Prognostic significance of high hyperdiploid and triploid/tetraploid adult acute myeloid leukemia
Conflict of interest: Nothing to report.
Abstract
To ascertain the clinical implications of high hyperdiploid (HH; 49–65 chromosomes) and triploid/tetraploid (TT; >65 chromosomes) adult acute myeloid leukemia (AML), all such cases were retrieved from the Swedish AML Registry. Of the 3,654 cytogenetically informative cases diagnosed between January 1997 and May 2014, 68 (1.9%) were HH (n = 50)/TT (n = 18). Patients with HH/TT were older than those with intermediate risk (IR) AML (median 71 years vs. 67 years; P = 0.042) and less often had de novo AML (63% vs. 79%; P = 0.004); no such differences were observed between HH/TT and complex karyotype (CK) AML. The overall survival (OS) was similar between patients with HH/TT and CK AML (median 0.9 years vs. 0.6 years; P = 0.082), whereas OS was significantly longer (median 1.6 years; P = 0.028) for IR AML. The OS was shorter for cases with HH than with TT (median 0.6 years vs. 1.4 years; P = 0.032) and for HH/TT AMLs with adverse abnormalities (median 0.8 years vs. 1.1 years; P = 0.044). In conclusion, HH/TT AML is associated with a poor outcome, but chromosome numbers >65 and absence of adverse aberrations seem to translate into a more favorable prognosis. Thus, HH/TT AMLs are clinically heterogeneous and should not automatically be grouped as high risk.Am. J. Hematol. 90:800–805, 2015. © 2015 Wiley Periodicals, Inc.
Introduction
Gains of chromosomes, in particular trisomies 4, 8, 11, 13, 21, and 22, are frequent in adult acute myeloid leukemia (AML) 1. Cases with such trisomies as sole anomalies are often grouped as intermediate risk (IR), although a poorer outcome than what is usually seen in the IR group has been emphasized 2-11. Most AMLs with numerical abnormalities harbor 1–2 chromosome gains and hence have modal chromosome numbers of 47–48, i.e., they are low hyperdiploid. In contrast, high hyperdiploidy (HH; 49–65 chromosomes) and triploidy/tetraploidy (TT; >65 chromosomes) are rare 1. In fact, most HH and TT AMLs reported in the literature have been single case reports or small patient series providing limited data on the incidence and clinical implications of these karyotypic subgroups 12-20. However, a few larger series of HH and/or TT AMLs have been published, comprising 11 cases with HH 21, 25 with TT 22, 38 with HH 23, and 221 with HH 24; these studies have shown that AMLs with HH/TT constitute <2% of all adult AML cases, a frequency notably lower than the one observed (∼8%) in pediatric AML 25. Because AMLs with HH/TT by default have complex karyotypes (CK), at least if defined as ≥3 abnormalities, they are usually grouped as high risk. Indeed, although the results of studies addressing the prognostic impact of HH/TT to some extent have varied, partly because of different inclusion criteria and treatment protocols 21-24, they have generally associated HH/TT with low remission rates and short overall survival (OS). However, the types of chromosome abnormality present may modify the prognosis, as recently reported by the Leukaemia Research Cytogenetics Group (LRCG) who, after trichotomizing HH cases into those with numerical changes only, those harboring structural chromosome aberrations classified as IR, and those carrying HR abnormalities, could show that the incidences of relapse varied significantly among these three subgroups 24.
The aims of the present study, which is based on the population-based Swedish AML Registry, were threefold: (1) to investigate if AMLs with HH/TT differ clinically from those classified as IR or CK; (2) to ascertain whether Swedish HH/TT AMLs cytogenetically subgrouped according to LRCG 24 are prognostically distinct; and (3) to compare clinical features between HH and TT AMLs.
