Rituximab versus splenectomy in persistent or chronic adult primary immune thrombocytopenia: an adjusted comparison of mortality and morbidity
Conflict of Interest: Nothing to report.
Abstract
Splenectomy and rituximab are both recommended as second-line treatment in immune thrombocytopenia (ITP), but they have never been directly compared. We compared their efficacy and serious adverse outcomes in a retrospective cohort of 105 adult primary ITP patients exposed to one or other of these treatments. Primary outcome was composite: death from hemorrhage or from infection and hospitalization for bleeding or for infection. Secondary outcomes were overall mortality, hospitalization for bleeding, hospitalization for infection, as well as response and complete response (international definitions). Analyses were adjusted on a propensity score. Patients treated with rituximab (n = 43) were older and had more comorbidities than the splenectomized patients (n = 62). Mean follow-up was, respectively, 3 and 8.4 years. After adjustment on the propensity score, there was no difference between the two groups regarding the primary and other clinical outcomes. In the multivariate analysis, only a history of mucosal bleeding (HR 3.2 95% CI [1.2–8.5]) and a Charlson score ≥1 (HR 4.2 95% CI [1.8–9.6]) were associated with the primary outcome. These two factors were also associated with hospitalization for bleeding. As expected, response, complete response and maintenance rates were higher in the splenectomy group. Splenectomy compared with rituximab was independently associated with a response at 12 months (OR 4.4, 95% CI [1.7–11.8]). Then, adjusted analyses in this real-life cohort confirmed the better results of splenectomy compared with rituximab. Am. J. Hematol. 89:41–46, 2014. © 2013 Wiley Periodicals, Inc.
Introduction
Splenectomy and rituximab are two options for severe persistent (lasting from three to 12 months) or chronic (lasting more than 12 months) primary immune thrombocytopenia (ITP) 1-3. Splenectomy has been performed for a century, so it has been thoroughly evaluated. It is the reference treatment leading to a complete response rate of 85% in a few days 4. However, about one quarter of patients relapse during a 5-year follow-up 5-8. At a median follow-up of 20 years (range 10–43), about 60% of the patients are responders 9. Perioperative morbimortality is remarkably low in ITP patients, but the long-term infectious risk sees a 4 to 14-fold increase after splenectomy compared with the general population 10. Rituximab has been prescribed for ITP since the first decade of the 21st century 11. It induces a 40–50% response rate in splenectomy-candidate patients 12-14. Relapses are frequent, so the response rate decreases to 20% at 5 years 15, 16. Infectious risk in the largest published cohorts seems to be low. Only one serious infection (sigmoiditis) occurred during the two-year follow-up in a clinical trial including 60 patients and there was no evidence of an increased infectious risk in a five-year follow-up of 72 adult primary ITP patients 14, 16. However, these studies stemmed from reference centers, the patients were young, and they had few comorbidities. In 2007, a review of published cases of rituximab-treated patients (306 ITP patients ranging from 16 to 89 years of age) concluded that rituximab-induced adverse drug reactions were not rare: 21.6% of the patients experienced mild or moderate adverse events, 3.7% experienced severe or life-threatening events, and 9 patients (2.9%) died, including 2 by infection 17. In daily practice, rituximab is used in older, comorbid patients for whom splenectomy is avoided because of the risk of anesthesia. Rituximab is also more and more frequently used as a second-line therapy after corticosteroids instead of splenectomy even in younger patients because they prefer this treatment to surgery 18. However, the two strategies have not been compared directly over the long term for robust outcomes such as mortality, serious bleeding and serious infections. The primary objective of this study was to compare these outcomes in persistent or chronic ITP patients treated with either rituximab or splenectomy in real life practice. The secondary objective was to identify risk factors for outcome occurrence.
Materials and Methods
Patient selection
We selected all the patients splenectomized in our University hospital from 1997 to 2010 thanks to a request in the “Programme de Médicalisation des Systèmes d'Information” (PMSI). The PMSI is an electronic database that registers medical information for all hospitalized patients in both public and private French health facilities. The data reflects hospital activities and is used to calculate hospital funding. All the patients encoded as suffering from ITP (ICD-10th version code: D69.3) and splenectomized in our hospital from 1997 to 2010 were selected. The ITP patients exposed to rituximab were selected through the register of the Pharmacy Department of our hospital from 2002 to mid-2011. Every time rituximab is dispensed, the diagnosis is encoded. If a patient was exposed to splenectomy after rituximab or to rituximab after splenectomy, he was allocated to the group corresponding to the first treatment.
