Fludarabine and cytarabine versus high-dose cytarabine in consolidation treatment of t(8; 21) acute myeloid leukemia: A prospective, randomized study
Author contributions: L.R. collected and verified patient information, analyzed and interpreted data, and wrote the manuscript; H.X. collected clinical and immunophenotypic data, interpreted data, and wrote the manuscript; analyzed data, performed statistical analysis and verified patient information; L.W., H.C., S.L., W.Z., J.W., and J.Y. recruited the patients, performed diagnosis and treatment; and X.S. is the principal investigator, designed research, interpreted data, and critically reviewed the manuscript.
Ruiqi Li and Xiaoxia Hu contributed equally to this work.
Abstract
Acute myeloid leukemia (AML) patients with t(8;21) aberration often have favorable outcomes, however, relapse still occurs in 30–40% patients, with only 50–60% of patients with t(8;21) AML cured with regimens containing high-dose cytarabine (HD-Ara-C). To evaluate the effects of fludarabine and cytarabine (FA) consolidation therapy for t(8;21) AML patients, a prospective randomized study was performed. A total of 45 patients with t(8;21) AML after achieving complete remission (CR) were randomly assigned to receive four course consolidation with FA (n = 23) or HD-Ara-C (n = 22). Our study showed that at 36-months, relapse-free survival (RFS) was 81.73% in the FA arm and 50.73% in the HD-Ara-C arm (P = 0.04), overall survival (OS) was 91.1% and 48.4% (P = 0.01) in the FA arm and in the HD-Ara-C arm respectively; whereas cumulative incidence of relapse (CIR) was 18.27% and 47.39%, in the FA arm and in the HD-Ara-C arm respectively (P = 0.05). In our study, treatment with FA, MRD2 status (reduction ≥ 3-log) and absence of c-kit mutations were identified as independent prognostic factors for lower risk of relapse, improved RFS and OS. We also found RFS for patients without c-kit mutations was 100% in FA arm, and 57.8% in HD-Ara-C arm at 36 months (P = 0.005); OS of both groups at 36 months was 100% and 51.4%, respectively (P = 0.004), suggesting a benefit of consolidation therapy with FA for t(8;21) AML patients, especially, those without c-kit mutations (Clinicaltrials.org ID NCT# 02024308). Am. J. Hematol. 92:12–17, 2017. © 2016 Wiley Periodicals, Inc.
Introduction
The aberration of t(8;21) found in patients with acute myeloid leukemia (AML), which leads to RUNX1-RUNX1T1 (formerly known as AML1-ETO) rearrangement, is one of the most common cytogenetic abnormalities with a frequency of ∼7% in de novo adult AML patients 1. According to the World Health Organization classification, core-binding factor AML (CBF-AML), CBFα-AML[t(8;21)(q22;q22)AML] and CBFβ-AML [inv(16)(p13q22)/t(16;16)(p13;q22) AML] are recognized as unique entities within the category of “AML with recurrent genetic abnormalities” 2. According to high remission rates and survival probabilities, CBF-AML is considered as a favorable AML risk group, but relapse still occurs in 30–40% patients with only 50–60% of patients with t(8;21) cured with standard high-dose cytarabine (HD-Ara-C) based regimens 3. To improve treatment and outcomes, it is necessary to identify patients unlikely to respond to standardized therapies based on their genetic abnormalities; thereby developing novel and effective therapeutic approaches for such patients.
In recent years, numerous prognostic factors have been identified to be correlated with a high risk of relapse, such as age, hyperleucocytosis, CD56 expression, additional cytogenetic aberrations (such as loss of chromosome Y), gene mutations such as c-kit and FLT3-ITD, and minimal residual disease (MRD). As reported, c-kit is the most common secondary genetic alteration in CBFα-AML with a frequency of 12–46% 4, 5. Although the clinical significance of c-kit mutations in CBFα-AML has been intensively studied, the results from different study groups are conflicting. For example, in current NCCN guidelines, c-kit mutations are considered as a negative prognostic factor, while in the International European Leukemia Net (ELN) 6, c-kit mutational status is not considered as a prognostic factor.