Methods
Patients
The Swedish Adult Acute Leukemia Registry was founded in January 1997 by the Swedish Society of Hematology. The registration of all newly diagnosed adult patients with AML, de novo as well as secondary (excluding blast phase of chronic myeloid leukemia) and treatment-related AMLs, is compulsory in Sweden. The registry, which comprises 98% of all adult AML patients diagnosed between 1997 and 2014 and hence can be considered truly population-based, includes data on diagnostic karyotypes, comorbidity, performance status, intention-to-treat (intensive or palliative therapy), type of treatment, resource requirements, response to induction therapy, and survival. Local ethics review boards have approved data registration and analysis. In May 2014, the registry contained records from 5,870 AML patients, excluding those with acute promyelocytic leukemia. The cytogenetic data on these cases were ascertained from the seven laboratories performing cytogenetic analyses of AML in Sweden, and all retrieved karyotypic strings were reviewed, as previously reported 26. A total of 3,654 (62%) cases had informative karyotypes, i.e., they were cytogenetically abnormal or normal; the remaining were either karyotypic failures (n = 66; 1.1%) or had not been sent for cytogenetic analysis (n = 2,150; 37%).
Definition of high hyperdiploid and triploid/tetraploid AML
HH and TT were defined as the presence of 49–65 chromosomes and >65 chromosomes, respectively. Cases with any of the following cytogenetic abnormalities were excluded: t(1;3)(p36;q21), inv(3)(q21q26)/t(3;3)(q21;q26), t(3;5)(q25;q35), t(3;12)(q26;p13), t(6;9)(p22;q34), t(8;16)(p11;p13), t(8;21)(q22;q22), t(9;22)(q34;q11), der(11q23)/KMT2A (a.k.a. MLL) rearrangements, t(15;17)(q22;q21), and inv(16)(p13q22)/t(16;16)(p13;q22). These translocations/inversions are all well-known primary aberrations associated with particular morphologic, immunophenotypic, and clinical features; in addition, several of these aberrations are risk-stratifying in AML. Thus, it was decided to focus specifically on an HH/TT group without such primary changes. TT cases that represented duplications of near-diploid clones, either seen cytogenetically or inferred based on duplication of all structural changes in the TT clone, were also excluded. The HH/TT-positive cases were further subdivided into: (1) Adverse: presence of −5, del(5q) or other changes resulting in loss of 5q, −7, del(7q) or other changes resulting in loss of 7q, −17, and/or der(17p) together with chromosome gains; (2) Numerical: presence of only numerical aberrations; 3) Structural: presence of at least one non-adverse structural rearrangement in addition to numerical abnormalities.
Statistical analyses
The chi square test was used to compare the basic cytogenetic patterns (adverse, numerical, and structural abnormalities) between the HH and TT cases. Age and gender distributions, types of AML and therapy, and complete remission (CR) and early death (ED) rates in the HH/TT, IR, and CK groups were compared using Kruskal–Wallis one-way analysis of variance or Fisher exact probability test, as detailed in Tables 1 and 2. CR was defined as a bone marrow aspirate with less than 5% leukemic blasts and with evidence of regeneration of normal hematopoietic cells. ED was defined as death within 30 days from the start of intensive chemotherapy. OS was calculated from the date of diagnosis to death from any cause or the date of last follow-up. The follow-up times for the HH/TT, IR, and CK groups were 0–16.9 years (mean 1.8 years), 0–17.2 years (mean 2.7 years), and 0–16.6 years (mean 1.2 years), respectively. Kaplan–Meier survival curves were used to estimate median OS times, and the log-rank test was applied to compare OS between cases with HH and TT as well as between AMLs grouped as HH/TT (also subdivided into those with and without adverse abnormalities), IR, or CK; only patients who had received intensive therapy were included in the survival analyses. The statistical analyses were carried out using STATA v.13 (StataCorp LP, College Station, TX).
