.7 fatigue severity scale ↑ 4.5 in KCCQ OSS ↑ 2.6 in EQ-5D |
|||||||||
- Hb: hemoglobin; NYHA: New York Heart Association functional class; MLHFQ: Minnesota Living with Heart Failure Questionnaire (heart-failure specific instrument for health-related quality of life measurement); 6MWT: distance in the 6 minutes walking test; LVEF: left ventricular ejection fraction; NT-proBNP: amino-terminal segment of the type B natriuretic peptide; CRP: C reactive protein; KCCQ OSS: Kansas City Cardiomyopathy Questionnaire (heart-failure specific instrument for health-related quality of life measurement); KCCQ OOS: overall summary score of the KCCQ; EQ-5D: European quality of life 5 dimensions (generic instrument for health-related quality of life measurement).
Pathophysiology of Anemia
The pathophysiology of anemia in patients with CHF is multifactorial. Notably, the etiology of anemia is evaluated in only 60% of these 28. In one study, only 20% of anemic patients had a complete iron evaluation, yet 80% of these had iron deficiency 32. Some studies put the prevalence of ID is as high as50–70%6, 33 for ambulatory or hospitalized patients, respectively.Beyond iron deficiency, other factors such as hemodilution 34, inflammation 35, and the use of cardiovascular drugs36 also play a role.
CHF is associated with an increase in production of inflammatory cytokines, mainly TNF-α and IL-6. These have been associated with an inadequate production of endogenous erythropoietin in response to anemia, suppressed erythropoietic response of red cell precursors, and increased synthesis of hepcidin. The latter peptide is released by the liver in response to inflammatory stimuli and inhibits intestinal absorption of iron, along with iron sequestration in the reticulo-endothelial macrophage system, leading to ineffective erythropoiesis 35.
Anemia and Clinical Outcomes
All-cause mortality (RR 1.47), hospitalization (RR 1.28), and CHF hospitalization (RR 1.43, all P < 0.0001) are higher in anemic compared with nonanemic patients with CHF 19. The association of anemia with mortality has been reported to be a hazard ratio of 2.1 [95% CI 1.6–2.7] 12. In a randomized study with extended follow-up, multivariate analyses showed an adjusted, severity-dependent influence of anemia on mortality (hazard ratio 1.33 [1.07–1.66]) 30. Rehospitalization rate (Table 1) is also higher, risk increased by 3.3% for every g l−1 decrease in discharge hemoglobin. The combination of anemia, chronic kidney disease (CKD) and CHF has been termed the cardio-renal anemia syndrome. Adequate treatment of the three conditions can prevent progression of both CKD and CHF 37.
The impact of anemia on exercise tolerance and quality of life is also substantial.
Reduction in exercise capacity is in parallel to decreasing Hb levels (r = 0.24, P < 0.001) 38. Adjusted regression analysis demonstrated a significant relationship between hemoglobin and health-related quality of life through 12-months follow-up 39.
Lastly, anemia may increase healthcare management costs. In a study of 1,056 symptomatic patients with CHF, the adjusted costs per year alive were higher in anemic than nonanemic patients ($22,926 vs. $17,189; P = 0.04) 40. Another study reported a 25% increase in management costs for patients with CHF who were anemic 41.
Iron Deficiency and Clincial Outcomes
ID, with or without anemia, is an independent predictor of increased mortality and hospitalization (Table I Supporting Information). Improvement of myocardial function with iron replacement therapy in ID patients with CHF has been demonstrated by tissue doppler and stain rate imaging studies 42. Experimental evidence points to iron serving as a cofactor necessary for muscle function 43. Recently, a knock-out model for the transferrin receptor (Tfr1) in mice who had ID but not anemia, showed that such mice died within 2 weeks after birth (cardiomegaly, poor cardiac function, failure of mitochondrial respiration, and ineffective mitophagy). The mice could be rescued with large doses of iron (presumably when nontransferrin bound iron (NBTI) was generated, which the heart can take up) 44. These studies give insight that iron cofactors are synthesized by mitochondria, so that iron is necessary for mitochondrial function to supply energy for repeated muscle contraction; and that Tfr1 itself is critically important for normal heart function. Some examples of remaining questions to be answered: does iron replacement therapy need to be administered parentally, or would oral iron have the same effect; and is there a difference in response to iron therapy in patients who also have anemia of chronic inflammation? 45
Several studies have confirmed the adjusted negative effects of ID on endurance and quality of life. In multivariable regression models, ID but not anemia was associated with impaired health-related quality of life (HRQoL) 46. In fact, ID but not hemoglobin levels were independently associated with submaximal exercise capacity 47. Therefore, a normal hemoglobin level does not exclude ID, which should be investigated in all patients with CHF.
