Histological transformation to diffuse large B-cell lymphoma in patients with Waldenström macroglobulinemia
Abstract
Histological transformation to diffuse large B-cell lymphoma (DLBCL) rarely occurs in patients with Waldenström Macroglobulinemia (WM). We identified 20 patients out of a cohort of 1,466 WM patients who experienced histologic transformation. The 5, 10, and 15-year cumulative incidence rates were 1, 2.4, and 3.8%, respectively. Approximately half of the patients were naive to nucleoside analogues, and a quarter were previously untreated for WM at the time of transformation. More than 80% of patients presented with extranodal involvement, 65% with high IPI scores. DLBCL cells did not express CD10 but expressed BCL6 and BCL2. All patients were treated with chemoimmunotherapy. The median survival from histological transformation was 2.7 years. The median overall survival was shorter for transformed patients versus those who did not transform (estimated 9 vs. 16 years; P = 0.09). Histological transformation to DLBCL is rare, and is associated with inferior survival in WM. Am. J. Hematol. 91:1032–1035, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
Waldenström macroglobulinemia (WM) is a rare lymphoma characterized by the malignant growth of IgM-secreting lymphoplasmacytic lymphoma cells in the bone marrow and other organs 1. Although the survival outcomes of patients with WM have improved over the last decade 2, 3, there is an increased risk of developing second malignancies, including melanoma and cancers of the thyroid, lung and genitourinary tract as well as acute myeloid leukemia and diffuse large B-cell lymphoma (DLBCL) 4. Furthermore, the outcomes of patients with WM who develop DLBCL appear worse than for patients with DLBCL without antecedent WM 5.
Transformation to more aggressive histological lymphoma subtypes, such as DLBCL, has been described in patients with WM 6-9. However, the incidence and outcome of patients with WM who experience histological transformation remain largely unclear. The objective of this retrospective study was to describe the cumulative incidence, clinicopathological characteristics and outcomes of patients with WM who experienced histological transformation.
Methods
We performed a retrospective chart review in patients seen at our Center between January 2000 and December 2014. Patients with a clinicopathological diagnosis of WM 10, and a more aggressive histological lymphoma subtype diagnosed concurrently or after the diagnosis of WM were included in our study. Pertinent clinical and pathological data were gathered and analyzed using descriptive statistics. For WM response assessment, we used the response criteria updated at the 6th International Workshop on WM 11. For DLBCL response assessment, we used the 2007 Cheson response criteria, whenever possible 12. For the immunohistochemical classification of DLBCL, we used the algorithm proposed by Hans et al. 13. The presence of the MYD88 L265P gene mutation was detected by polymerase chain reaction methods, as previously described 14.
The time from the diagnosis of WM to histological transformation, defined as the time in years between the diagnosis of WM and the diagnosis of transformation, the survival from histological transformation, defined as the time in years from diagnosis of transformation to last follow-up or death, and the survival from WM diagnosis, defined as the time in years between WM diagnosis to last follow-up or death, were estimated using the Kaplan-Meier method 15. Univariate survival comparisons were made using the log-rank test 16. All calculations and graphs were obtained using STATA/SE 13.1 (StataCorp, College Station, TX).
Results
A total of 20 patients who had histological transformation were identified in a cohort of 1,466 WM patients. The clinical characteristics are shown in Table 1. All patients experienced histological transformation to DLBCL. The median time from diagnosis of WM to histological transformation was 4.4 years (95% CI 2.6–8 years). The cumulative incidence of histological transformation in WM patients was 1% at 5 years, 2.4% at 10 years, and 3.8% at 15 years (Fig. 1A). Fifteen patients had received treatment prior to histological transformation but five patients (25%) were treatment-naïve at the time of transformation. Histological transformation was diagnosed in six (33%) patients while on active WM-directed therapy. Of the 13 patients previously exposed to rituximab, 9 patients transformed >6 months from the most recent dose of rituximab, and 4 while on active rituximab therapy. Only two patients were not previously exposed to an alkylating agent and/or nucleoside analogue.
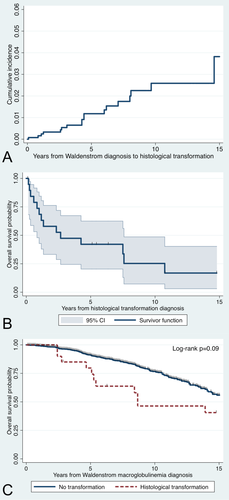
Cumulative incidence curve from Waldenström macroglobulinemia diagnosis to histological transformation (A); overall survival curve of WM patients from histological transformation (B); and overall survival curves of WM patients from diagnosis of WM (C). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| Characteristic | Number (% or range) |
|---|---|
| Median age at Waldenström diagnosis (years) | 62 (40–75) |
| Median age at transformation (years) | 70 (41–84) |
| Age ≥60 years | 17 (85%) |
| Male sex | 12 (60%) |
| ECOG performance status >1 | 6 (35%) |
| Elevated LDH levels | 10 (67%) |
| Extranodal involvement | 16 (84%) |
| Stage III or IV | 10 (59%) |
| Median number of lines of therapy prior to transformation | 1 (0–5) |
| Previous therapies | |
| Rituximab | 13 (87%) |
| Alkylating agents | 11 (73%) |
| Nucleoside analogues | 7 (47%) |
| Proteasome inhibitors | 6 (40%) |
- ECOG, Eastern cooperative oncology group; LDH, lactate dehydrogenase.