| No. of chromosomes | |||
|---|---|---|---|
| Features | 49–65 n = 50 (%) | >65 n = 18 (%) | P value |
| Cytogenetic abnormalitiesa | |||
| Adverse | 26 (52) | 7 (39) | 0.387b |
| Numerical | 9 (18) | 6 (33) | |
| Structural | 15 (30) | 5 (28) | |
| Age (years) | |||
| Median | 71 | 68 | 0.554c |
| 18–40 | 3 (6) | 1 (6) | |
| 41–60 | 8 (16) | 3 (17) | |
| >60 | 39 (78) | 14 (77) | |
| Gender | |||
| Male | 33 (66) | 15 (83) | 0.232d |
| Female | 17 (34) | 3 (17) | |
| WBC count (×109/l) | |||
| <10 | 16 (32) | 7 (39) | NA |
| 10–50 | 4 (8) | 1 (6) | |
| >50 | 2 (4) | 0 | |
| NK | 28 (56) | 10 (56) | |
| Percentage of BM blasts | |||
| <40 | 11 (22) | 2 (11) | NA |
| 40–70 | 8 (16) | 4 (22) | |
| >70 | 3 (6) | 2 (11) | |
| NK | 28 (56) | 10 (56) | |
| Type of AML | |||
| MDS-AML | 10 (20) | 3 (17) | 0.164e |
| t-AML | 11 (22) | 1 (5.6) | |
| De novo AML | 29 (58) | 14 (78) | |
| Type of therapy | |||
| Intensive | 32 (64) | 12 (67) | 1.00f |
| Hypomethylating agents | 1 (2) | 0 | |
| Palliative | 13 (26) | 5 (28) | |
| NK | 4 (8) | 1 (6) | |
| CRg | |||
| Yes | 18 (56) | 10 (83) | 0.160d |
| No | 14 (44) | 2 (17) | |
| EDg | |||
| Yes | 2 (6) | 0 (0) | 1.000d |
| No | 30 (94) | 12 (100) | |
| Allo SCT | |||
| Yes | 4 (8) | 4 (22) | NA |
| No | 15 (30) | 5 (28) | |
| NK | 31 (62) | 9 (50) | |
| Overall survivalg | |||
| Median (years) | 0.61 | 1.4 | 0.032h |
| 95% CI | 0.47–0.96 | 0.86-Ni | |
- Allo SCT = allogeneic stem cell transplantation; AML = acute myeloid leukemia; BM = bone marrow; CI = confidence interval; CR = complete remission; ED = early death (within 30 days from diagnosis); MDS = myelodysplastic syndrome; NA = not analyzed because of more than 50% uninformative cases; NK = not known; t-AML = treatment-related AML; WBC = white blood cell.
- a Cases grouped as “adverse” had −5, del(5q) or other changes resulting in loss of 5q, −7, del(7q) or other changes resulting in loss of 7q, −17, and/or der(17p), those grouped as “numerical” had numerical changes only, whereas those grouped as “structural” had non-adverse structural abnormality together with numerical changes.
- b Chi square test.
- c Kruskal–Wallis one-way analysis of variance.
- d Fisher exact probability test.
- e Fisher exact probability test: de novo AML versus MDS-AML/t-AML combined.
- f Fisher exact probability test: intensive versus palliative therapy.
- g Based only on patients receiving intensive therapy.
- h Log-rank test.
- i The data are not sufficient to calculate the upper confidence interval for the median survival time.
