Bortezomib, melphalan, prednisone (VMP) versus melphalan, prednisone, thalidomide (MPT) in elderly newly diagnosed multiple myeloma patients: A retrospective case-matched study
Conflicts of interest: Employment or Leadership Position: None. Consultancy or Advisory Role: Sara Bringhen, Merck Sharp & Dohme; Peter Gimsing, Janssen-Cilag, Novartis and Celgene; Sonja Zweegman, Celgene and Janssen-Cilag; Juan José Lahuerta, Celgene and Janssen-Cilag; Albert Oriol, Celgene, Janssen-Cilag and Novartis; Gunnar Juliusson, Merck Seorno and Genzyme; Anders Waage, Janssen-Cilag, Celgene and Mundipharma; Jesus San Miguel, Millennium, Celgene and Johnson & Johnson; Mario Boccadoro, Celgene and Janssen-Cilag; Pieter Sonneveld, Celgene, Janssen-Cilag, Novartis, Onyx, Millennium-Takeda; Antonio Palumbo, Celgene and Janssen-Cilag. Stock Ownership: None. Honoraria: Sara Bringhen, Celgene, Janssen-Cilag, and Novartis; Alessandra Larocca, Celgene, Janssen-Cilag; Maria Victoria Mateos, Celgene, Janssen-Cilag, Millennium and Novartis; Peter Gimsing, Janssen Cilag, Celgene, and MundiPharma; Sonja Zweegman, Celgene and Janssen-Cilag; Musto Pellegrino, Celgene and Janssen-Cilag; MaideCavalli, Celgene; Michele Cavo, Janssen-Cilag, Novartis; Antonio Palumbo, Celgene, Janssen-Cilag, Merck and Amgen; Massimo Offidani, Celgene and Janssen-Cilag. Research Funding: Pellegrino Musto, Celgene and Janssen-Cilag; Mario Boccadoro, Celgene and Janssen-Cilag. Expert Testimony: None. Other Remuneration: None.
Abstract
Novel agents in combination with melphalan and prednisone (MP) significantly improved progression-free survival (PFS) and overall survival (OS) in multiple myeloma (MM). Randomized trials comparing MP plus bortezomib (VMP) versus MP plus thalidomide (MPT) are lacking. Nine hundred and fifty-six elderly (>65 years) newly diagnosed MM patients from six European randomized trials were retrospectively analyzed and matched for age, albumin, and beta2-microglobulin at diagnosis, 296 patients were selected from the VMP groups, and 294 from MPT. Complete response rate was 21% in the VMP patients and 13% in the MPT patients (P = 0.007). After a median follow-up of 34 months (range, 1–92), VMP significantly prolonged both PFS (median 32.5 vs. 22.9 months, HR 0.65; 95% CI 0.52–0.82; P < 0.001) and OS (median 79.7 vs. 45.1 months, HR 0.44; 95% CI 0.32–0.59; P < 0.001) in comparison with MPT. The benefit in terms of OS of the VMP group was quite similar among patients with different risk factors defined by sex, ISS, ECOG performance status, or serum creatinine but not among patients ≥75 years. Multivariate analysis confirmed that VMP was an independent predictor of longer PFS and OS. In a control-case matched analysis, PFS and OS were prolonged in patients who received VMP in comparison with those treated with MPT. Am. J. Hematol. 89:355–362, 2014. © 2013 Wiley Periodicals, Inc.
Introduction
A meta-analysis of 27 randomized trials showed that melphalan plus prednisone (MP) was as effective as several combination chemotherapies in prolonging overall survival (OS) 1. In elderly multiple myeloma (MM) patients, MP has been the reference treatment for several decades.
Meta-analysis 2, 3 of data from six randomized trials 4-9 showed that combination of thalidomide and MP (MPT) improved progression-free survival (PFS) and OS compared with MP alone in transplant ineligible MM patients. Only two 5, 6 of the six 4-9 phase III trials comparing MPT vs. MP demonstrated OS benefit. Overall, MPT regimen led to 17% risk reduction of death compared with MP and increased median OS by 6.6 months 3.
The clinical relevance of the combination bortezomib plus MP (VMP) has been explored in large randomized trials 10-15. In the VISTA trial, VMP was superior to MP, with risk reductions in progression (52%) and in death (31%) 10-12. In two subsequent studies, a reduced intensity schedule (once-weekly) of bortezomib significantly decreased the incidence of peripheral neuropathy without any negative effect on PFS or OS 14, 15. In elderly patients, first-line therapy including novel agents enhanced survival, although to a lesser degree 16 mainly due to treatment-related toxicities. Thus, the concomitant presence of multiple diseases in these patients greatly influences treatment decisions and requires judicious screening and necessary support 16.