Conversely, a retrospective analysis suggested that improved event-free survival (EFS) in CBF-AML was due to induction treatment with fludarabine and Ara-C (FA) or FA + granulocyte colony stimulating factor (G-CSF) (FLAG), rather than idarubicin and Ara-C (IA) 7, 8. It has been reported that in the Medical Research Council (MRC) AML 15 trial, two cycles of induction therapy with FLAG-idarubicin (FLAG-Ida), followed by two cycles of HD-Ara-C consolidation can improve the survival time of patients with favorable-risk AML 9. To evaluate the effects of FA versus HD-Ara-C on consolidation therapy for AML, as well as the prognostic value of c-kit mutations, we conducted a prospective randomized study by recruiting 45 patients with previously untreated CBFα-AML after achieving complete remission (CR). This clinical trial was registered at www.clinicaltrials.org (NCT# 02024308).
Methods
Patients and study designs
This study recruited fifty patients aged 13–66 years and diagnosed as CBFα-AML in our institute between September 2009 to May 2015. CBFα-AML was defined by the presence of either the t(8;21) (q22;q22) translocation by karyotype and/or fluorescence in situ hybridization (FISH) analysis and/or evidence of RUNX1-RUNX1T1 fusion transcript. Our study was an open label, randomized-control, comparative study. The sample size of the present study was pre-calculated, according to a probable relapse-free survival (RFS) difference of 20% between FA and HD-Ara-C arms. Eligible patients were randomly assigned by computer software to received FA and HD-Ara-C treatment at a ratio of 1:1. Therefore, 45 patients with CBFα-AML who achieved CR after induction chemotherapy were randomly assigned in the trial (Fig. 1). Our study, approved by the Ethics Committee of Changhai Hospital, was conducted in accordance with the Declaration of Helsinki. We obtained written informed consent from all recruited patients (or their legal guardians). All patients were followed up from the time when their diagnosis was made until the end of November 2015.
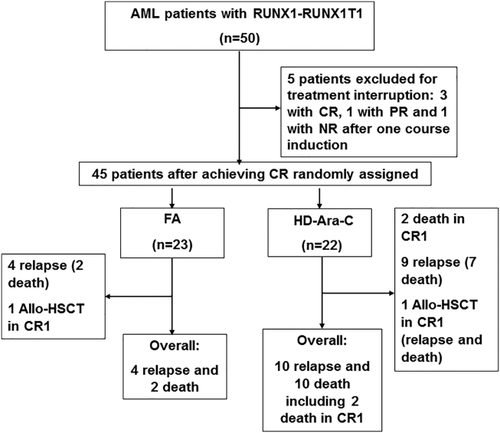
Trial flowchart.
Induction therapy
All patients with CBFα-AML received induction therapy with DA or IA regimens (D: daunorubicin 60 mg m−2 per day 1–3; I: idarubicin 8 mg m−2 per day 1–3; A: cytarabine 100–200 mg m−2 per day 1–7). If patients did not achieve CR, up to two cycles of induction therapy were allowed, i.e. patients who achieved partial remission (PR) with one cycle of induction were re-induced with the same regimen. If patients did not achieve PR with one cycle of induction, they received HAG regimen which comprised of homoharringtonine (HHT, 1 mg day−1, intravenously, on days 1–14); cytarabine (25 mg m−2 per 24 hr divided into twice, subcutaneously, on days 1–14), and G-CSF (5 µg kg−1 day−1 from day 0 until the neutrophil counts above 1.5 × 109 cells/L) 10.
Consolidation therapy
Patients who achieved CR were randomly assigned into the consolidation group of FA or HD-Ara-C arms with four monthly cycles. In this study, FA regimen was comprised of fludarabine (30 mg m−2 by 30-min infusion on days 1–5) and cytarabine (1.4 g m−2 infusion starting 3.5 hr after fludarabine for over 4 hr on days 1–5), while HD-Ara-C contained cytarabine (2 g m−2/12 h for six doses). According to our trial design, if the patients with c-kit mutations had matched related or unrelated donors, they received allogeneic hematopoietic stem cell transplantation (allo-HSCT) after one course of consolidation therapy with myeloablative conditioning regimen. Further, trimethoprim-sulfamethoxazole and acyclovir were routinely used for prophylaxis for pneumocystis carinii and herpes virus infections, respectively, in both groups after each consolidation course.