| HH/TT n = 68 (%) | IR n = 2,006 (%) | P value | CK n = 692 (%) | P value | |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median | 71 | 67 | 0.042a | 69 | 0.360a |
| 18–40 | 4 (6) | 166 (8) | 41 (6) | ||
| 41–60 | 11 (16) | 504 (25) | 157 (23) | ||
| >60 | 53 (78) | 1336 (67) | 494 (71) | ||
| Gender | |||||
| Male | 48 (71) | 1,047 (52) | 0.003b | 384 (55) | 0.020b |
| Female | 20 (29) | 959 (48) | 308 (45) | ||
| WBC count (×109/l) | |||||
| <10 | 23 (34) | 366 (18) | NA | 208 (30) | NA |
| 10–50 | 5 (7) | 203 (10) | 62 (9) | ||
| >50 | 2 (3) | 174 (9) | 30 (4) | ||
| NK | 38 (56) | 1,263 (63) | 392 (57) | ||
| Percentage of BM blasts | |||||
| <40 | 13 (19) | 291 (15) | NA | 166 (24) | NA |
| 40–70 | 12 (18) | 217 (11) | 85 (12) | ||
| >70 | 5 (7.4) | 217 (11) | 42 (6) | ||
| NK | 38 (56) | 1,281 (64) | 399 (58) | ||
| Type of AML | |||||
| MDS-AML | 13 (19) | 200 (10) | 0.004c | 97 (14) | 0.791c |
| MPN-AML | 0 (0) | 67 (3) | 51 (7) | ||
| t-AML | 12 (18) | 153 (8) | 95 (14) | ||
| De novo AML | 43 (63) | 1,586 (79) | 449 (65) | ||
| Type of therapy | |||||
| Intensive | 44 (65) | 1,544 (77) | 0.050d | 467 (67) | 0.650d |
| Hypomethylating agents | 1 (1.5) | 28 (1) | 18 (3) | ||
| Palliative | 18 (26) | 359 (18) | 164 (24) | ||
| NK | 5 (7.4) | 75 (4) | 43 (6) | ||
| CRe | |||||
| Yes | 28 (63) | 1,123 (73) | 0.229b | 233 (50) | 0.085b |
| No | 16 (36) | 421 (27) | 234 (50) | ||
| EDe | |||||
| Yes | 2 (5) | 109 (7) | 0.765b | 52 (11) | 0.300b |
| No | 42 (95) | 1,435 (93) | 415 (89) | ||
| Allo SCT | |||||
| Yes | 8 (12) | 340 (17) | NA | 76 (11) | NA |
| No | 20 (29) | 570 (28) | 193 (28) | ||
| NK | 40 (59) | 1,096 (55) | 423 (61) | ||
| Overall survivale | |||||
| Median (years) | 0.9 | 1.6 | 0.028f | 0.6 | 0.082f |
| 95% CI | 0.56–1.10 | 1.41–1.75 | 0.51–0.74 |
- Allo SCT = allogeneic stem cell transplantation; AML = acute myeloid leukemia; BM = bone marrow; CI = confidence interval; CK = cytogenetically complex (≥3 abnormalities); CR = complete remission; ED = early death (within 30 days from diagnosis); HH/TT = high hyperdiploid (49-65 chromosomes) or triploid/tetraploid (>65 chromosomes); IR = intermediate risk; MDS = myelodysplastic syndrome; MPN = myeloproliferative neoplasm; NA = not analyzed because of more than 50% uninformative cases; NK = not known; t-AML = treatment-related AML; WBC = white blood cell.
- a Kruskal–Wallis one-way analysis of variance.
- b Fisher exact probability test.
- c Fisher exact probability test: de novo AML versus MDS-AML/MPN-AML/t-AML combined.
- d Fisher exact probability test: intensive versus palliative therapy.
- e Based only on patients receiving intensive therapy.
- f Log-rank test.
Results
Frequency and cytogenetic/morphologic features of HH/TT
Among the 3,654 cytogenetically informative AML cases, 68 (1.9%) were HH (n = 50; 1.4%) or TT (n = 18; 0.5%); the karyotypes of these cases are given in Supporting Information Table I. Of the 68 HH/TT cases, 33 (49%) harbored adverse abnormalities, 15 (22%) numerical changes only, and 20 (29%) non-adverse structural aberrations in addition to numerical anomalies. The distributions of these cytogenetic subgroups did not differ significantly between the cases with HH and TT (Table 1). Apart from the group “not otherwise specified” (n = 25; 37%), the most common FAB types were M2 (n = 12; 18%), M4 (n = 12; 18%), and M5 (n = 6; 9%) (Supporting Information Table II).
