KIT D816V and JAK2 V617F mutations are seen recurrently in hypereosinophilia of unknown significance
Conflict of interest: The authors declare no conflict of interest
Abstract
Myeloproliferative neoplasms with eosinophilia are commonly characterized by a normal karyotype and remain poorly defined at the molecular level. We therefore investigated 426 samples from patients with hypereosinophilia of unknown significance initially referred for screening of the FIP1L1-PDGFRA (FP) fusion gene also for KIT D816V and JAK2 V617F mutations. Overall, 86 (20%) patients tested positive: FP+ in 55 (12%), KIT D816V+ in 14 (3%), and JAK2 V617F+ in 17 (4%) patients, respectively. To gain better insight into clinical characteristics, we compared these cases with 31 additional and well-characterized KIT D816V+ eosinophilia-associated systemic mastocytosis (SM-eo) patients enrolled within the “German Registry on Disorders of Eosinophils and Mast cells.” Significant differences included younger age, male predominance, and higher eosinophil counts for FP+ cases while abdominal lymphadenopathy, ascites, and serum tryptase levels >100 μg/l were characteristic for those with KIT D816V. Leukocytes, hemoglobin, and splenomegaly did not differ significantly. A median of three additional mutations, most frequently TET2 and SRSF2, were identified in 12/13 KIT D816V+ SM-eo patients with available material indicating a more complex molecular pathogenesis. Median survival was not reached for FP+ cases but was only 26 and 41 months for KIT D816V+ SM and JAK2 V617F+ MPN-eo, respectively. Eosinophilia of ≥2 × 109/l was identified as discriminator for inferior survival in KIT D816V+ and/or JAK2 V617F+ patients (median survival 20 months vs. not reached, P = 0.002). Thus, there is a clear prognostic and therapeutic rationale for detection of KIT D816V and JAK2 V617F in the diagnostic work up of eosinophilia. Am. J. Hematol. 90:774–777, 2015. © 2015 Wiley Periodicals, Inc.
Introduction
In the diagnostic work-up of hypereosinophilia of unknown significance (HEUS), eosinophilia is frequently considered to be a reactive condition due to allergic reactions, infections, or autoimmune disorders. In a minority of cases, eosinophilia is caused by hematologic disorders. These cases are subcategorized into clonal stem-cell disorders (eosinophilia-associated myeloproliferative neoplasms [MPN-eo] or chronic eosinophilic leukemia [CEL]) and non-clonal eosinophilia caused by aberrant production of eosinophilopoietic cytokines (e.g. Interleukin 3 [IL-3], IL-5, granulocyte-macrophage colony-stimulating factor [GM-CSF]) by clonal T-lymphocytes 1, 2.
In MPN-eo, the most common molecular aberrations are tyrosine kinase (TK) fusion genes with involvement of PDGFRA, e.g. FIP1L1-PDGFRA (FP), PDGFRB, e.g. ETV6-PDGFRB, or FGFR1, e.g. ZNF198-FGFR1, leading to a constitutively activated TK activity of the fusion protein 3-7. With the exception of the cytogenetically invisible FP fusion gene, which is created by an interstitial deletion at chromosome band 4q12, all other fusion genes are usually suspected by an abnormal karyotype with characteristic balanced reciprocal translocations or other rearrangements involving certain chromosomal bands, e.g. 8p11-2 as an indicator for a rearrangement of FGFR1 8. FP is by far the most frequently identified fusion gene and therefore molecular testing by RT-PCR (reverse transcription polymerase chain reaction) or FISH (fluorescence in situ hybridization) is usually performed early in the diagnostic work-up of HEUS also due to excellent response rates of targeted treatment with imatinib 9.
