Haptoglobin testing in hemolysis: Measurement and interpretation
Abstract
Haptoglobin is primarily produced in the liver and is functionally important for binding free hemoglobin from lysed red cells in vivo, preventing its toxic effects. Because haptoglobin levels become depleted in the presence of large amounts of free hemoglobin, decreased haptoglobin is a marker of hemolysis. Despite its ubiquity and importance, a paucity of literature makes testing difficult to interpret. This review highlights the many physiological roles that have been recently elucidated in the literature. Different methodologies have been developed for testing, including spectrophotometry, immunoreactive methods, and gel electrophoresis. These are covered along with their respective advantages and disadvantages. As there is no single gold standard for hemolysis, validation studies must rely on a combination of factors, which are reviewed in this article. Pitfalls and limitations of testing are also addressed. False positives can occur in improper specimen preparations, cirrhosis, elevated estrogen states, and hemodilution. False negatives can occur in hypersplenism and medications such as androgens and corticosteroids. Haptoglobin testing in the setting of inflammation is additionally discussed as interpretation can be difficult in this setting. Given the widespread use of haptoglobin testing, it is vital that clinicians and laboratory staff understand the principles and correct interpretation of this test. Am. J. Hematol. 89:443–447, 2014. © 2013 Wiley Periodicals, Inc.
Background
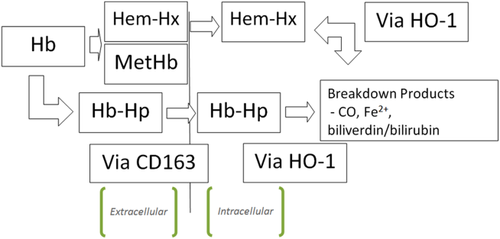
Haptoglobin was first described by Polonovsky and Jayle in 1938 as a “plasma substance” that binds hemoglobin 1. When red cells are lysed in vivo, free hemoglobin binds to circulating haptoglobin; the hemoglobin-haptoglobin complex is then degraded by the reticuloendothelial system (Fig. 1). By scavenging free hemoglobin, haptoglobin plays a critical role in preventing free hemoglobin-induced vascular dysfunction or injury. Decreased haptoglobin is a marker for hemolysis because haptoglobin levels become depleted in the presence of large amounts of free hemoglobin 2, 3. Because of the widespread use and diagnostic importance of haptoglobin measurement, it is vital that clinicians and laboratory staff understand the principles and correct interpretation of test results.
Biology of haptoglobin
Haptoglobin is an alpha-2-glycoprotein synthesized mainly by hepatocytes consisting of both alpha and beta subunits linked by disulfide bonds forming dimers 4. The human haptoglobin (HP) gene exists in two major allelic forms leading to three major genotypes: Hp1-1, Hp2-1, and Hp-2-2, that separate on alkaline starch gel electrophoresis due to different molecular weights 5. Their properties vary in binding strength, anti-oxidative ability, and rate of clearance. Binding strength is greatest with Hp1-1, but the Hp-2 genotypes can bind a larger number of hemoglobin alpha-beta dimers. Complexes of Hp2-2 and hemoglobin have higher affinity for the CD163 receptor, the monocyte-macrophage receptor that facilitates its clearance.
Although haptoglobin is primarily produced in the liver, protein expression has also been detected in the lung, and mRNA has been detected in the kidney, spleen, thymus, and heart 6, 7. It is an acute phase reactant with production increased by inflammatory cytokines such as IL-1 and IL-6 8.
Haptoglobin is functionally important for binding free hemoglobin, preventing its toxic effects. Free hemoglobin scavenges nitric oxide, which is an important regulator of smooth muscle relaxation, endothelial adhesion molecule expression, platelet activation, and platelet aggregation 9. Furthermore, free hemoglobin is hydrophobic, interacting with and disrupting lipid membranes and other lipophilic structures 10. The iron in free heme produces free radicals by reacting with endogenous hydrogen peroxide 11.
