Histologic transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma
Conflict of interest: None of the authors has declared any competing financial interests.
Abstract
Although generally considered a clinically indolent neoplasm, CLL/SLL may undergo transformation to a clinically aggressive lymphoma. The most common form of transformation, to DLBCL, is also known as Richter syndrome. Transformation determines the course of the disease and is associated with unfavorable patient outcome. Precise detection of transformation and identification of predictive biomarkers and specific molecular pathways implicated in the pathobiology of transformation in CLL/SLL will enable personalized therapeutic approach and provide potential avenues for improving the clinical outcome of patients. In this review, we present an overview of the pathologic features, risk factors, and pathogenic mechanisms of CLL/SLL transformation. Am. J. Hematol. 91:1036–1043, 2016. © 2016 Wiley Periodicals, Inc.
Introduction
The concept of transformation in patients with chronic lymphocytic leukemia (CLL) was initially suggested in a case report published in 1928 by Maurice Richter (Supporting Information Fig. 1). He described the autopsy findings of a 46-year-old man with non-tender swelling of the left side of the neck, which gradually increased in size. Lymphadenopathy was accompanied by occasional pain in the epigastric and suprapubic regions and weight loss. The laboratory findings included lymphocytosis, and degenerated white blood cells in the peripheral blood smear. Significant autopsy findings included generalized lymphadenopathy, hepatosplenomegaly, and a small ulcer in the ileum. Microscopic examination of the lymph nodes and liver showed two histologic lesions termed as “leukemic cells” and “tumor cells.” The leukemic cells were described as small lymphoid cells and the tumor cells as large lymphoma cells. The bone marrow was comprised mostly of small neoplastic lymphocytes, whereas the ileal lesion consisted of large lymphoma cells. As such, the simultaneous occurrence of two lesions of different histologic grades was demonstrated, although their genetic relationship was not established 1.
In 1964, Lortholary et al. in France coined the term Richter's syndrome (Supporting Information Fig. 2). The authors presented four autopsy reports of patients presenting initially with CLL. The patients presented with fever, weight loss, lymphadenopathy, hepatosplenomegaly, and/or lymphocytosis. The CLL was treated initially with either radiotherapy alone, or radiotherapy and prednisone. All patients eventually developed a malignant lymphoma such as so-called reticular cell sarcoma (most likely diffuse large cell lymphoma) or Hodgkin disease (lymphoma). A mixed proliferation of small neoplastic lymphoid cells and large lymphoma cells were observed on histologic sections of the lymph nodes. Fifteen published articles from 1928 until 1963, including the case report by Richter, were reviewed by Lortholary and colleagues as a part of their manuscript that described cases of concurrent CLL/SLL and a histologically high-grade malignant lymphoma. The authors proposed that such an association be known as Richter syndrome and this term remains in current use 2.
Richter syndrome (RS) refers to a setting in which a patient with CLL/SLL also develops diffuse large B-cell lymphoma (DLBCL) and, most often, is a manifestation of histologic transformation. Richter transformation is another term often used as a synonym. RS is also loosely applied to CLL/SLL patients who develop less common lymphoid neoplasms such as Hodgkin lymphoma (HL), plasmablastic lymphoma or B-lymphoblastic leukemia/lymphoma. Whereas, histologic transformation occurs in patients with other types of low-grade B-cell neoplasms 3, the term RS is only used in the context of CLL/SLL.
In this review, we present an overview of the pathologic features, risk factors and pathogenic mechanisms of RS.
Clinical Features
Transformation of CLL/SLL into DLBCL or another uncommon type of aggressive lymphoma is typically suspected when a patient with CLL/SLL develops sudden onset of B symptoms, often accompanied by enlarging lymph nodes detected by physical examination or imaging studies. Commonly, work-up for such a scenario includes 18-fluorodeoxyglucose-positron emission tomography (18FDG-PET/CT) which detects a relative increase in metabolic activity, expressed quantitatively as standardized uptake value (SUV). In a patient with suspected RS, the identification of any lesion (nodal or extranodal) with increased 18FDG avidity (SUV > 5.0) by PET/CT is an indication for tissue evaluation. Whereas the absence of lesions detectable by 18FDG-PET is highly sensitive in excluding RS, with a negative predictive value of up to 97%, the positive predictive value of 18FDG-PET/CT for RS is generally proportional to the SUV; an SUV > 10 is predictive of the presence of aggressive lymphoma with 80% confidence, and an SUV > 13 with >90% confidence 4-7.