In an international multicenter cohort of patients with CHF 48, the clinical correlates and prognostic implications of ID (ferritin <100 μg L−1 or 100–299 μg L−1 in combination with a transferrin saturation <20%), anemia and renal dysfunction (estimated glomerular filtration rate <60 mL min−1/1.73 m2) were analyzed. The presence of IDA, anemia due to CKD, or a combination was observed in 69.3% of patients. During a median follow-up of 1.92 years, 29.2% died. Multivariate hazard models revealed ID to be the key determinant of prognosis, either individually (P = 0.04) or in combination with either anemia (P = 0.006), CKD (P = 0.03), or both (P = 0.02). The presence of IDA amplifies mortality risk, either alone or in combination with CKD.
Evaluation and Management of Anemia
An algorithm for the evaluation and management of anemia in patients with CHR is illustrated in Fig. 1. Serum ferritin assay and transferrin saturation are important in the assessment of patients with iron restricted erythropoiesis. When transferrin saturation is low (<20%) and the ferritin level is high (>300 ng ml−1), anemia of inflammation is generally considered. Overlap of these two common causes of anemia (ID and inflammation) has made the use of transferrin saturation and ferritin difficult to interpret 35. The % hypochromic reticulocytes and mean hemoglobin reticulocyte concentration (CHr) are assay options for the identification of iron restricted erythropoiesis. Among reticulocyte variables, a CHr value <30 pg appears to be the most predictive value for response to intravenous iron. The transferrin receptor may have a role in some medical centers where it is available 35, 49. However, the soluble transferrin receptor assay is offered as an in-house test in very few labs and is mostly a send-out and therefore not readily available. On the other hand, CHr is offered on almost every large hematology analyzer and can easily be added to in-house testing.
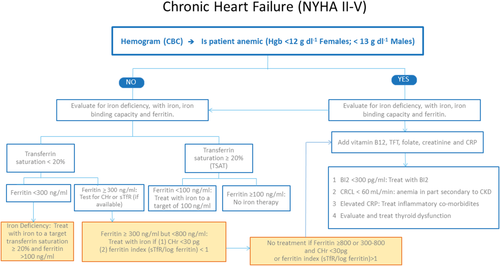
Outlines a strategy for evaluating iron deficiency in patients with and without anemia, who have chronic heart failure. It emphasizes the critical role of iron deficiency in the anemia in patients associated with CHF, along with the occurrence of iron deficient erythropoiesis even when the ferritin is >100 ng ml−1. CHr: reticulocyte hemoglobin concentration. sTfR: soluble transferrin receptor. TFT: thyroid function tests. CRCL: creative clearance. CRP: C-reactive protein. [Color figure can be viewed at wileyonlinelibrary.com]
The identification of ID with or without anemia is important for the management of patients with CHF. An algorithm for the evaluation and management of anemia in patients with CHF is presented in the Fig. 1. Anemia is defined using the WHO criteria, as a hemoglobin value <120 g L−1 in females and 130 g L−1 in males. ID is defined according to the criteria described as above 5. Anemia secondary to iron restricted erythropoiesis can be due to an absolute iron deficiency; iron sequestration mediated by up-regulation of hepcidin;50 and/or due to erythropoietin-stimulated erythropoiesis 51. If absolute ID is diagnosed, it is mandatory to rule out gastrointestinal pathology, including malignancy, as a source of chronic blood loss. Serum creatinine and glomerular filtration rate must be assessed to evaluate for CKD. Finally, thyroid function testing should be part of routine assessment of patients with CHF, with or without anemia 52.
When serum ferritin and transferrin saturation values are inconclusive, to diagnose or exclude iron deficiency, further evaluation may be necessary. A response to a therapeutic trial of oral iron confirms absolute iron deficiency. However, lack of response to oral iron therapy should require a trial of intravenous (IV) iron, since in the presence of inflammation and upregulation of hepcidin, absorption of iron from the intestinal tract is impaired 49. In a study of patients with a clinical diagnosis of iron deficiency anemia based on ferritin and transferrin saturation, after an initial 14 day-run in to identify nonresponders (<1 g dl−1 Hb increase) to oral iron therapy, only 21% of these early non responders responded to four additional weeks of oral iron therapy, compared to 65% of patients treated with IV iron therapy 53, 54. Additionally, lack of response to oral iron may be due to noncompliance, impaired absorption due to edema of the GI mucosa, or use of H2-blockers or protein pump inhibitors. A recent study55 found that even once daily oral iron therapy is associated with increased hepcidin levels that inhibit effective absorption of iron, therefore supporting a strategy of alternate day oral iron supplementation 49.