The medical files of the selected patients were then reviewed to check the ITP diagnosis according to the international definition 1. Then, we restricted the study to the cases of adult persistent or chronic primary ITP only.
Outcomes
Primary outcome was composite and included death by hemorrhage or by infection and hospitalization for bleeding or for infection. Secondary outcomes were overall mortality, mortality by bleeding, hospitalization for bleeding, hospitalization for infection, response at 3 and 12 months, complete response at 3 and 12 months, loss of response, loss of complete response. Response was defined by a platelet count of ≥ 30 G/L and by the absence of bleeding twice 7 days apart 19 in the absence of other treatment for ITP (including corticosteroids). Complete response was defined by a platelet count of ≥100 G/L and the absence of bleeding twice 7 days apart 19 in the absence of other treatment for ITP (including corticosteroids). Loss of response was defined by a platelet count of < 30 G/L on two consecutive days (<100 G/L for loss of complete response) or occurrence of bleeding or introduction of another treatment for ITP 19. Postsplenectomy local complications during the 3 months following splenectomy (on-site infection, eventration, symptomatic portal vein thrombosis) were also assessed.
Data collection
Data was collected in the medical files and by calling the patients and their general practitioners by phone in August 2012.
Statistical analyses
Comparisons between groups were made using the chi2 or the Fisher exact test for categorical variables and the T-test or the Wilcoxon-Mann-Withney test for the continuous variables (α = 5%). Outcomes were described by Kaplan-Meier curves and compared using the log-rank test (α = 5%). The index date was the date of splenectomy or the date of first rituximab infusion. Follow-up was censored when the outcome occurred, at last follow-up date, or when a patient was exposed to another ITP treatment (particularly, when sequentially exposed to splenectomy after rituximab or vice versa). In the splenectomy group, the follow-up was also censored at the longest follow-up in the rituximab group, to avoid any diagnosis bias. In the absence of guidelines to contraindicate splenectomy, the decision to perform splenectomy or to treat with rituximab in this real life cohort was taken after a multidisciplinary individual benefit-to-risk ratio assessment and a patient-tailored discussion. As a result, we calculated a propensity score so as to allow the comparison between rituximab and splenectomy groups. A propensity score is aimed at taking into account the factors that may have influenced treatment allocation. From inclusion variables, a score is calculated for each patient, which corresponds to the probability of being exposed to a given treatment. Adjusting on this score restores the equipoise probability for each patient to belong to a given arm versus the other. As a result, it allows nonrandomized comparative studies to get closer to a randomized design 20, 21. In this cohort, the propensity score calculation included the following variables: year of index date, age, gender, comorbidity Charlson score, disease duration, bleeding signs at diagnosis, history of mucosal bleeding, ITP treatment before splenectomy or rituximab. The Charlson score has been validated in various clinical situations, including in the elderly. It is a scoring system based on a list of 18 conditions (each scored from 1 to 6 points), which predicts the comorbidity-linked 10-year mortality 22. There was no collinearity among the variables included in the propensity score calculation. The score proved efficient in restoring the equipoise of all the variables regarding treatment allocation (Supporting Information Table SI). All the analyses were adjusted on this propensity score.
When study power was sufficient, we conducted Cox or logistic regression models to assess the role of the following covariates in outcome occurrence: treatment group, Charlson score, gender, disease duration, platelet count at diagnosis, history of mucosal bleeding, and exposure. Covariables associated with the outcome with a P-value < 0.20 were included in multivariate models (manual backward procedure). Nevertheless, age and treatment group were forced into multivariate models. We also performed sensitivity analyses for the primary outcome stratifying on age (<65 years vs. ≥65 years) and restricting analyses to the period 2002–2012. Statistical analyses were performed using SAS 9.3™ software (SAS institute, Cary, NC).
Results
Patient selection
The selection process is reported in Fig. 1. A cohort of 105 patients treated by splenectomy (n = 62) or with rituximab (n = 43) as second-line therapy was finally built.