Of the 33 cases with adverse abnormalities, 15 (45%) had partial loss of 5q, 13 (39%) monosomy 5, 13 (39%) monosomy 17, ten (30%) monosomy 7, five (15%) partial loss of 7q, and three (9%) 17p rearrangements. Additional chromosome gains, seen in at least 15% of cases, were +11 (n = 15; 45%), +22 (n = 13; 39%), +1, and +8, and +21 (n = 11; 33%), +13 and +14 (n = 8; 24%), +10, +19, and +20 (n = 7; 21%), +9 (n = 6; 18%), and +Y and +15 (n = 5; 15%).
Recurrent gains in the 15 cases with only numerical changes comprised, in decreasing frequency order, +8 (n = 9; 60%), +13 (n = 7; 47%), +14 and +22 (n = 5; 33%), +9 and +21 (n = 4; 27%), +4, +11, and +15 (n = 3; 20%), and +10, +18, and +20 (n = 2; 13%).
Among the 20 cases with non-adverse structural changes in addition to numerical changes, the most common (at least 15%) gains were +8 (n = 11; 55%), +13 (n = 5; 25%), +9 and +15 (n = 4; 20%), and +4, +14, +20, and +21 (n = 3; 15%). Rearrangements involving 11q (not 11q23) were the only recurrent structural change, seen in three (15%) of the cases.
The frequency distributions of autosomal chromosome gains in the three subgroups are given in Fig. 1. Because statistical analyses would entail 22 separate comparisons, resulting in high false discovery rates due to multiple testing, no such calculations were performed. However, based on visual inspection, some specific gains seem to be particularly common in the adverse group, such as trisomies 1 and 11 (Fig. 1).

Frequency distributions of autosomal chromosome gains in the three subgroups Numerical (N = 15; blue bars), Structural (N = 20; red bars), and adverse (N = 33; green bars). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Overall survival differs between patients with HH and TT AML
As seen in Table 1, the cases with HH or TT did not differ significantly with regard to age, gender, types of AML and therapy, and CR and ED rates. However, the OS differed significantly between patients with HH and TT (median 0.6 years vs. 1.4 years; P = 0.032; Fig. 2).
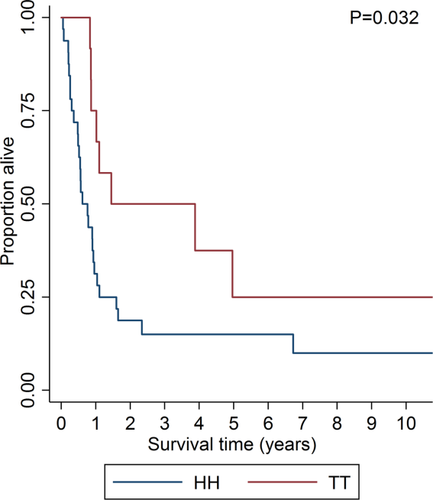
Overall survival (OS) for AML patients with high hyperdiploidy (HH; 49–65 chromosomes; median OS 0.6 years; blue curve) and triploid/tetraploidy (TT; >65 chromosomes; median OS 1.4 years; red curve) treated with intensive therapy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Overall survival differs between patients with HH/TT AML with and without adverse abnormalities
Of the 68 HH/TT cases, 33 (49%) had adverse chromosome changes and 35 (51%) had non-adverse aberrations (“numerical” and “structural” combined; the OS did not vary significantly between these two: median 1.0 years vs. 0.9 years; P = 0.338). The adverse and non-adverse groups did not differ with regard to CR rates (59% vs. 67%; P = 0.749) or ED rates (0% vs. 7%; P = 0.515). However, the OS was significantly longer for those with non-adverse changes than for those with adverse abnormalities (median 1.1 years vs. 0.8 years; P = 0.044; Fig. 3).