Laboratory studies commonly associated with RS include a substantially elevated serum lactate dehydrogenase (LDH) level >1.5 times the upper limit of normal and an elevated serum beta-2 microglobulin (β2M) level >2 mg/L. Hypercalcemia, new onset of absolute lymphocytosis ≥5.0 × 109/L, and thrombocytopenia <100 × 109/L often accompany RS, but these findings are not by themselves a reliable predictor of RS 8-19.
Histologic Variants of Richter Transformation
Diffuse large B-cell lymphoma
DLBCL is the most frequent histologic variant of histologic transformation in patients with CLL/SLL. The development of DLBCL occurs 1.8–4.0 years from the time of initial CLL/SLL diagnosis and can arise before or subsequent to CLL/SLL therapy. Among patients with newly diagnosed CLL/SLL, the annual incidence rate of DLBCL is 0.5%; whereas among patients who have received treatment for CLL/SLL the annual incidence rate of DLBCL is ∼1% 15. Up to 10.7% of all CLL/SLL patients progress to DLBCL (RS-DLBCL) 11, 12, 14, 15, 20-23 (Supporting Information Table I). The incidence rate for DLBCL reported in the literature is highly variable, likely attributable to many factors including the heterogeneity of the patient population studied, duration of clinical follow-up, and diagnostic criteria used to define development of DLBCL (clinical/imaging vs. biopsy-proven). DLBCL may arise in any demographic subset of CLL/SLL patients, but is most common in men over 60 years of age. (Table 1)
| Study | Age | Gender | LDH (>1.5 times the upper limit) | β2M | TK1 | Absolute lymphocyte count ≥ 5.0 × 109/L | Platelets <100 × 109/L | |
|---|---|---|---|---|---|---|---|---|
| Male % | Female % | |||||||
| Tsimberidou et al. 24 | 53% (>60 years) | NR | NR | 47% | 40% (>3 × UNL) | NR | 37% (≥5 × 109/L) | 57% (<100 × 109/L) |
| Rossi et al. 12 | 70 years (64–73) | 64.7 | 35.3 | 35% | 3 mg/L (2.3–3.7) | NR | NR | 204 (150–232) |
| Tsimberidou et al. 13 | 66 (34–77) | 50 | 50 | 55% | 55% (>2 × ULN) | NR | 20% (>30 × 109/L) | NR |
| Rossi et al. 9 | 65% (>60 years) | 62.7 | NR | 45% | NR | NR | 51.2% (≥ 5 × 109/L) | 30.5% (<100 x 109/L) |
| Fan et al. 14 | 37.5% (>60 years) | 62.5 | 37.5 | 31% | 4.35 mg/L (2.0–16.7) | 37.5% (≥2.0 pmol/L) | 18.75% (≥5 × 109/L) | NR |
| Chingrinova et al. 10 | 70% (>60 years) | 56 | NR | 50% | NR | NR | 44% (≥5 × 109/L) | 24% (<100 x 109/L) |
| Parikh et al. 15 | 61 years (21–85) | 78 | NR | NR | 3.1 mg/L (1.2–13.1) | NR | 9.1 x 109/L (0.7–415.5) | 190 (71–493) |
- NR, not reported; UNL, upper normal limit; LDH, lactate dehydrogenase; β2M, beta 2 microglobulin; TK1, Thymidine kinase 1.