MPT and VMP regimens are now regarded the new standard of care for untreated MM patients ≥65 years. No randomized trial comparing MPT vs. VMP has been performed. In this case-matched study of individual patient data (matched for age, albumin and beta2-microglobulin) from six randomized trials, we assessed the impact of treatment on outcome in elderly untreated MM patients receiving MPT or VMP.
Methods
Patient selection
Patients with untreated MM, ineligible for autologous transplantation, enrolled in the VMP or MPT arms of six published European phase III trials—GISMM-2001, HOVON49, NMSG, PETHEMA-GEM05MAS65, GIMEMA-MM0305, and TMSG—were evaluated 4, 7-9, 13, 14. We evaluated only patients >65 years since the studies had different eligible age ranges. We analyzed data only from European groups who wished to participate in the study. Data of IFM 99-06, IFM 01/01, and VISTA trials were not available for this analysis. Details on treatment regimens (Table 1) and results have previously been reported 4, 7-9, 13, 14. To increase comparability among treatment arms, we matched patients in homogenous strata defined by k-means clustering 17 that assigned each observation to the nearest mean in the data space here consisting of three variables considered influential on main outcomes: age, albumin, and beta2-microglobulin at diagnosis, treated as continuous variables. The K-means method identified and then clustered those patients with the nearest average distance in the metric of a set of predefined variables. Thus, from an overall source population of 956 patients, 590 patients in either MTP or VMP arms were selected retrospectively.
| Trial | Country | No. of pts | Inclusion year | Age, y | SD stage | WHO status | Schedule | No. of cycles/interval (weeks) | Maintenance |
|---|---|---|---|---|---|---|---|---|---|
| GISMM-20014 | Italy | 331 | 2002-05 | > 65 | II, III | 0–4 | M: 4 mg/m2 days 1–4; P: 40 mg/m2 days 1–7; ± Thal: 100 mg/day | 6/4 | Thal: 100 mg until relapse for pts treated with MPT |
| HOVON498 | The Netherlands/Belgium | 333 | 2002-07 | > 65 | Ib, II, III | 0–3 | M: 0.25 mg/kg days 1–5; P: 1 mg/kg days 1–5; ± Thal: 200 mg/day | 8/4 | Thal: 50 mg until relapse for pts treated with MPT |
| NMSG127 | Nordic | 357 | 2002-07 | > 65 | I-III symptomatic | 0–4 | M: 0.25 mg/kg days 1–4; P: 100 mg days 1–4; ± Thal: 200–400 mg/day | Until plateau/6 | Thal: 200 mg/day from plateau until relapse for pts treated with MPT |
| TMSG9 | Turkey | 114 | 2006-09 | > 55 | I-III symptomatic | 0–2 | M: 9 mg/m2 days 1–4; P: 60 mg/m2 days 1–7; ± Thal: 100 mg/day | 8/6 | No |
| GIMEMA-030513 | Italy | 511 | 2006-09 | > 65 | I-III symptomatic | 0–2 | M: 9mg/m2 days 1–4; P: 60mg/m2 days 1–4; Vel: 1.3 mg/m2 days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1–4 and on days 1, 8, 22, and 29 during cycles 5–9; ± Thal: 50 mg/daya | 9/6a | Vel: 1.3 mg/m2 every 14 days + Thal: 50 mg/day for 2 years or until progression or relapse for pts treated with VMPT |
| PETHEMA-GEM05MAS6514 | Spain | 260 | 2006-08 | > 65 | I-III symptomatic | 0–2 | Vel: 1.3 mg/m2 days 1, 4, 8, 11, 22, 25, 29, and 32 during cycle 1 and on days 1, 8, 22, and 29 during cycles 2–6; M: 9mg/m2 days 1–4; P: 60mg/m2 days 1–4; vs. Vel: 1.3 mg/m2 days 1, 4, 8, 11, 22, 25, 29, and 32 during cycle 1 and on days 1, 8, 22, and 29 during cycles 2–6 P: 60 mg/m2 days 1–4; Thal: 100 mg/day | 6/6 (5 for cycle 1) | Patients completed the 6 cycles were randomly assigned to maintenance schedule: Vel: 1.3 mg/m2 days 1, 4, 8, 11 every 3 months P: 50 mg every 48 h or Vel: 1.3 mg/m2 days 1, 4, 8, 11 every 3 months Thal: 50 mg daily for up to 3 years or until progression or relapse |
- M: melphalan; P: prednisone; Thal: thalidomide; Vel: bortezomib.
- a After the inclusion of the first 139 patients, both VMPT and VMP induction schedules were changed to nine 5-week cycles and Vel was administered on days 1, 8, 15, and 22 during cycles 1 to 9.