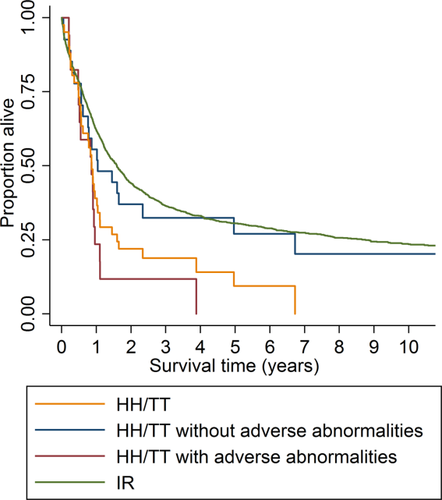
Overall survival (OS) for AML patients with high hyperdiploidy or triploid/tetraploidy (HH/TT; ≥49 chromosomes; yellow curve), HH/TT without adverse abnormalities (blue curve), HH/TT with adverse abnormalities (red curve), and with intermediate risk (IR; green curve). The OS was significantly longer for HH/TT cases without adverse changes than for those with adverse abnormalities (median 1.1 and 0.8 years, respectively; P = 0.044) and the OS was significantly longer for IR cases than for those with HH/TT (median 1.6 and 0.9 years, respectively; P = 0.028). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
HH/TT versus CK AML
Among the 3,654 cytogenetically informative AML cases, 692 (19%) had CK and were hence classified as high risk AML. The age distribution, frequency of de novo AML, type of therapy given, CR and ED rates, and OS did not differ significantly between the patients with HH/TT and CK AMLs (Table 2). However, there was an overrepresentation of males in the HH/TT group (71% vs. 55%; P = 0.020).
HH/TT versus IR AML
A total of 2,006 (55%) of the 3,654 AML cases were grouped as IR. There were no significant differences between the HH/TT and IR AMLs with regard to type of therapy given and CR and ED rates (Table 2), whereas patients with IR AML were younger (median 67 years vs. 71 years; P = 0.042), more often females (48% vs. 29%; P = 0.003), and had a higher frequency of de novo AML (79% vs. 63%; P = 0.004). Furthermore, OS was significantly longer in the IR group (median 1.6 years vs. 0.9 years; P = 0.028; Fig. 3).
Discussion
In the present population-based series of adult AML, HH and TT were identified in 1.4% and 0.5% of cases, respectively; these frequencies are on a par with those in previous studies 21, 23, 24. Furthermore, the most prevalent chromosome gains, i.e., +8 (46%), +11 and +13 (29%), +22 (28%), +21 (26%), +14 (22%), +9 (21%), and losses, i.e., −5 and −17 (19%) and −7 (15%), are also similar to those previously reported in HH/TT AML 21, 23, 24. Thus, we conclude that our patient cohort, the second largest series of HH/TT AML to date, is representative of this rare cytogenetic subgroup of adult AML.
There were no relevant age differences between patients with HH/TT (median 71 years) and CK AML (median 69 years), whereas those with IR AML were somewhat younger (median 67 years) (Table 2). The median age of patients with HH and/or TT AML in previous studies has been lower—58 years 24, 64 years 22, 65 years 23, and 70 years 21; this most likely reflects the population-based nature of our cohort. In fact, close to 80% of the patients with HH/TT AML were older than 60 years (Tables 1 and 2). Thus, the karyotypic features HH and TT in adult AML seem to be associated with high age.
HH/TT AML was more common in males (71%) compared with those grouped as IR (52%) or CK (55%) (Table 2). A male preponderance has also been noted in some 21, 22, but not all 23, 24, previous studies. However, considering that relatively few patients were included in our study, the observed gender difference may well be fortuitous; a possibility further supported by the fact that the proportions of males with HH/TT AML and non-HH/TT AML are next to identical (57% vs. 56%) among all published cytogenetically abnormal AML cases 1. In contrast, HH/TT in pediatric AML has been reported to be more common in girls 25. In childhood AML, HH/TT has also been associated with acute megakaryoblastic leukemia (AMKL) 24, 25; this morphologic subtype comprised 30% of all HH/TT AML cases in a recent NOPHO-AML study 25. However, only one patient had AMKL in our cohort. Instead, most of the morphologically characterized HH/TT AML cases were of the FAB types M2, M4, or M5 (Supporting Information Table II). Interestingly, a high prevalence of monocytic subtypes has also been noted in previous studies 22-24. Because some of these 23, 24 included AMLs with KMT2A rearrangements and considering that such abnormalities are strongly associated with monocytic/monoblastic leukemia 1, the inclusion of KMT2A-positive cases may partly explain the high frequency of M4/M5 in these studies. We did not include HH/TT AMLs with 11q23 abnormalities, so the frequent occurrence of M4/M5 in our series is thus not due to the presence of such changes; however, it should be stressed that we did not screen for cryptic KMT2A rearrangements, which in one study were reported to be present in 15% of HH AML 23.