Evaluation for minimal residual disease (MRD) and criteria for response
Bone marrow samples were collected at the time for diagnosis and before each cycle of chemotherapy. To monitor the RUNX1-RUNX1T1 transcripts in patients with CBFα-AML, their MRD levels were serially examined by real-time quantitative polymerase chain reaction, as described previously 11. Results were expressed as a [fusion gene/ABL1] × 100 transcript ratio. The examination of MRD level was performed using BM samples, which were collected before initiation of the first, second, third and fourth consolidation courses, namely MRD1, MRD2, MRD3, and MRD4, respectively. After completion of treatment, MRD evaluation was performed every 3 months for 3 consecutive years.
The criteria for the response to treatment, including CR, PR and relapse were defined according to previous reports 12. In addition, complete molecular response (CMR) was defined as a transcript ratio ≤0.001% 13, and patients with molecular recurrence were defined by an MRD ratio increase of more than 1-log on two successive samples 14.
End points and statistical methods
The primary end point used in our study was relapse and relapse-free survival (RFS). The secondary end point was overall survival (OS). Here, OS was defined as the time from study entry until death or date last known alive; RFS was defined as the time from induction CR until relapse or death; and cumulative incidence of relapse (CIR) was defined as the time from the CR date to date of relapse, death, or date last known alive, for which death in CR was considered as relapse.
Clinical variables across treatment groups were compared using two statistical methods: (1) χ2 or a two-sided Fisher exact test for categorical variables; (2) nonparametric Mann–Whitney U test for continuous variables. P values below 0.05 were considered to be statistically significant. Estimated probabilities for OS and RFS were calculated by the Kaplan–Meier method 11, and the log-rank test was used to assess differences between survival curves. Estimates of CIR were calculated only for patients achieving a CR, and Gray's k-sample test was applied to evaluate differences of relapse rates between patients 15, Both sub-distribution hazard ratio of relapse (SHR) and hazard ratio (HR) were given with 95% confidence interval (CI). We also calculated estimation and comparison of stratum-specific survival distributions across stratified groups. This included treatment arms, MRD-2 response and c-kit mutations. For multivariate analysis of prognostic factors, Cox proportional hazards regression models using forward selection were constructed for analysis of OS and RFS, meanwhile proportional hazards assumption was graphically checked. Furthermore, multivariable models using Gray's method were constructed for CIR. Variables with P value <0.1 in univariate analysis were selected and used in multivariate models. The differences in CR rates and MRD responses between treatment groups were compared using Fisher's exact test. All statistical analyses were performed using software R (version 3.1.3).
Results
Patient characteristics
Fifty patients with AML (median age 42 years, range 13–66) were diagnosed and recruited during the study period. RUNX1-RUNX1T1 transcript was detected in these patients. 24% (12/50) patients were found to have c-kit mutations. Among them, seven patients had exon 17 mutations, three with exon 8 mutations, and two with hybrid mutations.
All except two patients achieved CR (CR rate: 96%, 48/50), however three CR patients (6.25%, 3/48) were excluded from this study, due to discontinuation of therapy. Five cases with PR after the first cycle of induction therapy achieved CR by second cycle of induction. Five cases with non-response (NR) were re-induced with HAG regimen and achieved CR. Two cases (one with PR and another with NR) discontinued reinduction therapy. Taken together, 69.57% (16/23) and 81.82% (18/22) of patients in FA arm and HD-Ara-C arms achieved complete remission, respectively, after the first course of induction therapy (P = 0.339).
Finally, 45 patients recruited in the study entered the randomized study. The cytogenetic status of 98% patients (44/45) in randomized study was known, however, the RUNX1-RUNX1T1 fusion transcript was detected in two patients with a normal karyotype. 40.91% (18/44) of patients had additional cytogenetic abnormalities, in which loss of Y chromosome was the most common, accounting for 31.82% (14/44). The actual rates of realization of proposed consolidation were 91.30% (21/23) in FA arm and 95.45% in HD-Ara-C arm (21/22), respectively. The characteristics of patients by treatment arms are shown in Table I.