Overall, 296 patients were selected from the VMP groups, 180 and 116 from GIMEMA-MM0305 13 and PETHEMA-GEM05MAS65 trials 14, respectively. Moreover, 125/296 cases received bortezomib once-weekly, 38 received twice-weekly, and 133 received twice-weekly for the first few cycles (range 1–4 cycles) and subsequently once-weekly. A further 294 cases were chosen from the MPT arms, 80 from HOVON-49 8, 89 from GISMM-2001 4, 96 from NMSG 7, and 29 from TMSG trial 9. Patients were treated between 2002 and 2009 with median follow-up of 34 months (range 1–92) for the whole cohort, 34 (1–81) for the MPT group, and 34 (1–92) for VMP. Primary and secondary endpoints were OS and PFS, respectively, according to International Myeloma Working Group (IMWG) criteria. Selection bias was investigated comparing survival between selected and discarded cases in the two study arms: no differences were detected for the VMP group, while there was a slightly worse survival of the discarded cases among the MPT group (data not shown). Therefore, the comparison of the VMP group was against the better-performing selection of cases from the MPT group, with a possible underestimation of the net effect and with no inflation of the results.
The institutional review board at each participating center approved trials that were performed in accordance with the Declaration of Helsinki. All patients provided written informed consent. Trials were registered at ClinicalTrials.gov or controlled-trials.com: NCT00232934 4, NCT00218855 7, ISRCTN90692740 8, NCT01063179 13, NCT00443235 14, and NCT00934154 9. This retrospective study was approved by each single institutional review board responsible for the original prospective trial.
Assessment
Data were collected at each coordinating center, sent to a central coordinating center, reviewed for consistency and completeness, and entered into a new database. Age, gender, creatinine value, Durie-Salmon stage, ISS score, ECOG Performance Status (PS), serum calcium, and Ig isotype together with date of progression or date of last follow-up, date of death or of last follow-up, best response achievement, and grade of adverse events (AEs) according to National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0 were collected. In HOVON-49 and GEM05MAS65 trials, responses were initially determined using the European Group for Blood and Marrow Transplantation criteria 18 and re-evaluated using IMWG criteria 19. For GISMM-2001, NMSG, TMSG, and GIMEMA-MM0305 trials, responses were assessed using IMWG criteria 19. The relapse criteria utilized are reported in Supporting Information Table 1.
Statistical analysis
OS was defined as the time from study entry to death due to any cause, and PFS as the time from study entry until progression or death due to myeloma; in both cases, patients still alive were censored at the date of last contact.
Kaplan-Meier curves for PFS and OS were calculated for the chemotherapy regimens (VMP vs. MPT) 20. Variables influencing OS and PFS were initially screened with Cox univariate analysis. Independent effects of possible determinants were then analyzed in multivariate Cox proportional hazards models 21 stratified on the k-means clusters. Cox univariate analysis was performed for the following prognostic factors: chemotherapy regimen (VMP vs. MPT), age at diagnosis (≥75 vs. <75 yrs), gender, Durie-Salmon stage, ISS score, PS (≥2 vs. 0–1), serum creatinine (>1.2 vs. ≤1.2 mg/dl), serum calcium (>2.35 vs. ≤2.35 mg/dl), Ig isotype, and best response achievement (CR/VGPR/PR vs. <PR). Best response achievement was treated as a time-dependent variable, including in the Cox models an interaction term built as product of the dichotomous dummy variable for treatment and the observed follow-up time at the event.
Patient characteristics were tested using the Pearson χ2 test for categorical variables and the Mann-Whitney test for continuous ones. All reported P-values were two-sided, at the conventional 5% significance level. Data were analyzed as of December 2012 using IBM SPSS (v20.0.0, IBM Corporation, New York) and R v2.15.1 (The R Foundation for Statistical Computing, Vienna).
Results
Starting with 956 cases, we first eliminated cases lacking beta2-microglobulin and albumin data, and the remaining 817 cases were complete for age and beta2-microglobulin and albumin. Out of 817 cases, 590 were selected by k-means clustering. Baseline characteristics were well balanced between the two groups, although a higher percentage of worse PS cases were present in the VMP group (Table 2). Median age was 72 years (IQR, 69–76 years); about 30% of patients were ≥75 years in both groups.