The richest literature in regards to chronic hemolysis causing vasculopathy is seen in sickle cell disease, where its effects are linked with pulmonary hypertension, leg ulceration, priapism, and cerebrovascular disease 12. In a model of haptoglobin-null mice, a strong hemolytic stress causes renal injury due to oxidative damage 13. In haptoglobin and hemopexin double-null murine models, a non-lethal hemolytic stress causes liver inflammation, cirrhosis, and splenomegaly 2. Activation of Kupffer cells in the liver increases pro-inflammatory cytokines such as TNF-alpha and IL-1beta, which eventually leads to fibrosis. Splenomegaly occurs hours after induced hemolysis, which suggests that red blood cell accumulation and adhesion may be the cause 2, 14.
Haptoglobin is protective by irreversibly binding free hemoglobin. Hemoglobin-haptoglobin complexes are rapidly cleared from circulation via monocytes and tissue macrophages via CD163 receptors 15. Free haptoglobin has a half-life of 5 days, whereas hemoglobin-haptoglobin complexes have a half-life of minutes 16. Lysozomes degrade globin moieties while heme is converted by heme oxygenase-1 (HO-1) into Fe2+, CO, and biliverdin. Biliverdin is eventually converted to bilirubin via biliverdin reductase. It is thought that these metabolites may have anti-inflammatory effects. Fe2+ also induces ferritin, which binds to the free iron, preventing it from generating reactive oxygen species 3.
Haptoglobin also has physiological roles other than metabolism of hemoglobin. Locally synthesized haptoglobin may provide antioxidant and antimicrobial effects 17. Haptoglobin can suppress proliferation of lymphocytes and B-cell mitogenesis as well as modulating macrophage function by inhibiting viral hemagglutination and prostaglandin H synthase 18. By altering the distribution of helper T-cells, haptoglobin can function as an immune system modulator and may be partially responsible for certain infections, allergies, and autoimmune disorders 19. Haptoglobin has also been found to induce angiogenesis, most notably in the Hp2-2 subtype 20.
Methods of haptoglobin testing
Haptoglobin measurement relies upon altered physical properties when bound to hemoglobin versus when circulating as a free protein. Peroxidase activity of hemoglobin in the hemoglobin-haptoglobin complex can be determined in solution, after gel filtration, spectrophotometry, or after gel electrophoresis 21-23. The hemoglobin-haptoglobin complex can also be measured after acid denaturation 24. Three common methods used to measure haptoglobin include spectrophotometry, immunoreactive methods, and gel electrophoresis.
Spectrophotometry can identify hemoglobin-haptoglobin complexes in hemolysis with variable sensitivity and specificity 25. Shim et al. describe measuring light absorption at two different wavelengths (432 and 408 nm) in serum reduced with sodium hydrosulphite to determine the concentration of hemoglobin-haptoglobin complexes. Calculating the change of absorption and the ratio of these changes can be used determine the presence of hemoglobin-haptoglobin complexes, free hemoglobin, and free haptoglobin. The concentration of free hemoglobin and hemoglobin-haptoglobin complexes are determined by ΔA408 in severe hemolysis. The protocol for slight hemolysis is modified for improved sensitivity and specificity. Limitations include the potential interference of bilirubin and chylomicrons in samples tested to measure haptoglobin as a function of light transmittance or use of toxic reagents depending on the methodology used 26.
Immunoreactive methods use antisera against haptoglobins to produce a precipitated product that is measured by radial immunodiffusion, nephelometry, or turbidimetry. Radial immunodiffusion involves suspending haptoglobin and antibodies in a medium, such as agar gel. The size of precipitin complexes is used to calculate the haptoglobin concentration. However, the antisera must be made to react with different haptoglobin types. Differences in molecular size between the haptoglobin phenotypes can affect the diffusion rate, skewing the radial immunodiffusion assay. Corrections can only be made if the phenotype is known 27. Several commercial kits are available. With nephelometry and turbidimetry, a light beam is applied to haptoglobin-hemoglobin-antibody complexes suspended in solution, and a detector measures the different properties of the reflected light. Nephelometry, however, does require having a nephelometer as well as specific reagents. Nephelometry is more accurate than spectrophotometry but, as with all immunoreactive methods, antisera against all phenotypes are required 28. There is some evidence that measurement of haptoglobin using turbidimetry may be as reliable 29. Traditional ELISA methods have been developed but also require specific antisera 17.