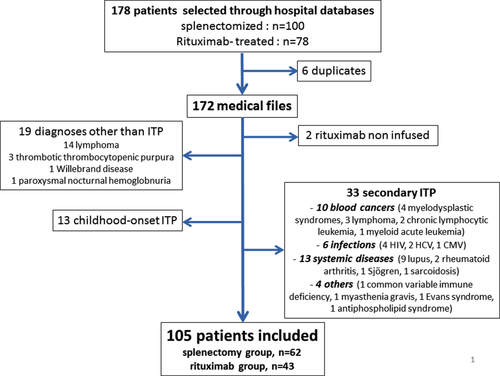
Patient characteristics
Patient characteristics are described in Table 1. Rituximab was used mainly to treat persistent ITP whereas splenectomy was used to treat chronic ITP. Patients treated with rituximab were older and had more comorbidities than patients treated by splenectomy. Platelet count at diagnosis and frequency of mucosal bleeding did not differ between the groups. Rituximab was used at a dose of 375 mg/m2/week for four weeks for all patients.
| Splenectomy n = 62 | Rituximab n = 43 | P | |
|---|---|---|---|
| Age (mean ± SD) (years) | 38.5 ± 14.1 | 66.5 ± 18.5 | <0.0001 |
| Female, n (%) | 47 (75.8%) | 19 (44.2%) | 0.001 |
| Disease duration (median ± IQR), (years) | 1.3 ± 2.7 | 0.6 ± 2.4 | 0.01 |
| Platelet count at diagnosis (mean ± SD) × 109/La | 26.2 (± 24.2) | 17.7 ± 20.1 | 0.09 |
| Bleeding at diagnosis, n (%)b | 34 (54.8%) | 28 (65.1%) | 0.04 |
| History of mucosal bleeding, n (%) | 34 (54.8%) | 25 (58.1%) | 0.7 |
| Charlson score at index date (mean ± SD) | 0.3 ± 0.7 | 1.1 ± 1.2 | <0.0001 |
| 0 | 51 (82.3%) | 17 (39.5%) | – |
| 1 | 4 (6.4%) | 12 (27.9%) | – |
| 2 | 6 (9.7%) | 8 (18.6%) | – |
| ≥ 3 | 1 (1.6%) | 6 (14.0%) | – |
| ITP drugs before index date, n (%) | 62 (100%) | 43 (100%) | 1 |
| Corticosteroids | 62 (100%) | 43 (100%) | 1 |
| Intravenous immunoglobulins | 30 (48.4%) | 26 (60.5%) | 0.2 |
| Vinblastin/vincristine | 1 (1.6%) | 6 (13.9%) | 0.02 |
| Hydroxychloroquine | 2 (3.2%) | 3 (7.0%) | 0.4 |
| Dapsone | 4 (6.4%) | 2 (4.6%) | 1 |
| Danazol | 5 (8.1%) | 10 (23.3%) | 0.03 |
| Cyclosporine | 0 (0%) | 1 (2.3%) | 0.4 |
| Romiplostim | 1 (1.6%) | 1 (2.3%) | 1 |
| Eltrombopag | 0 (0%) | 1 (2.3%) | 0.4 |
- a Missing data for 23 patients in the splenectomy group and 6 patients in the rituximab group.
- b Missing data for three patients in the splenectomy group.
Mean follow-up was 8.4 ± 4.7 years in the splenectomy group and 3.0 ± 1.9 years in the rituximab group. Fourteen patients were lost to follow-up in the splenectomy group (mean follow-up, 3.4 years) and two in the rituximab group (mean follow-up, 4 years). At the time of loss of follow-up, all the patients in the splenectomy group had a sustained complete response, except for two patients (no response, and loss of response). In the rituximab group, one patient had a sustained complete response and the other a sustained response at this time.
Outcomes
With the longest follow-up in each group, primary outcome occurred in 14 patients in the rituximab group and 11 in the splenectomy group. Two patients died in the splenectomy group versus eight in the rituximab group (P < 0.001). In the splenectomy group, one patient died from cancer and the other from unspecified cause (according to the general practitioner, this patient had no sign of bleeding or infection). In the rituximab group, the cause of death was sepsis (n = 1), pneumonia (n = 2), cancer (n = 1), ischemic stroke (n = 1), bleeding (n = 2), and unspecified cause not related to bleeding or infection (n = 1). The two bleeding-related deaths were cerebral bleeding and urinary tract bleeding. Details regarding deaths are provided in Supporting Information Table SII. After adjustment on the propensity score, there was no difference between the two groups regarding the primary outcome, overall mortality, hospitalization for bleeding, or hospitalization for infection (Fig. 2 and Supporting Information Fig. S1).