| VMP (n = 296) | MPT (n = 294) | ||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | p |
| Age, years | |||||
| Median | 72 | 72 | |||
| IQR | 69–76 | 69–76 | |||
| ≥75 | 115 | 30 | 115 | 30 | 0.9 |
| Male, sex | 147 | 50 | 165 | 56 | 0.1 |
| Isotype | |||||
| IgG | 184 | 62 | 200 | 68 | |
| IgA | 76 | 26 | 70 | 24 | 0.039 |
| Light chain | 34 | 11 | 17 | 6 | |
| Data missing | 2 | 1 | 7 | 2 | |
| ECOG Performance status | |||||
| 0–1 | 83 | 28 | 189 | 64 | |
| 2–4 | 96 | 32 | 89 | 30 | <0.0001 |
| Data missing | 117 | 40 | 16 | 6 | |
| International Staging System stage | |||||
| I | 90 | 30 | 72 | 25 | |
| II | 129 | 44 | 159 | 54 | 0.038 |
| III | 77 | 26 | 63 | 21 | |
| Data missing | 0 | 0 | |||
| Creatinine | |||||
| Median | 1.00 | 0.97 | |||
| ≥1.2 mg/dl | 81 | 27 | 91 | 31 | 0.33 |
| Data missing | 0 | 0 | |||
| Albumin | |||||
| Median | 3.7 | 3.6 | |||
| ≤3.5 mg/dl | 119 | 40 | 126 | 43 | 0.51 |
| Data missing | 0 | 0 | |||
| β2-microglobulin | |||||
| Median | 3.7 | 3.8 | |||
| ≥3.5 mg/L | 125 | 42 | 131 | 45 | 0.49 |
| Data missing | 0 | 0 | |||
- IQR, interquartile range; ISS, International Staging System; ECOG, Eastern Cooperative Oncology Group; VMP, bortezomib-melphalan-prednisone; MPT, melphalan-prednisone-thalidomide.
Response rate
A greater proportion of patients in the VMP group had a CR, VGPR, or PR after induction therapy (Table 3). The CR rate was 21% in the VMP group and 13% in the MPT (P = 0.007), the ≥PR rate was 78% in the VMP group and 69% in the MPT (P = 0.01).
| Response | VMP (n = 296) n (%) | MPT (n = 294) n (%) | P value |
|---|---|---|---|
| Best response according to International Uniform Response Criteria | |||
| Complete, very good partial or partial response | 231 (78) | 202 (69) | 0.01 |
| Complete response | 62 (21) | 37 (13) | 0.007 |
| Very good partial response | 58 (20) | 54 (18) | 0.7 |
| Partial response | 111 (37) | 111 (37) | 0.95 |
| Stable disease | 58 (20) | 70 (24) | 0.214 |
| Progressive disease | 2 (1) | 5 (2) | 0.25 |
| Not available | 5 (2) | 17 (6) | 0.009 |
- VMP, bortezomib-melphalan-prednisone; MPT, melphalan-prednisone-thalidomide.
Progression-free survival
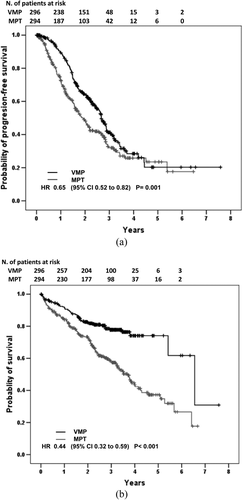
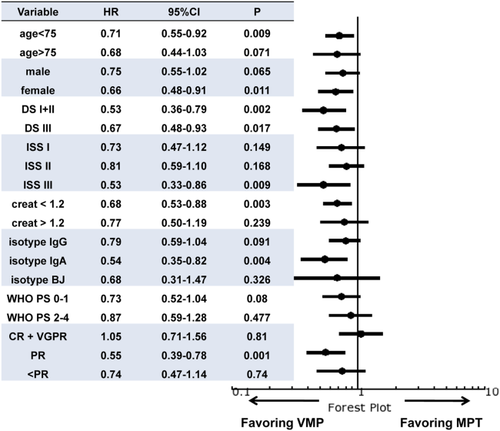
After a median follow-up of 34 months (range, 1–92), 318 patients (54%) relapsed or died. For the entire population, median PFS was 29 months (IQR, 14.8–53.9); median PFS was 32.5 months in the VMP group, and 22.9 months for MPT patients (P = 0.001). The three-year PFS rate was 41.3% in VMP cases and 32.8% in MPT cases (Fig. 1, Panel A). VMP led to 35% reduced risk of progression or death compared with MPT. A statistically significant PFS benefit with VMP was observed in patients <75 years, in females, in all subgroups of patients defined by Durie-Salmon, ISS stage III, creatinine <1.2 mg/dL, IgA isotype and in cases achieving PR (Fig. 2). A multivariate model showed that for VMP achievement of a better response and PS at baseline were independent predictors of prolonged PFS (Table 4). When patients were stratified by trial, cases treated with VMP in PETHEMA-GEM05MAS65 showed a statistically better PFS than cases treated with MPT in NMSG, HOVON-49, and TMSG trials but were not different than cases treated with MPT in GISMM-2001, while cases treated with VMP in GIMEMA-MM0305 showed a statistically better PFS than cases treated with MPT in HOVON-49 trial, were not different than cases treated with MPT in NMSG and TMSG trials, and were worse than cases treated with MPT in GISMM-2001 (Supporting Information Figure 1, Panel A).