| FA | HD-Ara-C | P | |
|---|---|---|---|
| No. patients | 23 | 22 | |
| Male/female, n | 12/11 | 12/10 | 0.87 |
| Median age, y (range) | 46 (23–62) | 37.5 (13–64) | 0.11 |
| Median WBC count, ×109/L (range) | 9.9 (0.92–51.76) | 12.72 (0.65–149.15) | 0.58 |
| Median HGB level, g L−1 (range) | 68 (42–126) | 70.5 (50–135) | 0.69 |
| Median platelet count, ×109/L (range) | 35 (4–155) | 29 (10–74) | 0.19 |
| Median blasts in bone marrow (%) (range) | 54 (16–93) | 58.5 (21.5–95.5) | 0.71 |
| De novo AML, No, (%); Secondary AML,a no. (%) | 21 (91.3); 2(8.7) | 22 (100); 0 (0) | 0.49 |
| Additional cytogenetic abnormalities (Loss of Y), no. (%) | 7 (30.43) | 7 (31.82) | 0.92 |
| Gene mutation, no. (%) | |||
| Mutated c-kit | 6 (26.09) | 4 (18.18) | 0.72 |
| CD56 positive, no. (%) | 16 (72.73) | 16 (76.19) | 0.79 |
| lactate dehydrogenase (LDH) | 396 (131–3545) | 488 (109–3285) | 0.56 |
| Median fusion transcript ratio at diagnosis (%) | 83.03 (34.86, 892.7) | 160.45 (29.32,609.31) | 0.41 |
- a Secondary AML: AML associated with chemotherapy agents. AML = acute myeloid leukemia, FAB = French-American-British, WBC = white blood cells, HGB = hemoglobin, FA = fludarabine and cytabine, HD-Ara-C = high dose cytarabine.
Clinical outcomes
The median follow-up of patients in this study was 37.5 months (range, 5.5–77.5 months). During that period of time, 12 patients died, including 2 who achieved CR1 (HD-Ara-C arm). One patient died early due to perianal infection after consolidation therapy and another died of pulmonary infection during re-induction chemotherapy for molecular relapse. Relapse occurred in 14 patients, including 4 in FA arm and 10 in HD-Ara-C arm. Among the 14 relapsed cases, 10 patients died (2 in FA arm; 8 in HD-Ara-C arm). With respect to further therapy, 8 patients received allo-HSCT with a myeloablative conditioning regimen, including 2 patients with c-kit mutations in CR1 (one each in FA and HD-Ara-C arm), 4 in CR2 (1 in FA arm; 3 in HD-Ara-C arm) and 2 in relapse (HD-Ara-C arm). Among 10 cases with c-kit mutations, 8 patients did not receive allo-HSCT during CR1, including 4 cases abandoning further therapy, 3 cases without matched donors and 1 case continuing to be in remission for 2 months.
Clinical outcomes of patients in the study are illustrated in Fig. 2. Data analysis showed that at 36 months, CIRs were 18.27% (95%CI, 5.473–36.976%) in patients of FA arm, but 47.39% (95%CI, 24.47–67.30%) in those of HD-Ara-C arm, (Fig. 2A). Although the difference of CIRs between the two arms did not reach statistical significance, there is a marked trend in an increased CIR in the HD-Ara-C arm (SHR: 0.339, 95%CI (0.109, 1.06), P = 0.05 by the Fine and Gray' test). Moreover, at 36 months, RFS was 81.73% (95% CI, 67–99.7%) in FA arm, and 50.73% (95% CI, 32.8–78.5%) in HD-Ara-C arm. Comparison showed RFS in FA arm was higher than that in HD-Ara-C arm (HR: 0.323, 95%CI (0.101, 1.032), P = 0.04 by the log-rank test) (Fig. 2B). Finally, OS of the patients with CR was 91.1% (95%CI, 80–100%) in FA arm at 36 months, but 48.4% (95%CI, 30–78%) in HD-Ara-C arm, suggesting that survival was higher in the FA arm (HR:0.185, 95%CI (0.041, 0.846), P = 0.01 by the log-rank test) (Fig. 2C). Enrollment of 45 patients (23 on FA and 22 on HD-Ara-C) was at least 99% power to detect a 0.205 hazard ratio of FA vs HD-Ara-C at a two-sided significance level of 0.05 with 48-month accrual time and 84-month total time in a power calculation for OS.
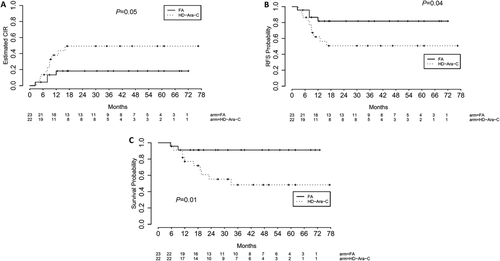
Clinical outcomes by treatment arms. A: Cumulative incidence of relapse (CIR); B: Relapse-free survival (RFS); C: Overall survival (OS) from CR. FA = fludarabine and cytabine, HD-Ara-C = high dose cytarabine.