Iron Therapy
Table II (Supporting Information) summarizes currently available oral and intravenous iron preparations. Although inexpensive and convenient, oral iron therapy has several limitations. Medications and food interactions can lead to precipitation of Fe3+ in the gastrointestinal tract (GI), causing symptoms such as vomiting, dyspepsia, constipation, and heartburn. Such adverse effects lead to patient noncompliance with oral iron supplements.
Six different IV iron preparations are currently available: low molecular weight (LMW) iron dextran (Infed), iron gluconate, iron sucrose, ferumoxytol, ferric carboxymaltose, and iron isomaltoside. The advantage of iron dextran is its low cost and ability to be given in doses >1000 mg by infusion. However, LMW iron dextran carries a boxed warning regarding the risk of anaphylaxis reactions, so must be given as a test dose prior to administration of the full dose. Safety reports have mandated eliminating the use of the high-molecular-weight iron dextran (Dexferrumnow off the market) because of a high incidence of adverse reactions. Low molecular weight iron dextran (Infed) is approved in pediatric and adult patients for treatment of iron-deficiency anemia unresponsive to oral iron therapy. On balance, the current formulations are supported by literature that shows equivalent safety and efficacy.
Iron sucrose is approved for use in patients with CKD and IDA.It is given as slow intravenous infusion at a maximum individual dose of 200mg. A test dose is not required. Ferric gluconate is also indicated for the treatment of IDA in patients with CKD, given as slow intravenous infusion at a maximum individual dose of 125 mg. A test dose is not required. Ferumoxytol is approved for use in patients with CKD with a recommended dose of an initial 510 mg dose followed by a second 510 mg dose. A test dose is not required. Ferumoxytol must be administered as an intravenous infusion. The initial product labeling for the infusion rate of ferumoxytol was revised from rapid infusion, based on postmarketing surveillance documenting a large number of reactions with rapid infusion. Ferric carboxymaltose (FCM) is approved for the treatment of IDA in adults who have intolerance or are unresponsive to oral iron. The usual dose for FCM in the USA is two individual doses of 750 mg each, whereas in Europe 1000 mg is routinely administered each dose. Iron isomaltroside is approved in Europe (and 45 countries, but not in USA) and has been shown to be efficacious in patients with CHF.
Table 1 22-27 summarizes the clinical trials that have evaluated IV iron therapy for the treatment of anemia and/or ID in patients with CHF. Bolger et al. treated 16 patients with CHF and IDA with intravenous iron and found a significant increase in hemoglobin (from 11.2 ± 0.7 g dL−1 to 12.6 ± 1.2 g dL−1), improvement in the New York Heart Association (NYHA) functional class, improvement in HRQoL scores and a significant increase in the distance walked in the 6 min walking test (6MWT) 22.
Toblli et al. treated 40 patients with CHF and IDA randomized to intravenous iron or placebo. The group treated with iron demonstrated a significant increase in hemoglobin (10.3 ± 0.6 g dl−1 to 11.8 ± 0.7 g dl−1), improvement in renal function, improvement of HRQoL measured with the Minnesota living with heart failure questionnaire (MLHFQ), increase in the distance walked in the 6MWT, reduction in natriuretic peptides concentration, and improvement of left ventricular ejection fraction (LVEF) 23.
The effect of intravenous iron sucrose on exercise capacity in chronic heart failure (FERRIC HF) study was a multicenter, randomized, single blind study of 35 patients with CHF and IDA. Patients were randomized 2:1 for16 weeks of intravenous iron (200 mg weekly until ferritin >500 ng ml−1, 200 mg monthly thereafter) or no treatment. The primary end point was the change in absolute peak VO2. Intravenous iron repletion improved exercise capacity and symptoms in patients with CHF and the benefits were more pronouncedin anemic patients 24.