| Overall survival | Progression-free survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis No. of cases = 461 | Univariate analysis | Multivariate analysis No of cases = 463 | ||||||||||
| No of cases | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age at diagnosis (≥75 vs. <75 yrs) | 230/360 | 1. 28 | 0.58–2.83 | 0.535 | 1.31 | 0.71–2.44 | 0.386 | ||||||
| Gender (male vs. female) | 312/278 | 1.14 | 0.84–1.55 | 0.390 | 1.05 | 0.83–1.33 | 0.692 | ||||||
| Chemotherapy regimen (VMP vs. MPT) | 296/294 | 0.44 | 0.32–0.59 | <0.001 | 0.38 | 0.25–0.59 | <0.001 | 0.65 | 0.51–0.82 | <0.001 | 0.54 | 0.37–0.80 | 0.002 |
| Durie-Salmon stage (III vs. I+II) | 1.23 | 0.89–1.71 | 0.214 | 1.37 | 1.05–1.78 | 0.022 | 1.13 | 0.82–1.57 | 0.451 | ||||
| ISS score | 0.189# | 0.888# | 0.249# | ||||||||||
| II vs. I | 288/162 | 1.51 | 0.97–2.34 | 0.068 | 1.14 | 0.67–1.93 | 0.625 | 1.27 | 0.93–1.73 | 0.130 | |||
| III vs. I | 140/162 | 1.51 | 0.70–3.23 | 0.294 | 1.13 | 0.44–2.90 | 0.807 | 1.04 | 0.55–1.97 | 0.902 | |||
| ECOG PS (≥2 vs. 0–1) | 185/272 | 1.35 | 0.96–1.89 | 0.084 | 1.42 | 0.98–2.07 | 0.068 | 1.44 | 1.10–1.88 | 0.008 | 1.57 | 1.12–2.20 | 0.009 |
| Serum creatinine (>1.2 vs. ≤1.2 mg/dl) | 172/408 | 0.94 | 0.66–1.34 | 0.733 | 0.92 | 0.69–1.22 | 0.562 | ||||||
| Serum calcemia (>2.35 vs. ≤2.35 mg/dl) | 292/264 | 1.14 | 0.84–1.57 | 0.399 | 1.05 | 0.83–1.34 | 0.680 | ||||||
| Ig isotypes | 0.582# | 0.486# | |||||||||||
| IgA vs. IgG | 146/384 | 1.18 | 0.84–1.66 | 0.348 | 1.16 | 0.89–1.51 | 0.271 | ||||||
| BJ vs. IgG | 51/384 | 0.92 | 0.51–1.65 | 0.781 | 0.94 | 0.61–1.46 | 0.781 | ||||||
| Best responsea | <0.001# | <0.001# | <0.001# | <0.001 | |||||||||
| VGPR vs. CR | 112/99 | 4.64 | 1.91–11.31 | 0.001 | 9.74 | 3.08–30.83 | <0.001 | 5.03 | 2.77–9.14 | <0.001 | 11.18 | 4.61–27.10 | <0.001 |
| PR vs. CR | 222/99 | 13.19 | 4.32–40.26 | <0.001 | 38.41 | 8.14–181.40 | <0.001 | 11.77 | 5.21–26.21 | <0.001 | 39.67 | 11.60–135.71 | <0.001 |
| <PR vs. CR | 135/99 | 46.69 | 12.25–177.98 | <0.001 | 170.12 | 26.49–1092.6 | <0.001 | 32.81 | 12.03–89.51 | <0.001 | 97.77 | 22.19–430.85 | <0.001 |
- a Treated as a time-dependent variable; # overall p-values for the whole variable with its dichotomous dummies accommodating its multinomial modalities.
- All Cox models were estimated stratifying the cohort on k-means cluster.
- ISS, International Staging System; PS, Performance status; D&S, Durie and Salmon staging system; CI, Confidence Interval; HR, Hazard Ratio; BJ, Bence Jones; VMP, bortezomib-melphalan-prednisone; MPT, melphalan-prednisone-thalidomide; CR, complete response; VGPR, very good partial response PR, partial response.