Factors associated with RS-DLBCL predisposition
A number of factors are likely involved in predisposing CLL/SLL patients to developing RS-DLBCL, as are summarized in Table 2. On the basis of stereotyped BCR, CLL/SLL can be segregated into various subsets. In one study, a subset associated with the IGHV4-39/IGHD6-13/IGHJ5 stereotype was associated with the highest risk for RS, and stereotyped BCR as well as IGHV4-39 were independent predictors of RS. Interestingly, the stereotyped BCR of RS-DLBCL is not observed in de novo DLBCL 25. Telomeres play an important role in ensuring genetic stability and regulating critical cellular functions, including proliferation, and replicative senescence 28. Others have reported that the 5-year risk of developing RS may be higher in patients with CLL/SLL associated with shorter (<5,000 bp) telomere length (18.9% vs. 6.4%). In the same study, it was observed that significantly shorter telomeres are present in CLL/SLL with unmutated IGVH genes, as well as in CLL/SLL associated with increased telomerase activity. These findings point to a higher number of antecedent cell replications and thus a higher chance to acquire additional genetic alterations 26. Other biological risk factors of CLL/SLL transformation to RS-DLBCL include expression of CD38 10, 14, 15, ZAP-70 10, 12-15, and CD49d 15. Cytogenetic abnormalities, such as del(11q22.3), del(17p13), del(15q21.3), del(9p21), and/or add(2p25.3), have been also associated with RS 10, 15, 27. Heritable germline polymorphisms in BCL2 29, CD38 30 and LRP4 31 have been reported to impart a higher risk for transformation, although the functional mechanisms underlying these associations remain to be determined.
| Frequency in RS patients (%) | References | ||
|---|---|---|---|
| Biological characteristics | Unmutated IGVH | 89 | Tsimberidou et al. 13 |
| 66.7 | Fan et al. 14 | ||
| 76 | Parikh et al. 15 | ||
| Stereotyped B-cell receptors | 49.3 | Rossi et al. 25 | |
| Short telomere length (≤5,000 bp) | 47 (90/191) | Rossi et al. 26 | |
| ZAP-70+a | 89 | Tsimberidou et al. 13 | |
| 66.7 | Fan et al. 14 | ||
| 61 | Chigrinova et al. 10 | ||
| 58 | Parikh et al. 15 | ||
| CD38+a | 93 | Fan et al. 14 | |
| 57 | Parikh et al. 15 | ||
| 46 | Chigrinova et al. 10 | ||
| CD49d+a | 77 | Parikh et al. 15 | |
| Genetic characteristics | del(11q) | 20 | Tsimberidou et al. 13 |
| 26.7 | Fan et al. 14 | ||
| 21 | Parikh et al. 15 | ||
| del(17p) | 33 | Tsimberidou et al. 13 | |
| 26.7 | Fan et al. 14 | ||
| 25 | Parikh et al. 15 | ||
| 40 | Fabbri et al. 27 | ||
| del (9p21) | ∼30 | Fabbri et al. 27 | |
| add(2p25.3) | 14 | Chigrinova et al. 10 | |
| TP53b disruptionc | 60 (12/20) | Chigrinova et al 10 | |
| 47 | Rossi et al. 9 | ||
| 36 | Fabbri et al. 27 | ||
| MYCb aberrations | 18 | Chigrinova et al. 10 | |
| 26 | Rossi et al. 9 | ||
| CDKN2A disruption | ∼30 | Fabbri et al. 27 | |
| Trisomy 12b | 40 | Tsimberidou et al. 13 | |
| 26.7 | Fan et al. 14 | ||
| 21 | Parikh et al. 15 | ||
| ∼30 | Fabbri et al. 27 | ||
| NOTCH1b | Fabbri et al. 27 |
- a By immunohistochemistry or flow cytometry.
- b Among the four most commonly altered aberrations ∼90% of RS cases.
- c Includes deletion and/or mutation.
Additional factors predictive of the development of RS remain largely unknown. Konoplev et al. 23 showed that CLL/SLL patients with increased serum thymidine kinase 1 (TK1), a key cellular enzyme involved in DNA synthesis, are at higher risk for developing RS. Increased serum TK1 level is associated significantly with male sex, elevated serum β2M level, unmutated IGVH, ZAP-70 expression, and lack of del(13q14.3). Furthermore, RS patients with a higher serum TK1 level tended to be at a higher risk of death. These observations were further corroborated by Szantho et al 32 who showed that increased serum TK1 levels in CLL patients are associated with advanced Rai stage, CD38 and ZAP-70 expression, and higher levels of absolute lymphocytosis.