The majority of patients were 60 years or older at the time of transformation (85%), and the International Prognostic Index score was >2 in 11 patients (65%). Nodal involvement by DLBCL was seen in 25% of patients. Extranodal involvement was common (84%), and the most common extranodal sites involved by histological transformation were bone marrow (25%), bone (19%), genitourinary tract (19%), body cavities (19%), orbits (13%), gastrointestinal tract (13%), and/or central nervous system (6%). The median IgM level at histological transformation was 2,060 mg/dl (range 364–7,000 mg/dl), with an increase in serum IgM level of at least 25% from baseline observed in six patients (50%).
DLBCL cells showed positive expression of CD10 in 7% (n = 1/15), BCL6 in 78% (n = 7/9), and MUM1 in 100% (n = 2/2) of tested cases. Positive expression of BCL2 was seen in 89% (n = 8/9) of tested cases, and MYC in 50% (n = 1/2). The median Ki67 expression (n = 13) was 90% (range 50–99%). EBV-encoded RNA expression was negative as assessed by in situ hybridization in 3 of 3 tested cases. Light chain expression was concordant between WM and DLBCL cells in 75% (n = 9/12) of tested cases. The MYD88 L265P gene mutation was present in both WM lymphoplasmacytic cells and DLBCL cells in 2 of the 2 tested cases.
The most common frontline regimens used to treat WM patients in histological transformation were R-CHOP in 15 patients (75%) and R-EPOCH in 2 (10%). One patient (5%) received CHOP alone, one patient (5%) received ICE, and one patient (5%) who developed CNS lymphoma received high-dose methotrexate. Thirteen patients (77%) experienced complete response to frontline treatment, and six (30%) underwent hematopoietic stem cell transplantation (HSCT) for histological transformation at some point during the course of their disease; five patients underwent HSCT following chemotherapy after DLBCL relapse and one patient in first complete remission.
With a median follow-up from WM diagnosis of 7 years, the median survival time from transformation was 2.7 years (95% CI 0.83–7.58 years; Fig. 1B). The estimated median OS of WM patients who experienced histological transformation was shorter than patients who did not experience transformation (8.7 vs. 16 years; log-rank P = 0.09; Fig. 1C). At the time of this report, 14 patients (70%) have died. The most common cause of death was DLBCL progression (n = 10; 71%). Other causes of death included WM progression (n = 1; 7%), acute myeloid leukemia (n = 1; 7%), and unknown causes (n = 2; 14%). There was no survival benefit in patients who were previously untreated for WM at the time of transformation (log-rank P = 0.47), or in patients who underwent HSCT as part of the treatment for transformation (P = 0.13).
Discussion
In the present manuscript, we describe our experience of 20 patients with WM who experienced histological transformation to DLBCL. Our study raises several points of interest. First, the cumulative incidence of histological transformation is approximately 2% at 10 years from WM diagnosis. Second, histological transformation can occur at anytime during the course of the disease, at diagnosis, before any treatment for WM is instituted, during response to WM therapy, and even 20 years after the diagnosis of WM. Third, at histological transformation most patients presented with extranodal disease and high-risk features. Fourth, histological transformation is associated with a median survival less than 3 years, and appears to adversely impact the survival of patients with WM.
The cumulative incidence and the timing of histological transformation in patients with WM have been incompletely evaluated. Our study shows a 10-year rate of transformation of 2.4%, which is lower than in chronic lymphocytic leukemia and follicular lymphoma, in which the rate of transformation was 5% at 10 years and 20% and 8 years, respectively 17, 18. In an earlier retrospective study, a higher risk of histological transformation was reported in WM patients who were treated with nucleoside analogues (n = 193) with a cumulative incidence of 10% at 10 years, versus patients who were not exposed to nucleoside analogues (n = 136) 6. A more recent randomized study in WM patients comparing chlorambucil (n = 170) to fludarabine (n = 169) showed a cumulative incidence of aggressive lymphomas of 8% in the fludarabine arm and 11% in the chlorambucil arm at 6 years 9. The differences between studies can be explained by differences in case selection as the study by Leleu included WM patients diagnosed about a decade ago, and the study by Leblond included European patients who participated in a randomized clinical trial. The low risk of transformation to DLBCL should not preclude WM patients from receiving appropriate effective therapy when needed.
The present study expands on the prior evidence by reporting the occurrence of histological transformation not only in patients who were not exposed to nucleoside analogues but also in patients who were previously untreated for their WM. These findings suggest an inherent increased risk of developing other primary and/or secondary lymphoproliferative disorders in patients with WM. A previous population-based study has suggested an increased risk of developing DLBCL in patients with WM 4. To further support the hypothetical increased susceptibility of WM patients on developing other more aggressive lymphomas is the number of patients who transformed while on and responding to active WM-directed treatment. Additional studies will focus on examining the whole genome of WM patients who experienced histological transformation looking for signals of genomic instability that could explain such susceptibility.