Overall survival
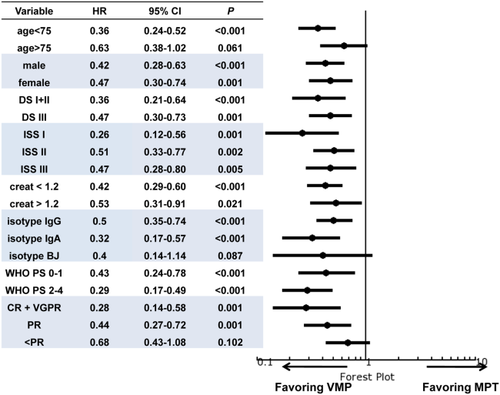
At time of analysis, 197 patients (33%) had died. In the entire population, the median OS was 61.9 months (IQR, 26.5–79.7). Median OS was 79.7 months in the VMP group and 45.1 months in the MPT group (P < 0.001). The three-year OS was 77.8% in VMP cases and 58.5% in MPT cases (Fig. 1, Panel B). VMP led to a 56% reduced risk of death compared with MPT. VMP led to a statistically significant benefit in terms of OS in all subgroups defined by gender, Durie-Salmon stage, ISS, creatinine level, PS, age <75 years, IgG or IgA isotype, and by achievement of ≥VGPR or PR (Fig. 3). Moreover, although we observed in patients ≥75 years a positive trend for VMP cases, in this setting VMP did not statistically impact on OS compared with MPT (HR 0.63; 95% CI 0.38–1.02; P = 0.061). However, a landmark analysis of OS for 318 relapsed patients, calculated from time of relapse, showed a significant longer survival in favor of VMP (three-year OS rate was 61.7 in VMP cases and 24.8% in MPT cases; HR 0.51; 95% CI, 0.35–0.75; P < 0.0001). Overall, age ≥75 years did not increase the risk of death in the subgroup of patients receiving either VMP (HR 2.06; 95% CI, 0.69–6.16; P = 0.198) or MPT (HR 0.62; 95% CI 0.14–2.85; P = 0.54). Upon multivariate analysis, VMP treatment, quality of response, and PS resulted in independent predictors of better outcome (Table 4). When patients were stratified by trial, VMP cases in PETHEMA-GEM05MAS65 showed a statistically longer OS than MPT cases in NMSG and HOVON-49 trials, but not different than cases treated with MPT in GISMM-2001 and TMSG trials, while cases treated with VMP in GIMEMA-MM0305 showed a statistically better OS than cases treated with MPT in HOVON-49, NMSG, TMSG, and GISMM-2001 trials (Supporting Information Figure 1, Panel B).
Frequency of adverse events
Rates of treatment-related death were similar between VMP and MPT: 18 patients (5.8%) died in the VMP group and 22 (7%) in the MPT (P = 0.51) group with the major cause of death being infection (five patients in VMP and six patients in MPT). The incidence of at least one grade 3–4 hematologic AE was significantly higher in patients receiving VMP compared with MPT (42.6% vs. 31.9%; P = 0.008). Grade 3–4 thrombocytopenia was slightly more frequent in VMP cases (11.9% vs. 7.4%; P = 0.077). The incidence of at least one grade 3–4 non-hematologic AE was significantly lower in VMP patients compared with MPT (31.9% vs. 42.6%; P = 0.008). Consistently, grade 3–4 cardiac complications were fewer in VMP cases (3.2% vs. 9%; P = 0.004). Similarly, VMP was associated with a lower incidence of severe infections (8.1% vs. 14.8%; P = 0.011) and severe dermatologic events (1.6% vs. 5.5%; P = 0.015) compared with MPT therapy. In the VMP group, 16 cases (5%) of deep vein thrombosis (DVT) occurred; in the MPT group, 21 cases (7%) of DVT and 5 cases (2%) of pulmonary embolism were detected (P = 0.11). A slightly higher rate of grade 3–4 sensory neuropathy and/or neuralgia was reported in VMP cases (11.3% vs. 7.1%; P = 0.095). The incidence of other severe nervous system events was significantly lower in VMP patients compared to MPT (2.3% vs. 6.8%; P = 0.011). The proportion of patients requiring therapy interruption was significantly different in the two groups (VMP 17% versus MPT 33%, P = 0.0001).
Discussion
In MM patients, survival varies according to host and disease characteristics. Treatment choice is crucial in the improvement of response rate and survival, preserving quality of life. Over the last decade, the use of thalidomide, bortezomib, and lenalidomide significantly prolonged survival 22. However, this advantage was much less pronounced in patients >60 years, while no survival improvement was observed in patients >70 years 23, 24. This difference may be due to frailty, co-morbidities, or disabilities, which often limit the management of elderly patients 16. Because vulnerable elderly patients are underrepresented in clinical trials, our study may be less representative of the overall elderly population. Nevertheless, this is the largest analysis conducted on elderly MM patients to date.