The Ferinject ™ Assessment in Patients with iron deficiency and chronic heart failure (FAIR-HF) was a multicenter, randomized, double-blind, placebo-controlled phase III in ambulatory patients with CHF and ID, with or without anemia 26. The treatment group received FCM 200 mg weekly, followed by 200 mg of FCM monthly. The double primary endpoint included changes in the Patient Global Assessment (PGA) and in NYHA functional class at week 24. Secondary objectives were HRQoL (Kansas City Cardiomyopathy Questionnaire (KCCQ) and EQ-5D instrument) and the distance walked in the 6MWT, assessed at weeks 4 and 12 and 24. Outcome end-points also included impact on hospitalizations and mortality. Fifty percent receiving FCM reported being much or moderately improved, compared with 28% receiving placebo, according to the PGA. Among the patients assigned to FCM, 47% had an NYHA functional class I or II at week 24, as compared with 30% assigned to placebo. The distance covered in 6-min test was also significantly higher in patients treated with FCM. No differences were seen in the rate of death or adverse events. Improvement in these parameters was seen in ID patients with and without anemia.
A sub-study of the FAIR-HF56 analyzed with greater detail the effects of intravenous iron repletion with FCMforHRQoL assessed at baseline and after 4, 12, and 24 weeks of therapy. FCM significantly improved VAS and KCCQ (mean differences from baseline in KCCQ overall, clinical and total symptom scores, P < 0.001 vs. placebo) at all-time points. At week 24, significant improvement vs. placebo was observed in mobility (P = 0.004), self-care (P < 0.001), pain/discomfort (P = 0.006), anxiety/depression (P = 0.012), and usual activity (P = 0.035). Effects were present in both anaemic and non-anaemic patients. Additional data from the FAIR-HF have shown beneficial effects of intravenous iron treatment for renal function in patients with CHF 57. Compared with placebo, FCM therapy was associated with an improvement in eGFR.
The beneficial effects of iron repletion with intravenous iron in patients with CHF with ID observed in the FAIR-HF study were confirmed in the Ferric Carboxymaltose on Performance Evaluation in Patients with iron deficiency in Combination With Chronic Heart Failure (CONFIRM-HF) 27, designed to confirm long-term safety and efficacy. The CONFIRM-HF was a multi-center, double-blind, placebo-controlled trial in ambulatory symptomatic patients with CHF with LVEF ≤45%, elevated natriuretic peptides, and ID. Patients were randomized to treatment with FCM or placebo for 52 weeks. The primary end-point was the change in 6MWT distance from baseline to week 24. Secondary end-points included changes in NYHA class, PGA, 6MWT distance, HRQoL, Fatigue Scores, and on the hospitalization rate for worsening CHF. Treatment with FCM significantly prolonged 6MWT distance at week 24. The treatment effect of FCM was consistent in all subgroups and was sustained to week 52. Throughout the study, the improvement in NYHA class, PGA, HRQoL, and Fatigue Score in patients treated with FCM was significant. Treatment with FCM was associated with a significant reduction in the risk of hospitalizations for worsening CHF. The number of deaths (FCM: 12, placebo: 14 deaths) and the incidence of adverse events were comparable between both groups. Thus, treatment of symptomatic, iron-deficient patients with CHF with FCM over a 1-year period resulted in sustainable improvement in functional capacity, symptoms, and QoL and reduction in the risk of hospitalization for worsening CHF.
Cost effectiveness of treatment with intravenous iron in patients with CHF and iron deficiency has been evaluated in several studies using data from the FAIR-HF trial and modeling it according several European health systems. In these models, the use of FCM for the treatment of ID in patients with CHF was cost-effective since the incremental cost-effectiveness ratio of FCM use was below the threshold of cost per QALY gained suggested by the UK National Institute for Health and Clinical Excellence 58, 59. Overall, the evidence for the relative merits of oral versus intravenous iron, has been evolving, and now suggests that prompt intervention using IV iron therapy should now be considered 60, 61.
Conclusion
Routine detection and management of ID and anemia in patients with CHF remain unmet medical needs. Anemia and iron deficiency have been confirmed to be highly prevalent in patients with CHF. Intravenous iron therapy improves anemia, cardiac function and exercise tolerance, leading to improvement in quality of life. Anemia management has also been demonstrated to be cost-effective. Clinical care pathways to manage anemia in patients with CHF are recommended as best practices in order to improve patient outcomes. It is noteworthy that the recently published European Society of Cardiology Heart Failure Management guidelines has gained a recommendation II A level of evidence for the treatment of ID with IV iron therapy in patients with CHF and reduced ejection fraction 62. With the evidence presented, AMCO is calling for adoption of anemia detection and management guidelines in patients with CHF as best practices in order to improve patient outcomes.