Pathologic features of RS-DLBCL
Most cases of RS-DLBCL show diffuse effacement of lymph nodes or extranodal sites by sheets of large cells with centroblastic morphology; a minority of cases have immunoblastic features. Mitotic figures and apoptotic bodies are usually frequent, and a starry-sky pattern and tumor necrosis are common 33. Typically, the neoplastic cells are positive for the B-cell markers—CD19, CD20, CD22, and PAX5—as well as monotypic surface immunoglobulin light chain. CD38, ZAP70, and CD49d are often positive, whereas CD5 and CD23 expression is retained to a varying extent 14, 15. Approximately 80% of RS-DLBCL have a non-germinal center B-cell-like (non-GCB) immunophenotype (negative for CD10 and positive for MUM1), whereas about 20% have a GCB immunophenotype (positive for CD10 and/or BCL6+, MUM1−) 10, 34. Other features often seen in RS-DLBCL include TP53 overexpression and a high (>70%) Ki-67 proliferation index. (Fig. 1) Epstein-Barr virus (EBV)-encoded RNA transcripts may be detected in some cases 15, 33.
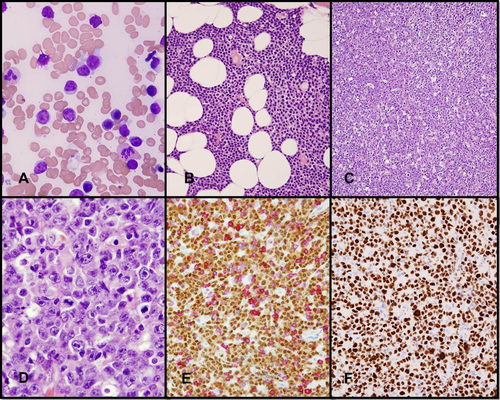
Diffuse large B cell lymphoma transformation of CLL/SLL. (A, B) CLL involving the bone marrow. (A) CLL with cytologically atypical morphology in a bone marrow aspirate smear with a population of small and medium-sized cells. (B) Bone marrow space replaced by sheets of small lymphocytes. A proliferation center is present. (C–F) Concurrent nasal mass biopsy involved by DLBCL. (C) Diffuse growth of large neoplastic lymphoid cells and a conspicuous starry sky pattern. (D) Neoplastic cells with immunoblast-like and centroblast-like morphology. (E) The neoplastic cells are positive for PAX5 and negative for CD5. (F) Ki-67 immunohistochemistry demonstrates a high proliferation rate. (A, wright-giemsa stain; B–D, hematoxylin-eosin; E and F, IHC with hematoxylin counterstain). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Clonal relationship
The clonal relationship between CLL/SLL and RS-DLBCL was first established through primary structural analyses of the IG genes 35. About 70–80% of RS-DLBCL cases are clonally related to the underlying CLL/SLL and can have similar unmutated or mutated immunoglobulin heavy chain variable region (IGVH) sequences 34. Commonly, RS-DLBCL arises from a dominant CLL/SLL clone after having acquired additional somatic mutations 10. The molecular characteristics of the B-cell receptor (BCR) have been suggested to have an impact on the risk for transformation. CLL/SLL patients with unmutated IGVH genes are at an increased risk for RS compared with those with mutated IGVH 9, 15. A fraction of CLL/SLL cases share common IGVH complementary determining region 3 motifs (stereotyped BCR) as a result of nonrandom recombination predispositions involving the V-D-J regions.
Molecular biology of RS-DLBCL
Despite morphologic and immunophenotypic overlap between RS-DLBCL and de novo DLBCL, these diseases diverge significantly at the molecular level 10, 27, 36.