The types of AML did not differ between the HH/TT and CK AMLs, whereas de novo AML was significantly more common in IR AML (Table 2). None of the HH/TT cases was secondary to a myeloproliferative neoplasm (MPN), whereas MPN AML comprised 3% and 7% of the cases grouped as IR and CK, respectively. This may well reflect the relatively few HH/TT-positive cases presented herein. However, it may be noteworthy that all secondary HH/TT AMLs in the three previous series with information on type of prior hematologic malignancy 21-23 occurred after a myelodysplastic syndrome (MDS), and not MPN. It is obviously premature to draw any firm conclusions at this stage, but, at present, MPN-AML seems to be underrepresented in cases with HH/TT.
The ED rate (5%) of the intensively treated patients with HH/TT AML did not differ significantly from what was observed in IR (7%) and CK (11%) AML (Table 2) and was also similar to what has been previously reported (5–9%) in HH/TT AML 22, 23. The CR rates of the patients receiving intensive treatment also did not vary between the HH (56%) and TT (83%) AMLs (Table 1) or between the HH/TT (63%) and the IR (73%) and CK (50%) cases (Table 2). Furthermore, the CR rate of the HH group was comparable with the 63% reported in the LRCG study 24. Thus, HH AML does not appear to be associated with a particularly poor early response to chemotherapy.
The OS did not differ between patients with HH/TT and CK AML, whereas it was significantly longer in the ones with IR AML (Table 2; Fig. 3). Thus, as a group, HH/TT is associated with a poor outcome, similar to CK AML. However, HH/TT AMLs are clearly prognostically heterogeneous. First, patients with TT fared significantly better than those with HH; the median survival of these two groups was 1.4 and 0.6 years, respectively (Table 1; Fig. 2). Second, and as also emphasized in two previous studies 23, 24, the types of abnormality present play a major prognostic role. Luquet et al. 23 reported that HH cases with only numerical changes, excluding those with cryptic KMT2A rearrangements, had a CR rate of close to 90% and that their outcome was not as unfavorable as for those with CK AML, suggesting that such HH AMLs should be classified as IR. This was confirmed by the LRCG who could subgroup the HH cases with structural changes into those with and without adverse abnormalities 24. Thus, they delineated three distinct subgroups with significantly different 5-year OS rates: numerical (28%), structural (18%), and adverse (7%). In the present study, the median OS was 1.0 years for the cases with only numerical changes, 1.1 years for those with non-adverse structural aberrations, and 0.8 years for those with adverse abnormalities, with the OS being significantly worse for the latter group compared with the one with numerical/non-adverse structural aberrations combined (Fig. 3).
In conclusion, HH/TT AML is associated with a poor outcome, but chromosome numbers >65 and absence of adverse aberrations seem to translate into a more favorable prognosis. Thus, we suggest that cases with HH/TT should not automatically be grouped as HR AML.
Author Contributions
VL designed the study and wrote the manuscript. AR planned and performed the statistical analyses and critically reviewed the manuscript. BJ reviewed and subgrouped the karyotypic strings and co-wrote the manuscript. PA, RB, ÅR-D, SL, LM, DS, BU, LW, AW, MH, and GJ provided clinical data and critically reviewed the manuscript.