MRD level for evaluating response to treatment
The transcript of RUNX1-RUNX1T1 fusion gene as a marker of minimal residual disease (MRD) was evaluated in 37 patients (FA arm: 22; HD-Ara-C arm: 15). The end point of MRD level reduction (targeted reduction level) was defined as a ≥ 3-log reduction between diagnosis and any checkpoints 16. The percentage of patients reaching targeted reduction level were 37.84% (14/37 cases) at MRD1, and 78.38% (29/37 cases) at MRD2. Furthermore, 72.97% of patients at MRD3 and 81.08% of patients at MRD4 reached targeted reduction levels. These values were similar to that at MRD2.
MRD level (MRD reduction ≥3-log vs. < 3-log) at MRD1 could not discriminate high-risk relapse patients (P = 0.11), but MDR level at MRD2 had the ability to discriminate against high-risk relapse patients (P = 0.01, as shown in Supporting Information Table SI). However, MRD level at other checkpoints (MRD3, MRD4) did not correlate with relapse risk. The percentage of patients who had c-kit mutations and reached a targeted reduction level at MDR2 (55.56%) was less than those without c-kit mutations (85.71%), however, the difference between these two groups was not significant (P = 0.08).
Univariate prognostic analysis
Univariate prognostic analyses for SHR, RFS, and OS are shown in Supporting Information Table SII. Using Cox proportional hazards regression models, four factors were demonstrated to have a significant impact or a marked trend toward a higher SHR, including (1) c-kit mutations (SHR, 5.39 [95%CI, 1.94-15], P = 0.001); (2) additional cytogenetic abnormalities (loss of Y chromosome): SHR 2.74, [95% CI, 0.991–7.6], P = 0.05; (3) higher WBC counts: SHR 2.38, [95%CI 0.913–6.18], P = 0.07; (4) higher bone marrow blasts (%): SHR 1.03, [95% CI 1–1.05], P = 0.04. The results of univariate analysis for RFS were similar to that of SHR, except that a higher LDH increased the HR of RFS. A similar analysis was also performed for OS of patients achieving CR. Two positive prognostic factors were identified for a longer survival: (1) MRD2 reduction ≥ 3-log: HR 0.134, [95% CI, 0.032–0.567], P = 0.006; (2) FA treatment: HR 0.0565, [95% CI, 0.041–0.846], P = 0.03.
Multivariate prognostic analysis
According to the results of univariate prognostic analysis, total eight covariates were selected for multivariate analysis, including: treatment arm, WBC (log), c-KIT mutations, MRD reduction ≥3-log (Table II).
| SHR | RFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SHR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Treatment arm (FA vs HD-Ara-C) | 0.170 | 0.0444, 0.647 | 0.009 | 0.108 | 0.017, 0.681 | 0.02 | 0.205 | 0.039, 1.075 | 0.06 |
| WBC (log)a | – | – | – | 3.746 | 0.822, 17.070 | 0.09 | 7.833 | 1.620, 37.866 | 0.01 |
| c-kit mutations (With vs. without) | 12.368 | 3.9332, 38.894 | <0.001 | 18.806 | 3.277, 107.938 | 0.001 | – | – | – |
| MRD2 reduction≥ 3-log(With vs. without) | 0.166 | 0.0410, 0.672 | 0.01 | 0.140 | 0.025, 0.783 | 0.03 | 0.067 | 0.010, 0.424 | 0.004 |
- Cox proportional hazards regression models using Forward selection adjusted other factors in univariate analysis. FA = fludarabine and cytarabine; HD-Ara-C = high dose cytarabine; SHR = subdistribution hazard of relapse; HR = hazard ratio, RFS = relapse free survival; OS = overall survival; LDH = lactate dehydrogenase. WBC = white blood cells, MRD = minimal residual disease. aWBC belongs to continuous data in the statistical analysis.