The molecular profile of RS-DLBCL is complex. Two major transformation models have been suggested to lead to development of RS from CLL/SLL 27. Most RS-DLBCL cases follow a “linear” model of transformation by which the transformed clone is a direct progeny of the “parent” CLL/SLL clone; whereas in a minority of cases, a “branched” model of evolution is implicated in which distinct genetic lesions direct a common precursor cell to give rise to the CLL and RS clones independently (Fig. 2). Leukemic presentation of RS is associated with the latter model.
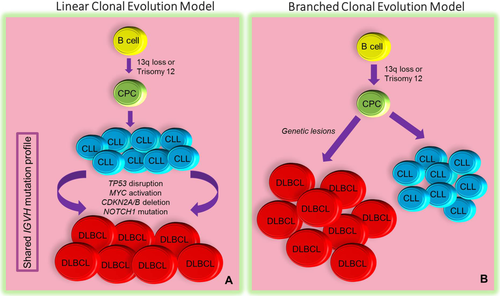
Patterns of clonal evolution in CLL/SLL transformation. In the linear model (A) of transformation, more common among patients with RS-DLBCL, DLBCL originates directly from the major CLL/SLL clone following the acquisition of additional genetic alterations. In this model, the DLBCL and CLL/SLL clones show similar IGVH mutation profiles. In the branched model (B), clones derive directly from a common precursor cell (CPC) by way of acquiring distinct genetic lesions. Note that both clones share a subset of alterations with the CPC and with each other in addition to harboring distinct genetic lesions. In both models, a minor subclone arising from the CPC and already equipped with transformation-specific gene signatures may be present at the time of diagnosis and may later declare itself as bona fide transformation. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The most common chromosomal copy number alteration (CNA) linked to the development of RS is loss of 17p (∼40%) resulting in inactivation of TP53, which in turn facilitates the development of additional genetic lesions, including additional genome-wide somatic mutations, and chromosomal rearrangements. Biallelic loss of 19p21/CDKN2A/B is frequently observed in RS (∼30%) and is associated with the “branched” model of evolution 10. Of note, disruptions of TP53 and CDKN2A often coexist with MYC-activating events. Other recurrent chromosomal CNAs implicated in the pathogenesis of RS include trisomy 12, del(7q31.31-36.6), del(8p), and del(14q23.2-q32.33) as well as high-level amplifications of chromosomes 8, 11, 13, and 18 10, 27, 37. Absence of del(13q14) also has been associated with an increased risk for transformation to RS. Rearrangements involving the MYC locus (8q24), very rare in CLL/SLL, may be gained during the course of disease, and have been suggested as a secondary event in disease progression, particularly in patients who harbor 8q24/MYC rearrangement in the context of a complex karyotype 12, 38. Another possible mechanism of MYC involvement in CLL/SLL, also associated with a shorter clinical course, is 8q24/MYC amplification. Brown and colleagues sequenced exon 1 of MYC in 188 CLL/SLL samples and found one case with a MYC p.T58A mutation, and a heterozygous insertion mutation that duplicates nine amino acids of the N-terminal interaction and transactivation domain in another sample. Although MYC amplification is a frequent event, considered to be acquired during transformation, this study showed that MYC amplification in CLL/SLL without high-grade transformation may occur 39.
Several genes involved in a host of cellular and biological programs have been identified to be recurrently mutated in RS 40-44. Genes involved in proliferation, apoptosis, and cell cycle regulation seem to stand out. The most commonly mutated genes implicated in the pathogenesis of RS include TP53 (tumor suppressor), NOTCH1 and MYC (proliferation), and CDKN2A/B (cell cycle regulation), together accounting for up to 90% of alterations seen in RS-DLBCL 27. Unlike TP53 and CDKN2A/B, NOTCH1 mutations, and other lesions associated with MYC activation are mutually exclusive 41. Notably, TP53 mutations and activating NOTCH1 mutations are less common in de novo DLBCL. Additional signaling pathways involved in the emergence of RS-DLBCL include the MAPK, Wnt, NF-κB, and TGF-β pathways. Cases of de novo DLBCL and RS-DLBCL share a minor subset of genes as a part of their genetic signatures—including MYC activation (GCB-derived DLBCL), MYD88 mutations, and CDKN2A/B loss (activated B-cell-derived DLBCL). However, RS-DLBCL lacks other abnormalities common in de novo DLBCL, such as inactivation of CREBBP/EP300, β2M, PRDM1/BLIMP1, and TNFAIP3/A20, as well as BCL2 and BCL6 translocations 27.