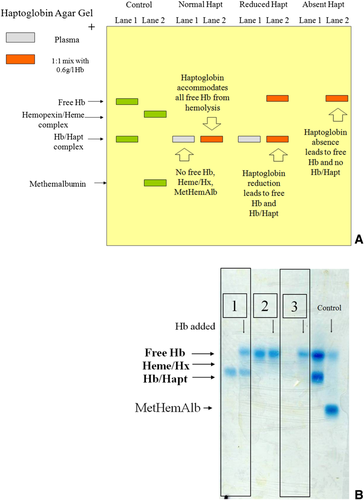
In our laboratory, we use a gel electrophoresis method modified from Ali et al., which has been validated against immunological and spectrophotometric methods 25, 28. Free hemoglobin, haptoglobin-hemoglobin complexes, hemopexin-heme complexes, and methemalbumin are separated by alkaline electrophoresis. A heme-specific stain, o-Tolidene, and hydrogen peroxide allow visualization of the heme-containing components 30. Several heme-specific stains are available, but o-Tolidene was chosen due to its high sensitivity 31. The haptoglobin level is reported in a semi-quantitative fashion (i.e., present, reduced, or absent) (Fig. 2A and B). Compared with quantitative methods, where interpretation of levels at the lower limit of normal or slightly below can be difficult, we believe this semi-quantitative reporting provides information that is easier to interpret. Unfortunately, this method is not available commercially. It is a manual technique with no automation, requiring technical intervention for interpretation of results, which introduces potential for errors by inexperienced operators.
Validity
Unfortunately, there are few studies on the validity of haptoglobin as a marker for hemolysis. As there is no “gold standard” test to confirm a diagnosis of hemolysis, validation studies must rely on a combination of clinical factors and correlation with other laboratory markers that may have limited specificity. Two studies are reviewed here.
One study used immunoreactive nephelometry to detect haptoglobins, compared retrospectively with clinical diagnosis of intravascular hemolysis, as determined from clinical data and laboratory markers that did not include haptoglobin. The authors found that a haptoglobin level below 25 mg/dL had 83% sensitivity and 96% specificity for intravascular hemolysis 32.
Another study by Kormoczi et al. quantified haptoglobin with spectrophotometry and compared these results with hemolysis as defined by other laboratory markers, anemia, and sometimes a known history of hemolytic disease 16. The sensitivity and specificity of a haptoglobin level below 28 mg/dl were 91.8% and 98.4%, respectively.
Limitations
There are limitations of haptoglobin as a marker for hemolysis regardless of the methodology used to measure it. Decreased haptoglobin levels can occur in the absence of hemolysis, due to cirrhosis of the liver, disseminated ovarian carcinomatosis, pulmonary sarcoidosis, and elevated estrogen states 32, 33. In patients undergoing liver transplant, haptoglobin levels normalize on day 14 post-transplant in concert with improved liver function. Hemodilution and blood transfusions can result in falsely reduced haptoglobin concentration 16, 34. Ahaptoglobinemia is common in the newborn period but by 3 months of age, haptoglobin is present in the majority of infants 17. Of note, both extravascular and intravascular hemolysis can cause reduced haptoglobin and thus the test cannot reliably be used to differentiate 16. Preanalytical errors, such as improper phlebotomy technique or specimen handling, may cause in vitro hemolysis and a falsely reduced haptoglobin. Different reference ranges have been reported depending on haptoglobin type, age, sex, and pregnancy but IFCC-standardized reference ranges are available 17.
Increased haptoglobin levels can occur with hypersplenism, megaloblastic anemia, or the use of drugs such as androgens and corticosteroids 35, 36. Kormoczi et al. found that in patients without hemolysis, ongoing inflammation caused plasma haptoglobin concentrations to rise threefold. However, among patients with active hemolysis in the presence of strongly increased CRP (>5 mg/dl), the majority had reduced haptoglobin levels (33 of 35 patients, vs. 2 of 35 patients with normal haptoglobin levels) 16.