Multivariate prognostic analysis showed that treatment regimen (FA versus HD-Ara-C), MRD2 status (reduction ≥ 3-log vs. reduction < 3-log) and c-kit status (mutated versus wild-type) were independent prognostic factors that influence SHR of relapse and RFS. Among those factors: (1) FA treatment decreased the CIR, as compared to HD-Ara-C (P = 0.05) (Fig. 2A); (2) CIR at 12 months in patients with MRD2 reduction ≥ 3-log (10.795%) was significantly lower than those with MRD2 < 3-log (53.125%, P < 0.001, Supporting Information Fig. S1A); (3) Patients with c-kit mutations were prone to relapse, as compared to those without c-kit mutation (CIR at 36 months: 73.333% versus 21.497%, P = 0.001 Supporting Information Fig. S2A). The RFSs for treatment regimens, MRD2 status and c-kit mutation status are shown in Fig. 2B, Supporting Information S1B and S2B, respectively.
Treatment regimen, MRD2 status and WBC count were identified by multivariate analysis as three independent prognostic factors that influence OS. Furthermore, none of patients in FA arm without c-kit mutations relapsed, thus OS of these patients was 100% (Supporting Information Table SIII, S4 and Fig. S3).
Toxicity profile
In our study, myelosuppression was similar in both consolidation treatment arms. During first course of consolidation therapy, the median duration of agranulocytosis was 8 days in both groups (P = 0.17), and the median duration of platelets <20 × 109/L was 7 and 6.5 days in FA arm and HD-Ara-C arm, respectively (P = 0.61) (Supporting Information Table SV). The median duration of agranulocytosis was 7.5 days in FA arm and 6 days in HD-Ara-C arm respectively (P = 0.23) after the second course of consolidation therapy; and the median duration of platelets <20 × 109/L was also similar in two arms (P = 0.55) (Supporting Information Table SV). The intensity and incidence of liver dysfunction were similar in two arms (P > 0.05) (Supporting Information Table SV). The frequency of invasive fungal disease in FA arm was 30.43% (7/16 cases), and similar frequency of that in HD-Ara-C arm 27.27% (6/16) was found (P = 0.82).
Discussion
This trial was a pilot study to prospectively compare the effects of FA versus HD-Ara-C on consolidation therapy for adult CBFα-AML patients. Our study demonstrated that FA consolidation could decrease relapse risk and improve the RFS and OS of AML patients with t(8;21), in particular, those without c-kit mutations, compared to patients treated with HD-Ara-C. Conversely, FA consolidation did not show prolonged myelosuppression or infection. Our results are consistent with previous reports that FA had advantages over HD-Ara-C in CBFα-AML. A retrospective comparison study indicated that compared with IA treatment, a FLAG-based regimen provided higher EFS in patients with CBFα-AML. Among 107 patients achieving CR, relapse occurred in 32% of cases treated with FLAG, and 33% of cases with FA, but 52% cases with IA/IAG 8. The Medical Research Council AML15 trial showed that 8-year survival was 95% for AML patients in a favorable risk group and they received FLAG-Ida induction followed by two courses of HD-Ara-C, however, only 23 cases from all recruited patients were in this group 9. Recently, therapy combined with FLAG and low dose of gemtuzumabozogamicin (FLAG-GO) for induction and consolidation achieved 78% 3-year OS and 85% RFS 17. These results were in line with our findings (81.73% 3-year RFS and 91.1% OS), although GO was incorporated and the frequency of c-kit mutations was only 9% in this cohort. Twenty-four hour doses of fludarabine and cytarabine in the FLAG-GO regimen were reduced compared with the 5 day standard FA regimen 17, which may account for the decreasing the effect of FA consolidation therapy in CBFα-AML patients. Based on previous reports and our data, consolidation therapy with FA should be considered for all CBFα-AML patients.
The rationale for designing an FA regimen in AML was based on preclinical data, which showed that the anti-leukemic effects of treatment are positively associated with increasing intracellular 5′ triphosphate of cytarabine (ara-CTP) levels induced by fludarabine and G-CSF. Randomized studies have also indicated that consolidation therapy with repeat high-doses of cytarabine could reduce the likelihood of relapse 18, indicating sensitivity to cytarabine in CBFα-AML. Increasing ara-CTP levels through treatment with HD-Ara-C was also the reason for improving survival in AML patients. Unfortunately, treatment with high dose of Ara-C (>2 g m−2) is associated with significant cytotoxic effects, which also decrease the ability of leukemic cells to transform cytarabine into ara-CTP 19. As fludarabine administration prior to cytarabine increased intracellular ara-CTP accumulation, this may be the principal mechanism by which fludarabine in combination with intermediate-dose cytarabine improves survival in CBF-AML patients 20.