Overall, TK fusion proteins other than BCR-ABL in chronic myeloid leukemia (CML) constitute only a relatively small proportion of mutational events in MPN, whereas point mutations in JAK2 (V617F), CALR, MPL, KIT (D816V), ASXL1, TET2, SRSF2, RUNX1, NRAS, KRAS, CBL, IDH1/2, EZH2, LNK, etc. play a pivotal role in the pathogenesis of BCR-ABL negative MPN 10, 11. Not much is known about the frequency of these point mutations in FP negative MPN-eo with normal karyotype 12, 13.
To date, treatment options for patients without underlying imatinib-sensitive PDGFRA or PDGFRB TK fusion genes are limited. However, for patients with KIT D816V or JAK2 V617F point mutations, the TK inhibitors ruxolitinib or midostaurin may offer potential targeted treatment 14, 15. We therefore sought to explore the frequency of FP, KIT D816V, and JAK2 V617F in patients who were primarily referred for routine testing of FP and to identify the clinical characteristics of specific disease subtypes.
Materials and Methods
Patients and samples
Initially, peripheral blood (PB) or bone marrow (BM) samples from 426 patients (male, n = 281; female, n = 145) with HEUS were screened for FP, KIT D816V, and JAK2 V617F. Clinical characteristics were obtained from positive patients. An additional 31 patients with previously diagnosed KIT D816V+ eosinophilia-associated (eosinophils >1 × 109/l) systemic mastocytosis (SM-eo, SM-CEL) were added for a more thorough comparison of clinical characteristics. The study design adhered to the tenets of the Declaration of Helsinki and was approved by the relevant institutional review board (Medical Faculty Mannheim, University of Heidelberg). All patients gave written informed consent.
Mutational analysis
DNA was extracted following standard procedures. Total RNA was extracted from leukocytes after red cell lysis according to the manufacturers protocol using TriZol (Life Technologies, Darmstadt, Germany) or the CsCl gradient centrifugation method as previously described 16. RNA was reverse transcribed using random hexamers and MMLV reverse transcriptase (Life Technologies). Nested RT-PCR for detection of the FP fusion gene, allele specific qualitative PCR (ARMS) from DNA for detection of the JAK2 V617F mutation, quantitative allele specific PCR (RQ-PCR) for detection of the KIT D816V mutation, and comprehensive mutational profiling of 17 candidate genes in KIT D816V+ patients were performed as previously described 17.
Statistical analysis
Correlation between variables was investigated by Mann–Whitney U test. Survival probabilities and median overall survival times were calculated by the Kaplan–Meier method. The significance level of the two-sided P values was 0.05 for all statistical testing procedures. Analyses were performed with Graphpad Prism 5.
Results
Screening of HEUS
Initially, 426 randomly selected patients with HEUS were screened for presence of FP, KIT D816V, and JAK2 V617F. Overall, 86 (20%) cases tested positive: FP in 55 (12%), KIT D816V in 14 (3%), and JAK2 V617F in 17 (4%) patients, respectively. All patients were screened for all three molecular aberrations. While FP and KIT D816V or JAK2 V617F were mutually exclusive, three KIT D816V+ patients were also JAK2 V617F+.
Clinical characteristics
The finding of KIT D816V suggested a previously unsuspected diagnosis of SM-eo in 14 cases. This was confirmed by BM trephine in 8/8 cases that were examined. In the remaining six patients without trephine biopsy, the median expressed KIT D816V allele burden (EAB) was 22% (range 2–40%) suggesting advanced disease 17. For the comparison of clinical characteristics, these 14 cases were therefore grouped together with a further 31 patients previously diagnosed with KIT D816V+ SM-eo/SM-CEL (Table 1). The most striking clinical differences between FP+, KIT D816V+, and JAK2 V617F+ patients were a higher median age in KIT D816V+ and JAK2 V617F+ patients (49 vs. 67. vs. 69 years, P < 0.001), male gender predominance in FP+ patients (P < 0.001), leukocytosis in FP+ patients (20.0 × 109/l vs. 9.7 × 109/l vs. 15.0 × 109/l, P < 0.05) and elevated median absolute eosinophil counts in FP+ vs. KIT D816V+ patients (7.1 × 109/l vs. 2.2 × 109/l, P < 0.0001). The highest individual eosinophil count of 99.5 × 109/l was observed in a KIT D816V+ patient with multiple additional mutations, e.g. ASXL1, RUNX1, and NRAS. Significantly elevated serum tryptase levels (normal value <11.4 μg/l) were detected in the majority of FP+ and KIT D816V+ patients, levels >50 μg/l were almost exclusively observed in KIT D816V+ SM patients (FP, 3/29; KIT D816V, 22/28).