In this retrospective case-matched study, data from 590 untreated MM patients receiving VMP or MPT were analyzed. VMP was associated with a significant reduced risk of both progression and death compared with the MPT, independently of sex, ISS, PS, or serum creatinine. Two major limitations should be considered when evaluating the net impact of VMP on OS. First, efficacy data of post-progression treatments are not available, and, second, the post progression therapeutic armamentarium is potentially different since the trials were conducted in different periods. However, a landmark analysis of OS for 318 relapsed patients, calculated from the time of relapse, showed a significant better prognosis for the VMP group.
Older patients commonly have more adverse prognostic factors and shorter survival 25. In the meta-analysis comparing MP with MPT 3, as well as in the VISTA study comparing MP with VMP 10-12, survival was worse in patients ≥75 years. In our study, age ≥75 years did not significantly increase the risk of death in patients receiving either VMP or MPT. Moreover, although a positive trend for VMP was observed, the type of therapy did not significantly impact OS in patients ≥75 years. These results should be interpreted with caution given the good fitness and the limited number of very elderly patients in our population (30% of the patients analyzed). This study suggests that VMP could be considered the first treatment choice in patients between 65 and 75 years, while we cannot conclude likewise for very elderly patients (≥75 years) since only a positive trend in terms of PFS and OS in favor of VMP was observed. Traditionally, PS measured the fitness of patients, but its role as a unique marker of functional status needs to be revised. The presence of co-morbidities is also a significant concern. In this analysis, poor PS cases represented only 30% of the whole study population. The benefit of VMP in terms of OS was also observed in this subset of patients, although the lack of information about co-morbidity and the number of unavailable PS values limits our understanding of the impact of patients' characteristics on clinical outcome.
Similarly, although both thalidomide and bortezomib pharmacokinetics are not affected by renal impairment and no dose reduction is required, our data showed that the benefit of VMP in terms of OS is independent of creatinine values. These results further support the recent IMWG consensus statement recommending VMP as first-line treatment in elderly patients with renal failure 26.
The ISS classification 27 along with cytogenetic status 28 is the most relevant prognostic factor in MM. ISS still remains an independent predictor of outcome and treatment of high-risk patients remains unsatisfactory. However, in our analysis the OS benefit of VMP was observed in all patient subgroups defined by ISS. Unfortunately, cytogenetic data were insufficient and could not be used to better dissect its prognostic impact.
In line with our results, several studies have recently emphasized the role of CR as an independent predictor of longer survival 29-31. In a recent retrospective study on 1,175 patients, CR achievement was associated with improved OS, with three-year OS rates of 91% in CR patients and 67–70% in those in VGPR or PR 30.
Both the toxicity profiles of VMP and MPT and treatment-related deaths were similar in the two groups. The overall incidence of grade 3–4 hematologic AEs was significantly higher in VMP patients, especially thrombocytopenia. Conversely, the incidence of infections and dermatologic and cardiac complications was higher in MPT patients. Therefore, cardiologic workup before starting thalidomide is appropriate to detect asymptomatic cardiologic abnormalities. A more careful assessment of fevers of unknown origin and prompt administration of antibiotic prophylaxis might reduce the risk of infection. The incidence of grade 3–4 sensory neuropathy and/or neuralgia was slightly higher in the VMP group; conversely, MPT patients showed a higher incidence of other severe nervous system events. Subcutaneous bortezomib could further improve the bortezomib toxicity profile 32. Finally, the incidence of thromboembolism was 5% in VMP patients and 9% in MPT patients.
To date, no randomized trial has directly compared VMP and MPT. A recent indirect comparison of VMP vs. MPT showed no differences between the two regimens for all outcomes, but a significant CR benefit with lower toxicity profile favoring VMP 33. Given the limits of a retrospective analysis and the lack of relevant data, such as post-relapse treatment and role of maintenance, co-morbidity and cytogenetics, this is the first direct comparison between the two schedules. Nevertheless, our data should be considered in the light of obvious heterogeneity in the patient population and treatment regimens among different trials analyzed in this study, including variability in dosing, duration of treatment, and maintenance.
In light of our findings, should we recommend VMP over MPT? It is difficult to draw a definitive conclusion given the retrospective design of our study. Moreover, our analysis was an indirect comparison of treatment, given that patients in MPT and VMP came from completely different trials. Despite this limit, we analyzed data derived from the most important European randomized trials 4, 7, 8, 13, 14, matched by age, albumin, and beta2-microglobulin levels, thereby shrinking influences of potential prognostic imbalances. We found that VMP is associated with better quality of response and longer PFS and OS in comparison with MPT. A critical point could be represented by maintenance therapy. Thalidomide maintenance was planned in 3/4 MPT protocols (HOVON-49 8, GISMM-2001 4, and NMSG 7 trials) we examined; conversely only the PETHEMA-GEM05MAS65 14 trial foresaw bortezomib maintenance, and this further supports VMP superiority. Finally, VMP's positive impact on PFS was further supported by two ad hoc sub-analyses in which trials with or without maintenance were evaluated separately by both univariate and multivariate analysis. Furthermore, it must be considered that the VMP arm observed a higher rate of cases with worse PS than the MPT arm. Considering the favorable toxicity profile and the efficacy of both VMP and MPT, along with the increased life expectancy of the general population and the enhanced PS of numerous old patients, clinicians could move from a more palliative therapy to a more intensive therapeutic approach in elderly MM patients. Uncertainty may remain in the selection of the more appropriate treatment in this setting; our results may help physicians make a more informed choice in favor of VMP.