Accelerated phase CLL
The term accelerated phase of CLL/SLL has been proposed to describe a biopsy specimen that shows increased large cells and a high proliferation rate, but without clear-cut histologic evidence of DLBCL. Patients may or may not have clinical symptoms of transformation. Patients with CLL/SLL in accelerated phase often, although not always, have a more aggressive clinical course. In a study by Gine et al., the authors suggest that the diagnosis of accelerated phase CLL/SLL can be based on any of three morphologic criteria: (1) expanded proliferation centers (broader than a ×20 microscopic field); (2) increased mitotic activity (>2.4 mitotic figures/proliferation center); or (3) an increased proliferation index within proliferation centers as indicated by high Ki-67 labeling (>40%). In their study, the median survival of patients with accelerated phase CLL/SLL was shorter than patients with typical CLL/SLL. In addition, the median survival from the time of biopsy according to the morphologic patterns of “non-accelerated” CLL, “accelerated” CLL, and DLBCL transformation was 76 months, 34 months, and 4.3 months, respectively 45.
Plasmablastic lymphoma
Plasmablastic lymphoma (PBL) is an aggressive B-cell malignancy, thought to be related to DLBCL, in which the lymphoma cells exhibit morphologic and immunophenotypic features of plasma cell differentiation 46 (Supporting Information Fig. 3). These neoplasms most commonly arise de novo in the setting of immunodeficiency, however, a few cases of plasmablastic lymphoma (RS-PBL) arising in patients with CLL/SLL has been reported 18, 19, 47. Most patients who develop RS-PBL are men, 52–77 years of age. A serum or urine paraprotein can be detected in some patients 18. In addition to typical plasmablastic features, residual CLL/SLL cells may be identified in some areas. The neoplastic cells of RS-PBL often express CD38, ZAP-70, CD138, BLIMP1, IRF4/MUM1, and XBP1. CD5, CD20, PAX5, and IRF8 are commonly negative. Molecular studies have shown monoclonal IGH 18 and MYC rearrangement 47. The prognosis of patients with RS-PBL is grim. The few CLL/SLL patients with RS-PBL reported in the literature died within 3–6 months after transformation 18, 19, 47.
B-lymphoblastic leukemia/lymphoma
B-lymphoblastic leukemia/lymphoma (B-LBL) is a neoplasm derived from progenitor B-lymphoid cells often involving bone marrow, peripheral blood, and less commonly lymph nodes 48. Acute lymphoblastic transformation of CLL/SLL (RS-LBL) is rare, although several cases have been reported. Most patients are men, with a reported age range of 42–76 years. The reported interval between CLL/SLL diagnosis and RS-LBL ranges from 2 months to 7 years; although simultaneous presentation of CLL/SLL and RS-LBL may occur 49-57. RS-LBL is characterized by numerous lymphoblasts in a background of cells typical of CLL/SLL. The blasts are intermediate size, with indented nuclei, fine chromatin, one or two small nucleoli, and scant cytoplasm. The neoplastic cells express HLA-DR, surface Ig, pan-B cell markers, and TdT 51, 52, 55-57; a TdT-negative case also has been reported 54. There is variable expression of CD5, CD10, CD22, and CD23 51, 52, 54-57.