| FIP1L1-PDGFRA+ | KIT D816V+ | JAK2 V617F+ | |
|---|---|---|---|
| Number of patients | 55 | 45 | 17 |
| Age (years) (median, range) | 49 (19–74) | 67 (33–81) | 69 (60–87) |
| Gender (m/f) | 54/1 | 26/19 | 10/7 |
| Leukocytes (×109/l) (median, range) | 20.0 (5.2–173.0) n = 55 | 9.7 (3.6–123.0) n = 44 | 15.0 (7.1–43.6) n = 17 |
| Eosinophils (×109/l) (median, range) | 7.1 (0.9–45.5) n = 54 | 2.2 (1.0–99.5) n = 44 | 3.8 (1.1–16.5) n = 16 |
| Hemoglobin (g/dl) (median, range) | 13.0 (7.9–15.8) n = 50 | 11.1 (6.8–14.7) n = 40 | 13.3 (6.9–17.3) n = 13 |
| Platelets (×109/l) (median, range) | 143 (11–600) n = 48 | 130 (27–945) n = 39 | 212 (50–597) n = 13 |
| Serum tryptase (µg/l) (median, range) | 29 (0–183) n = 29 | 162 (14–630) n = 28 | 105a (9–371) n = 3 |
| <20 | 10 | 1 | 1 |
| 20–50 | 16 | 5 | 0 |
| >50 | 2 | 3 | 0 |
| >100 | 1 | 7 | 1 |
| >200 | 0 | 12 | 1 |
| Splenomegaly | 36/44 (82%) | 31/35 (89%) | 6/8 (75%) |
| Ascites | – | 16/45 (42%) | – |
| Abdominal lymphadenopathy | – | 24/45 (63%) | – |
- a 2/3 patients were concomitantly JAK2 V617F+ and KIT D816V+.
Bone marrow histology and final diagnosis
Overall, advanced SM (aggressive SM [ASM] or mast cell leukemia [MCL]) with or without associated clonal hematologic non-mast cell lineage disorder (AHNMD) was diagnosed in 25/45 (56%) KIT D816V+ patients due to characteristic BM morphology and presence of one or more C-findings, e.g. anemia <10 g/dl (n = 10) and/or thrombocytopenia <100 × 109/l (n = 14), ascites (n = 14), histologically proven liver infiltration (n = 5) or weight loss (n = 6). In the absence of C-findings, the final diagnosis was indolent SM (ISM, n = 3), SM-AHNMD (n = 7), no subtyping (n = 3). In six patients, no underlying SM was concluded. There was no correlation between the KIT D816V EAB and absolute or relative numbers of eosinophils (n = 34, median 20%, range 0–57%). BM histology was available in 7/17 JAK2 V617F+ MPN-eo patients and suggested MPN-eo (n = 3) and MPN-eo with monocytosis (n = 1). In the three cases that were also KIT D816V+, morphological diagnoses were SM-CEL (n = 2) and myelodysplastic syndrome (MDS, n = 1).
Comprehensive mutational analysis
Comprehensive mutational profiling was performed for 13/45 (29%) KIT D816V+ patients with 12/13 (92%) patients being positive for at least 1 (median 3, range 0–4) additional mutation (TET2, n = 9; SRSF2, n = 5; CBL, n = 3; RUNX1, n = 3; EZH2, n = 2; ASXL1, n = 2; KRAS, n = 2; and JAK2 V617F, n = 2).