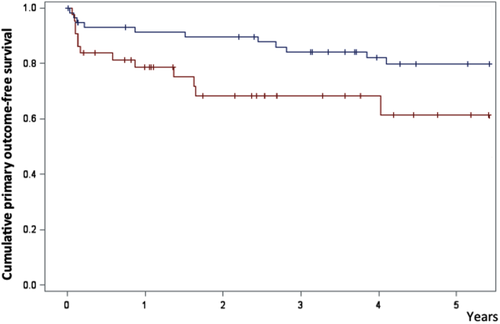
The primary outcome was associated with a history of mucosal bleeding (HR 3.2 95% CI [1.2–8.5]) and with a Charslon score ≥1 (HR 4.2 [1.8–9.6]) (Table 2). In sensitivity analyses restricted to patients aged under 65 and to the period 2002–2011, there was also no difference between the two groups (Supporting Information Table SIII). Hospitalization for bleeding was associated with the same variables, with a noteworthy weight of a history of mucosal bleeding (Table 3).
| Multivariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | |
| Rituximab vs. splenectomy | 2.71 [1.20–6.11] | 0.02 | – | – |
| Age ≥ 65 years vs. < 65 years | 2.52 [1.14–5.57] | 0.02 | ||
| Female gender | 0.60 [0.27–1.31] | 0.2 | – | – |
| Disease duration, ≥ 1 year vs. < 1 year | 0.56 [0.25–1.28] | 0.2 | – | – |
| History of mucosal bleeding | 3.27 [1.23–8.72] | 0.02 | 3.19 [1.20–8.51] | 0.02 |
| Charlson score ≥ 1 | 4.35 [1.90–9.94] | 0.0005 | 4.18 [1.83–9.56] | 0.0007 |
| Year of splenectomy or rituximab, by quartiles | – | 0.5 | – | – |
| < 2000 | 1 | – | ||
| ≥ 2000 and < 2006 | 1.46 [0.76–2.81] | 0.2 | ||
| ≥ 2006 and < 2009 | 1.22 [0.77–1.94] | 0.4 | ||
| ≥ 2009 | 1.29 [0.91–1.82] | 0.2 | ||
- Cox analysis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR [95% CI] | P | HR [95% CI] | P | |
| Rituximab vs splenectomy | 2.75 [0.99–7.59] | 0.05 | – | – |
| Age (for one year increase) | 1.02 [1.00–1.05] | <0.05 | – | – |
| Female gender | 2.14 [0.79–5.82] | 0.13 | – | – |
| Disease duration, ≥ 1 year vs < 1 year | 0.34 [0.11–1.05] | 0.006 | – | – |
| History of mucosal bleeding | 2.16 [0.70–6.69] | 0.18 | 11.90 [1.57–90.19] | <0.02 |
| Charlson score ≥ 1 | 3.76 [1.35–10.46] | 0.01 | 3.58 [1.29–9.95] | 0.01 |
| Other ITP drugs before index date (except corticosteroids) | 1.77 [0.57–5.84] | 0.3 | – | – |
| Year of splenectomy or rituximab, ≥ 2002 vs < 2002 | 2.36 [0.66–8.35] | <0.2 | – | – |
The frequency of hospitalizations for infection did not differ between treatment groups (Supporting Information Fig. S1). Seven patients treated with rituximab were hospitalized for infection (five pneumonia, one Staphylococcus septicemia, and one hepatitis E virus infection) versus six patients in the splenectomy group: two septicemia (Staphylococcus and Pneumococcus), and four enterobacteria infections (two digestive and two urinary tract infections).
Response rates at 3 months were 91.4% after splenectomy versus 69.8% after rituximab (P = 0.005). At 12 months, the response rates were 87.9% versus 59.0% (P = 0.001), respectively. Complete response rates were 82.8% versus 39.5% at 3 months (P < 0.0001) and 81.0% versus 35.9% at 12 months (P < 0.0001), respectively. The factors associated with a response at 12 months (either complete or not) are presented in Table 4. In multivariate analysis, splenectomy compared to rituximab was the sole variable associated with response at 12 months (OR, 4.4, 95% CI [1.7–11.8]). This was also the case when restricting the patients to those aged <65 years (Supporting Information Table SIII).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR [95% CI] | P | OR [95% CI] | P | |
| Splenectomy vs. rituximab | 4.43 [1.66–11.82] | 0.003 | 4.43 [1.66–11.83] | <0.003 |
| Age, ≥ 65 years vs. < 65 years | 0.32 [0.12–0.84] | 0.02 | – | – |
| Female gender | 1.32 [0.51–3.38] | 0.5 | – | – |
| Disease duration, ≥ 1 year vs. < 1 year | 0.88 [0.36–2.25] | 0.8 | – | – |
| History of mucosal bleeding | 0.77 [0.30–1.98] | 0.6 | – | – |
| Charlson score ≥ 1 | 0.50 [0.19–1.29] | 0.1 | – | – |
| Year of splenectomy or rituximab, ≥ 2002 vs. < 2002 | 0.37 [0.11–1.19] | 0.1 | – | – |
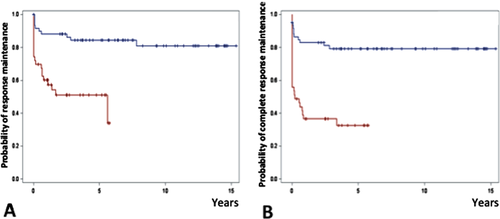
Maintenance of response and maintenance of complete response rates remained significantly higher in the splenectomy group after adjustment on the propensity score, whether the whole population was considered or whether the analysis was restricted to the patients who initially achieved a response or a complete response, respectively (Fig. 3 and Supporting Information Fig. S2). Median duration ± interquartile range (IQR) of response in responders was 81.0 ± 106.5 months (median follow-up: 90.0 ± 101.7 months) in the splenectomy group and 20.1 ± 43.2 months (median follow-up: 29.7 ± 43.4 months) in the rituximab group.