Early studies supported clonal evolution from CLL/SLL to RS-LBL based on surface immunoglobulin expression, cytogenetic findings 51, 55, and molecular analysis of the IG heavy and light chains 50, 52, 54, 57. A recent study described an analysis of paired CLL/SLL and B-LBL specimens from two patients with Philadelphia chromosome-positive B-LL and showed that the CLL/SLL and B-LBL cells used different IGHV families and were clonally unrelated. In addition, the IGHV gene was unmutated in both CLL/SLL and B-LBL cells in one of these two cases, and IGHV was mutated in CLL/SLL cells but not in B-LBL cells. These data suggest that B-LBL can occur in patients with CLL/SLL associated with either mutated or unmutated IGHV. Furthermore, the authors suggested that B-LBL may represent a secondary neoplasm and not evidence of histologic transformation in a subset of patients 56. Cytogenetic studies also have shown the presence of 8q24 translocation acquired at the time of transformation to B-LBL in some patients 51, 52. Some authors use the term Burkitt-like lymphoblastic transformation for those occurrence 57. Overall, compared with typical B-LBL, cases of blastic transformation of CLL/SLL show distinctive clinical features including a poor prognosis, and likely a different pathogenesis. In our opinion, classifying these cases as B-ALL or B-LBL without further comment may be misleading to clinicians and patients and, therefore, the pathology report needs to indicate that the neoplasm represents transformation of CLL/SLL and not de novo disease. We suggest using terms such as “TdT-positive blastoid lymphoma” or “high-grade TdT-positive blastic B-cell leukemia/lymphoma” for these tumors.
Hodgkin lymphoma
Less than 1% of patients with CLL/SLL develop Hodgkin lymphoma (RS-HL) 16, 58, 59. Patients with CLL/SLL are typically in the seventh decade of life (range, 30–88 years) when they undergo transformation to RS-HL and most are men. The median interval between CLL/SLL diagnosis and RS-HL is 4–6 years (Table 3). Of note, patients with CLL/SLL who are treated with purine nucleoside analogs (fludarabine, cladribine) and later on develop RS-HL seem to have worse outcomes compared with other CLL/SLL patients who develop RS-HL 61, 60. It has been postulated that exposure to immunosuppressive chemotherapy in patients with CLL may increase the risk for HL transformation. The presumed etiologic process is the marked and prolonged reduction of CD4-positive and CD8-positive T-cells, which likely permits the proliferation and accumulation of EBV-positive B-cells, eventually resulting in a high-grade lymphoma 61, 62.
| Study | Study period | Type of study | Age, years (range) | Gender | Median time to development of Hodgkin transformation, years (range) | Frequency of Hodgkin transformation | EBV positive RS cells | |
|---|---|---|---|---|---|---|---|---|
| Male % | Female % | |||||||
| Bockorny et al. 61 | 1975–2011 | Meta-analysis | 65.7 (34–85) | 76.5 | 23.5 | 4.3 (0–26) | 86 cases in 36 years | 70.6% |
| Parikh et al. 59 | 1995–2011 | Cohort | 68 (45–88) | 81 | NR | 6.2 (0–24.5) | 0.05%/year | 67% |
| Tadmor et al. 16 | 1996–2010 | Retrospective | 58 (30–77) | 75 | 25 | 5.9 (0.8–11.9) | 13% (16 of 119) | 50% |
- NR, not reported.
Hodgkin transformation is characterized by Hodgkin and Reed-Sternberg (HRS) cells in a polymorphous inflammatory background distinct from the CLL/SLL 17. (Fig. 3) The inflammatory background consists of T-cells and histiocytes, with or without abundant eosinophils. Tumor necrosis is a common finding 33. Any of the histologic subtypes of HL can be seen, with the mixed cellularity subtype being most common 61.