Hierarchical evaluation of leukemia-related risk factors, such as genetic anomalies, as well as response-related risk factors, for example MRD, has been applied in management of AML. Treatment according to MRD level (RUNX1-RUNX1T1 transcript level) has recently been stratified in patients with t(8;21) AML and has improved survival in high-risk patients who received allo-HSCT 21. According to our multivariate analysis, FA treatment and MRD2 reduction ≥3-log were identified as independent prognostic factors for risk of relapse, RFS and OS, while c-kit mutations were an independent prognostic factor for the risk of relapse and RFS, but not OS. The prognostic factors that came up to be significant in univariate and multivariate analysis for RFS/SHR are different in present study (Supporting Information Table SII, Table II). This is likely because of the small number of cases and multiple variables being tested. Previous studies found that MRD reduction <3-log after first course consolidation treatment was a significant predictor of relapse after achieving CR 16. However, findings in the AML05 trial suggest that MRD status after the second consolidation helped discriminate patients with a high-risk of relapse, based on the results from 116 eligible patients 21. In this trial, the relative lower intensity of induction and consolidation may be responsible for a delayed prognosis checkpoint. In our trial, we identified MRD2 reduction <3-log as an independent prognostic factor that influences patients with high SHR for relapse (Supporting Information Table SI, Table SIII; Fig. S1) Recently, Willekens et al. has demonstrated that the persistence of a detectable BM-MRD is not associated with an increased risk of relapse, while a detectable peripheral blood (PB)-MRD at the end of consolidation treatment is significantly associated with a higher risk of relapse and shorter survival times. During 2-year follow-up, the cumulative incidence of relapse is only 8.2% for patients with the persistence of peripheral blood complete molecular remission 13. The correlation of PB-MRD reduction with BM-MRD reduction, as well as the relation of PB-MRD reduction and complete remission with prognostic values need to be analyzed in future studies.
The prognostic values of c-kit mutations are controversial across studies 4, 5, 16. Duployez et al. recently reported that c-kit mutations were associated with a significantly high CIR in t(8;21) AML patients, especially in patients with a mutant allelic ratio of 35% or greater 22. In our study, c-kit mutations was identified as an independent prognostic factor that can increase relapse risk and decrease RFS, but have no impact on OS (Supporting Information Fig. S2). The differences in findings between studies may relate to the small numbers of patients, the wide range of cohorts, variations in mutations and different proportions of patients with a higher mutant allelic ratio. Furthermore, differences in race may also impact on the prognostic value of c-kit mutations 23. The majority of CBFα-AML patients with relapse could achieve second remission with salvage chemotherapy thereby increasing survival. This observation accounts for the reason c-kit mutations had no impact on OS in our trial. The results in our study showed the frequency of MRD2 reduction ≥3-log in patients with c-kit mutations was lower than patients without c-kit mutations. However, this difference was not significant, perhaps due to the lower number of patients (85.71% versus 55.56%, P = 0.08). Our findings are consistent with previous reports, in which patients with c-kit mutations did not readily achieve molecular remission 21.
Finally, MRD2 status correlated with c-kit mutation status and treatment regimens. Patients without c-kit mutations and an MRD2 reduction ≥3-log had the highest OS (84.3%) and lowest CIR (4.792%). In contrast, patients with c-kit mutations and MRD2 reduction ≥3-log suffered from a high CIR (70%), as shown in Supporting Information Table SIII, suggesting that a patient with MRD2 reduction ≥3-log cannot ameliorate the negative effects of c-kit mutations. Patients without c-kit mutation in FA arm did not relapse, therefore OS was 100%, and however, patients without c-kit mutations in HD-Ara-C arm had a high rate of relapse (40.28%) and a low OS (51.4%), as shown in Supporting Information Table SIV and Supporting Information Fig. S3. However, 66.67% of patients with c-kit mutations relapsed with FA treatment (Supporting Information Table SIV). These results suggest that FA therapy could confer higher RFS, and in turn, superior OS, for patients with CBFα-AML. However, treatment with FA treatment could not overcome the negative effects of c-kit mutations on CBFα-AML. Our results suggest that treatment with an FA regimen may be an optimal therapy for newly diagnosed CBFα-AML patients, in particular, those lacking c-kit mutations.
Acknowledgments
The sponsors did not influence how the study was conducted or the approval of the manuscript. They thank Wei Guo PhD and associate professor Cheng Wu from the Department of Health Statistics, Second Military Medical University for statistical analysis in our study.