Survival analysis
Follow-up data was available for 96 of 117 patients. Median overall survival (OS) was not reached in FP+ patients with 47/55 (85%) patients being in complete molecular remission while treated for median 78 months (range 1–213) with imatinib. The median survival was significantly shorter in KIT D816V+ (26 months, P < 0.0001) and JAK2 V617F+ (40 months, P < 0.0001) patients, respectively, with no significant difference between KIT D816V+ and JAK2 V617F+ patients (P = 0.5; Fig. 1). In a joint analysis of KIT D816V+ and JAK2 V617F+ patients, we identified eosinophilia of ≥2 × 109/l as discriminator for inferior survival (median survival 20 months vs. not reached, P = 0.002) by a minimal P-value approach (Table 1 and Fig. 2).
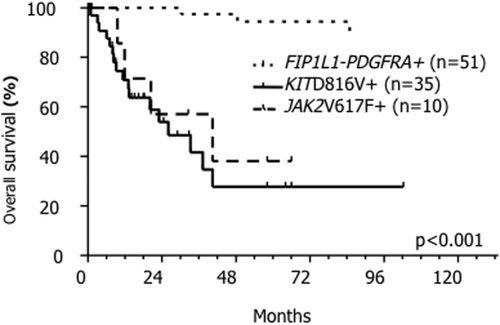
Kaplan–Meier estimate of overall survival in 96/117 mutation positive patients. FIP1L1-PDGFRA positive (FP+) patients are depicted with the spotted line, JAK2 V617F+ patients are depicted with the dashed line and KIT D816V+ patients are depicted with the continuous line. Median survival in FP+ patients was not reached, whereas it was 26 months in KIT D816V+ (P < 0.001) and 40 months in JAK2 V617F+ (P < 0.001) patients.
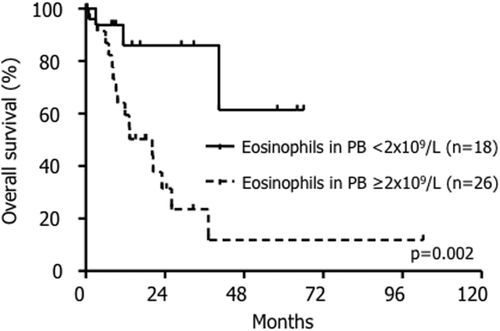
Kaplan–Meier estimate of overall survival (OS) in KIT D816V+ and/or JAK2 V617F+ patients. Patients with absolute eosinophil counts of ≥2 × 109/l in peripheral blood (PB) had a significantly shorter OS than patients with lower eosinophil counts (median survival 20 months vs. not reached, P = 0.002).
Discussion
The identification of the FP fusion gene and the rapid and durable responses on imatinib has revolutionized diagnosis and treatment of MPN-eo 3, 9, 18. The reported frequency of 2–15% FP positive cases in the diagnostic work-up of HEUS would be higher when the combination of characteristic clinical features are considered, i.e. such as male gender, elevated serum tryptase, and the characteristic BM features including increased numbers of loosely scattered mast cells and significant fibrosis. However, these features are not sufficient to definitely recognize FP cases and thus screening of a broader phenotype is required for accurate diagnosis. Alternative TK fusion genes with involvement of PDGFRB, FGFR1, or JAK2 are identified by corresponding chromosomal aberrations, e.g. reciprocal translocations, with rearrangement of 5q31-33, 8p11-12 or 9p24, respectively, in less than 3% of HEUS patients 4, 19, 20.