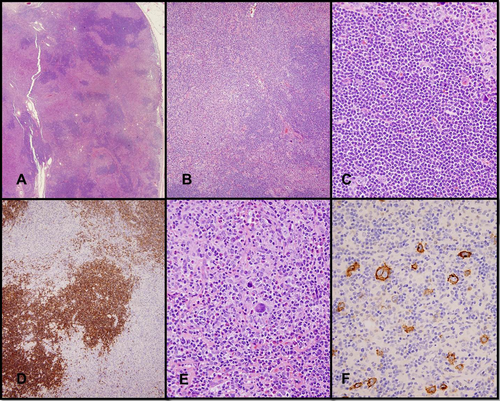
Hodgkin lymphoma transformation of CLL/SLL. (A and B) Lymph node involved by CLL/SLL (dark areas) and Hodgkin lymphoma (light/pale areas) indicating transformation. (C) Low-grade component composed of small lymphocytes (D) with CD5 expression. (E) HRS cells in a polymorphous inflammatory background composed of T cells, histiocytes, and eosinophils. (F) HRS cells are CD30+. (A–C, E, hematoxylin-eosin; D and F, IHC with hematoxylin counterstain). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
An unusual pattern that raises the possibility of HL transformation is the occurrence of HRS cells scattered in a monomorphous background of CLL cells. In some patients, follow-up biopsy specimens show unequivocal CHL as described above, but in other patients there is no subsequent HL. These lesions have been referred to in the literature as CLL/SLL with HRS cells 63-66. A recent study 67 suggests that this pattern can be considered as an early event in the transformation and appears to have the potential to progress to the type of RS-HL wherein HRS cells are in a polymorphous inflammatory background distinct from the CLL/SLL. The same study showed that the overall survival for these two types of morphology is similar.
By immunohistochemistry, HRS cells express CD30, CD15, and PAX5 (dim), and these cells are commonly positive for EBV (Table III) 34. HRS cells may be positive for CD20, usually with variable intensity, in 20–30% of cases 61.
In about 80% of patients with RS-HL, the CLL/SLL cells have mutated IGVH. This is a much higher frequency than that observed among patients who develop RS-DLBCL or have no transformation 34. In addition, it has been demonstrated that similar VH gene rearrangements can be seen in HRS and CLL/SLL cells, implying that the two populations of cells were derived from a common precursor B-cell 68, 69. A recent study showed that most of the CLL/SLL that is ZAP-70 positive is IGHV unmutated, whereas ZAP-70 negative cases are IGHV mutated. HRS cells from all IGHV unmutated (ZAP-70 positive) CLL/SLL cases were clonally unrelated, while HRS cells from IGHV mutated (ZAP-70 negative) CLL/SLL cases frequently share clonal origin with the associated CLL 67.
T-cell lymphomas
Rarely patients with CLL/SLL develop T-cell lymphomas. In general, the relationship between CLL/SLL and these neoplasms is poorly understood 70, 71. These rare patients are usually not included under the umbrella term of RS in the literature. A few relevant papers are cited, but these patients are not the focus of this review.
Treatment
Patients with RS generally have a poor prognosis. Most data for treatment for RS is derived from single-arm phase I–II studies, and retrospective analyses 13, 72-79. The standard therapy for RS is chemoimmunotherapy with a median survival after therapy of less than 1 year 36. MD Anderson Cancer Center has used oxaliplatin-based regimen (OFAR: oxaliplatin, fludarabine, Ara-c [cytarabine], rituximab) for patients with RS 13, 72. OFAR regimen led to an overall response rate of 50% with a 20% CR rate 72. However, the median progression-free survival (PFS) was only 4 months, and the median OS was 8 months. Langerbeins et al. reported outcomes in 15 patients with RS who received rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) 75. Two-thirds of the patients responded, with a median PFS of 10 months and a median OS of 21 months. Ibrutinib, a BTK inhibitor, is approved for patients with CLL 80, 81. Anecdotal reports have suggested the activity of ibrutinib in patients with RS 82-84. Pembrolizumab, a PD-1 monoclonal antibody, has also been reported to have encouraging activity in patients with RS 85. Several other targeted agents such as PI3K inhibitors, antibody-drug conjugates, or bi-specific antibodies may have a role in patients with RS, and need further evaluation. Allogeneic stem cell transplantation (allo-SCT) remains an important therapeutic modality for patients with RS 9, 24. Although allo-SCT is indicated for treating RS patients who undergo remission, and is the only modality that offers a potential for cure, many patients do not qualify because of their older age or inadequate response to induction therapy. For Hodgkin's transformation, treatment is with multidrug chemotherapy such as doxorubicin, bleomycin, vinblastine, dacarbazine 86.