Clinically, WM patients who experienced histological transformation presented with high IPI scores and high rate of extranodal disease. WM is typically associated with bone marrow involvement. Therefore, it is not surprising that the bone marrow was a common site of involvement by DLBCL transformation. The high rate of extranodal DLBCL could be explained by clonal evolution of WM favoring extranodal, extramedullary sites but additional research is needed to further clarify this finding. These high-risk features can explain the median OS of less than 3 years reported here, which is shorter than the expected median OS of 5 years in patients observed with de novo DLBCL in the rituximab era 19. In a recent population-based study, the outcome of WM patients who developed DLBCL was significantly worse than age and sex-matched individuals who developed de novo DLBCL without antecedent WM 5. Additionally, most of the cases of DLBCL diagnosed in WM patients showed lack of expression of CD10, a germinal center marker, suggesting that most of these cases might be of ABC-DLBCL subtype, which has been associated with a worse prognosis 13.
At this time, there is no evidence that patients with DLBCL who transformed from WM should be treated any different than patients with de novo DLBCL. There is mounting evidence, however, supporting the role of HSCT in DLBCL transformed from indolent lymphoma 20-23. Outcomes appear improved in support of autologous rather than allogeneic HSCT given the higher rates of non-relapse mortality associated with the latter. Autologous HSCT is associated with 5-year progression-free and overall survival rates of 30–40% and 50–60%, respectively. Allogeneic HSCT, conversely, was associated with rates of 20–40%. Therefore, autologous HSCT may be considered in patients with DLBCL that transformed from WM and responded to chemoimmunotherapy. The use of allogeneic HSCT should be reserved for clinical trials. Our recommendation for treatment would be in favor of chemoimmunotherapy with R-CHOP or R-EPOCH to eradicate the DLBCL component. Evaluation for autologous HSCT is encouraged. Maintenance rituximab can be considered after chemoimmunotherapy and HSCT to further suppress the WM component. A retrospective, nonrandomized study from our group has shown survival benefit from maintenance rituximab in patients with WM 24. A randomized study evaluating maintenance rituximab therapy versus observation in WM patients (MAINTAIN) is ongoing (NCT00877214).
Interestingly, a high proportion of transformed DLBCL patients showed expression of BCL2 in the malignant cells, offering a potential therapeutic target for BCL2 antagonists such as venetoclax 25. Also, we identified the MYD88 L265P mutation in both WM and DLBCL cells in two patients, suggesting that ibrutinib might be a potential therapeutic option in these cases. Ibrutinib therapy has also shown preferentially high rates of response in patients with ABC-DLBCL 26. A recent study has shown safety and preliminary efficacy of the combination of ibrutinib and R-CHOP in a phase 1B study on patients with CD20-positive non-Hodgkin lymphoma, of which 18 patients had DLBCL 27. Our study also suggests an increased expression of BCL6 and in some cases MYC. It is possible that the poor outcome seen in patients with DLBCL transformed from WM can be in part due to the development of a double-hit lymphoma (DHL) 28. DHLs have been associated with worse survival outcomes with standard chemoimmunotherapy 29.
Our study, however, is not without weaknesses. Although we examined a large number of patients with WM, our cohort might not be considered representative of the general population given the potential selection bias associated with including patients seen at a tertiary referral center. The data collection associated with our study was not prospective and suffers from missing data. Also, the number of patients reported is small and did not allow for multivariate analysis looking for factors predictive for transformation or prognostic for survival. Finally, there was not a formal clonal relation evaluation between the antecedent WM and the subsequent DLBCL although 75% of the cases had concordant light chain restriction. In the minority of discordant cases, there exists the possibility that the DLBCL component arose de novo rather than due to an actual histological transformation, or could be explained by class switching.
In conclusion, the incidence of histological transformation in WM patients is approximately 2% at 10 years, and can occur in treatment-naïve as well as heavily pretreated patients. Most patients present with poor prognostic markers and extranodal involvement, and the outcomes appear worse than the general population who develop de novo DLBCL in the rituximab era. Additional research is needed to improve our understanding behind the susceptibility of WM patients of transforming to DLBCL, and to develop novel therapies in order to positively impact the rates of response and survival of these patients.
Author Contributions
JJC and SPT designed the study. JJC and JG gathered the data. JJC analyzed the data and drafted the initial manuscript. All the authors critically read and approved the final manuscript.
Disclosures
JJC received honoraria from Alexion, Biogen, Celgene and Otsuka, and research funding from Abbvie, Gilead, Millennium, and Pharmacyclics. SPT received research funding and/or honoraria from Gilead, Janssen, Onyx, and Pharmacyclics. JG, KM, TD, and ZRH have no conflicts of interest to disclose.
Acknowledgment
Portions of this research were presented at the 2015 International Conference on Malignant Lymphoma, in Lugano, Switzerland.