Numerous new mutations in genes encoding for signaling molecules, transcription factors, epigenetic regulators, or splicing factors have been identified in patients with myeloid neoplasms. In some subtypes, the prevalence of mutations in one or more genes is >90%, e.g. in chronic myelomonocytic leukemia. Most likely due to the considerably greater clinical heterogeneity of HEUS, our understanding of the molecular pathogenesis of reactive eosinophilia, HES or MPN-eo without TK fusion genes is still limited. Nevertheless, we have identified KIT D816V and JAK2 V617F mutations in 3% and 4%, respectively, of HEUS samples, which were initially only referred to our laboratory for screening of FP. The finding of these mutations confirms an underlying myeloid neoplasia and identified cases with a poor prognosis.
The association with eosinophilia is a characteristic feature of advanced SM where it is observed in approximately 15–30% of patients 12, 21. In our series, 49% of the KIT D816V+ SM-eo/SM-CEL patients had significant anemia and/or thrombocytopenia as typical hematological C-findings defining aggressive SM. Additional characteristic C-findings in advanced SM included splenomegaly and ascites. While splenomegaly is also observed in the vast majority of patients with FP and JAK2 V617F associated MPN-eo, only SM patients presented with ascites and abdominal lymphadenopathy. The reasons why KIT D816V+ SM had not yet been diagnosed or even considered in the referred cases may be (a) the molecular testing of FP in PB was performed prior to a BM biopsy or (b) missed evaluation of serum tryptase and/or BM core biopsy or (c) missed diagnosis because of inappropriate BM evaluation. Because an elevated serum tryptase is so strongly characteristic for FP+ CEL or KIT D816V+ SM, its analysis is clearly recommended in every HEUS patient at diagnosis. In JAK2 V617F+ MPN-eo, elevated serum tryptase may hint upon the concurrent presence of KIT D816V as observed in 3 of 17 patients in our cohort.
In classical MPN, the BCR-ABL fusion gene is detected by definition in all CML cases whereas JAK2 V617F is seen in ∼95% of patients with PV and 60% of patients with MF or ET 22. While significant eosinophilia is a common feature for BCR-ABL1 positive CML at diagnosis, it is usually rare in JAK2 V617F+ MPN with less than 10 patients reported to our knowledge 23-25. In contrast to JAK2 V617F, eosinophilia in BM and/or PB is commonly observed when JAK2 is involved in fusion genes, e.g. PCM1-JAK2. The reasons for this are unknown although it is likely that the degree of activation of JAK2 is much stronger in a fusion gene compared to point mutation leading to anti-apoptotic signaling in eosinophils via GM-CSF and IL-5 activation 26, 27. In our series of 17 JAK2 V617F+ patients with eosinophilia, important differentiating features included the absolute eosinophil numbers that did not exceed 20 × 109/l and the concurrent presence of KIT D816V in three patients with associated elevated serum tryptase in two patients.
We here report on the largest cohort of HEUS patients to be systematically screened for FP, KIT D816V, and JAK2 V617F. Despite the rarity of those molecular aberrations, their identification is of clear diagnostic, prognostic, and therapeutic relevance because of the potential for targeted therapy 14, 15. The absolute number of eosinophils is significantly associated with poorer outcome in KIT D816V+ and JAK2 V617F+ patients which highlights the importance of adequate diagnosis and treatment in patients with eosinophilia. Preliminary data of early clinical trials on midostaurin in KIT D816V+ advanced SM have shown an exquisite sensitivity to eosinophils 28. Thoroughly performed assessment of organ involvement/dysfunction, serum tryptase, PB and BM morphology, cytogenetic analysis, specific mutational allele burden, and concurrent mutations will allow to gain further insights into individual pathogenesis, prognosis, and consequent therapeutic decisions between watch and wait, TK inhibitor, chemotherapy, or allogeneic stem cell transplantation.
Author Contributions
JS, RU, NN, MJ, SS and AF performed the laboratory work for the study. GM, AR, WKH provided patient material and information. KS, HPH and TG reviewed the bone marrow biopsies. JS, NCPC, AF and AR wrote the manuscript.
Acknowledgment
The authors thank Elena Felde and Maike Haas for excellent technical support.