Warkentin et al. analyzed cardiac surgery patients who underwent cardiopulmonary bypass, during which both hemolysis and inflammation are expected to occur. Serum haptoglobin concentrations significantly decreased after surgery in 56 of 58 patients. Of those patients, 41 (70.7%) had haptoglobin less than 25 mg/dl 37. Therefore, haptoglobin testing is theoretically less reliable for the detection of hemolysis during periods of inflammation, and individual results should be interpreted in the clinical context.
Conclusions
In summary, haptoglobin is a serum protein that binds toxic free hemoglobin released by lysis of red blood cells. Although different methods exist to measure haptoglobin, each has unique benefits and limitations. While we believe the methodology used in our lab provides results that are easily interpreted, the majority of commercial kits use immunoreactive methods and may be more suited to labs without experience using gel electrophoresis. Despite the ubiquity and importance of haptoglobin testing, there is a paucity of literature and therefore lack of understanding of this test. Clinicians must be aware of its limitations and interactions and interpret this test in the context of the clinical scenario.
| What is the test? |
| Haptoglobin is primarily produced in the liver and its main function is to bind free hemoglobin from lysed red cells in vivo. Because haptoglobin levels become depleted in the presence of free hemoglobin, decreased haptoglobin is a marker for hemolysis. |
| How is it measured? |
| Haptoglobin can be measured through multiple methods. Three common methods used to measure haptoglobin include spectrophotometry, immunoreactive methods, and gel electrophoresis. Spectrophotometry is potentially limited by interference of bilirubin and chylomicrons in samples tested to measure haptoglobin as a function of light transmittance. Immunoreactive methods are often used in commercial kits and require specific antisera against different haptoglobin phenotypes. Gel electrophoresis does provide results that may be easier to interpret but this method is not available commercially and requires operators experienced in the technique. |
| What are the normal range values? |
| Different reference ranges have been reported depending on haptoglobin type, age, sex, and pregnancy. It should also be noted haptoglobin is often not present in infants up to 3 months of age. The IFCC reported the standard reference range of haptoglobin in serum to be 0.3-2.0 g/L.a |
| What conditions or type of conditions is it used in? |
| Its utility is to determine whether or not hemolysis has occurred. Most typically, it provides information on intravascular hemolysis but extravascular hemolysis decreases haptoglobin as well. |
| What tests are helpful to do with it for a more complete picture? |
| It is helpful with other hemolytic markers such as a CBC, LDH, and an indirect bilirubin. An increased reticulocyte count will also determine if there is compensatory production of red blood cells through bone marrow erythropoesis. A peripheral blood smear and direct antiglobulin test may be useful to find specific causes for the hemolysis. |
| What tests provide similar information? |
| Free hemoglobin also binds to other proteins such as hemopexin and albumin. Therefore, determinations of hemoglobin complexed to these proteins can provide similar information about the presence of hemolysis. Free hemoglobin may also be measured. However, these other tests are not commonly used in clinical practice. |
| What tests can be avoided/eliminated? |
| In the broad differential for an acute drop in hemoglobin, a clinician must consider hemolysis and hemorrhage. If the acute drop in hemoglobin can be determined to be hemolysis rather than hemorrhage, imaging or invasive procedures to find a source may be avoided. Doing other non-specific markers for hemolysis when they may falsely positive for other reasons may also be avoided in certain clinical situations. |
| How does its use impact treatment? |
| By determining the presence of hemolysis, one can determine a differential based on immune and non-immune causes. It is more specific for the presence of hemolysis compared to other markers. |
- a Dati F, Schumann G, Thomas L, et al. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP Reference Material (CRM 470). International Federation of Clinical Chemistry. Community Bureau of Reference of the Commission of the European Communities. College of American Pathologists. European journal of clinical chemistry and clinical biochemistry: journal of the Forum of European Clinical Chemistry Societies 1996;34:517-20.