Author Contributions
Conception and design: Fortunato Morabito, Sara Bringhen, Alessandra Larocca, Maris Victoria Mateos, Roberto Passera, Mario Boccadoro, Antonio Palumbo,Massimo Offidani. Provision of study materials or patients: Fortunato Morabito, Sara Bringhen, Alessandra Larocca, Pierre Wijermans, Maria Victoria Mateos, Peter Gimsing, Carla Mazzone, Daniela Gottardi, Paola Omedè, Sonja Zweegman, Juan José Lahuerta, Renato Zambello, Pellegrino Musto, Valeria Magarotto, Martijn Schaafsma, Albert Oriol, Gunnar Juliusson, Chiara Cerrato, Lucio Catalano, Massimo Gentile, Ana Isabel Turel, Anna Marina Liberati, Maide Cavalli, Davide Rossi, Roberto Passera, Meral Beksac, Michele Cavo, Anders Waage, Jesus San Miguel, Mario Boccadoro, Pieter Sonneveld, Antonio Palumbo, Massimo Offidani. Collection and assembly of data: Fortunato Morabito, Sara Bringhen, Alessandra Larocca, Pierre Wijermans, Maria Victoria Mateos, Peter Gimsing, Carla Mazzone, Daniela Gottardi, Paola Omedè, Sonja Zweegman, Juan José Lahuerta, Renato Zambello, Pellegrino Musto, Valeria Magarotto, Martijn Schaafsma, Albert Oriol, Gunnar Juliusson, Chiara Cerrato, Lucio Catalano, Massimo Gentile, Ana Isabel Turel, Anna Marina Liberati, Maide Cavalli, Davide Rossi, Roberto Passera, Meral Beksac, Michele Cavo, Anders Waage, Jesus San Miguel, Mario Boccadoro, Pieter Sonneveld, Antonio Palumbo, Massimo Offidani. Data analysis and interpretation: Fortunato Morabito, Sara Bringhen, Massimo Gentile, Alessandra Larocca, Paola Omedè, Valeria Magarotto, Chiara Cerrato, Roberto Passera, Stefano Rosso, Mario Boccadoro, Antonio Palumbo. Manuscript writing: Fortunato Morabito, Sara Bringhen, Alessandra Larocca, Pierre Wijermans, Maria Victoria Mateos, Peter Gimsing, Carla Mazzone, Daniela Gottardi, Paola Omedè, Sonja Zweegman, Juan José Lahuerta, Renato Zambello, Pellegrino Musto, Valeria Magarotto, Martijn Schaafsma, Albert Oriol, Gunnar Juliusson, Chiara Cerrato, Lucio Catalano, Massimo Gentile, Ana Isabel Turel, Anna Marina Liberati, Maide Cavalli, Davide Rossi, Roberto Passera, Meral Beksac, Michele Cavo, Anders Waage, Jesus San Miguel, Mario Boccadoro, Pieter Sonneveld, Antonio Palumbo, Massimo Offidani. Final approval of manuscript: Fortunato Morabito, Sara Bringhen, Alessandra Larocca, Pierre Wijermans, Maria Victoria Mateos, Peter Gimsing, Carla Mazzone, Daniela Gottardi, Paola Omedè, Sonja Zweegman, Juan José Lahuerta, Renato Zambello, Pellegrino Musto, Valeria Magarotto, Martijn Schaafsma, Albert Oriol, Gunnar Juliusson, Chiara Cerrato, Lucio Catalano, Massimo Gentile, Ana Isabel Turel, Anna Marina Liberati, Maide Cavalli, Davide Rossi, Roberto Passera, Meral Beksac, Michele Cavo, Anders Waage, Jesus San Miguel, Mario Boccadoro, Pieter Sonneveld, Antonio Palumbo, Massimo Offidani.
Acknowledgments
The authors thank the patients who took part in the studies, the nurses De Lazzer Tiziana and Tresoldi Ornella, the data managers Federica Leotta and Tiziana Marangon, and the editorial assistant Giorgio Schirripa.