Of note, three rituximab-treated patients were re-treated after a first course. In the splenectomy group, three patients were exposed to rituximab later in the disease course. One nonresponder patient was exposed to rituximab 3 months after splenectomy due to serious bleeding. The two others were exposed to rituximab, respectively, two and eight years after splenectomy, due to severe bleeding in the context of loss of response. None of these three patients experienced serious infection nor died during follow-up. In the rituximab group, three patients were splenectomized after rituximab exposure during hospitalization for serious bleeding. Two of them were not responders three months after splenectomy, and the third relapsed four years later. None of these three patients experienced serious infection nor died during follow-up. The patients who failed on rituximab and who were not splenectomized were treated with TPO-receptor agonists (romiplostim, n = 5, eltrombopag, n = 5), dapsone (n = 2), danazol (n = 2), ciclosporin (n = 1), vinblastin (n = 1), and long-term corticosteroids (n = 2).
Splenectomy was performed by laparoscopy in 30 (47.6%) patients. Imaging techniques were not systematically used to search for portal vein thrombosis. Six patients (9.5%) had seven complications: three had symptomatic portal vein thrombosis, one had both eventration and peritonitis, and one had parietal abscess. All the complications except one portal vein thrombosis occurred in patients splenectomized by laparotomy.
Discussion
This study is, to our knowledge, the first direct and adjusted comparison of the outcome of patients treated by splenectomy or with rituximab in adult primary ITP. It reflects the “real life” use of splenectomy and rituximab. We assessed mortality, serious bleeding, and serious infections (hospitalizations) over a long follow-up period.
This study showed that a history of serious bleeding and comorbidities are major independent risk factors for serious clinical events, particularly serious bleeding. Exposure to rituximab or splenectomy was not a risk factor for these clinical outcomes after adjustment on the propensity score as well as after adjusting on covariables in multivariate regression models. The huge difference regarding the main outcome observed between the two groups may therefore be explained by channeling bias, as more comorbid patients were treated preferentially with rituximab rather than by surgery. Of note: the patients who died in the rituximab group, particularly from infection or bleeding, were older and had more comorbidities and consequently, they would have been refused for surgery.
In a study including 60 rituximab-treated patients, no deaths were observed during a two-year follow-up 14. However, the patients of this tertiary reference center clearly differed from ours. For instance, their mean age was 48 years compared with 66.5 in our study. Data on treatment of elderly ITP patients is lacking, while older ITP patients have a poor prognosis 5.
In our study, the response and loss of response rates among splenectomized patients are quite similar to those reported by others 4. In the rituximab group, the 35.9% complete response rate at one year was somewhat lower than expected, as it was 45.6% in a meta-analysis including 368 patients 13.
As expected, splenectomy was more effective than rituximab regarding response, complete response, maintenance of response, and maintenance of complete response rates. We could quantify that splenectomy was associated with a 4.4-fold probability of response at 12 months compared with rituximab. Little is known concerning the factors associated with response after ITP treatment. For splenectomy, the role of a younger age has been demonstrated, while a previous response to intravenous immunoglobulins has been discussed 4. In our multivariate analysis, age was not independently associated with the response at 12 months while a younger age was also associated with a complete response after rituximab 13.
The risk of infection after splenectomy is well known. It seems to be higher during the first years after surgery and uncorrelated to increased mortality during the first years of follow-up 6, 9, 10. We did not find any unusual infections among splenectomized patients 23. Regarding rituximab, there was no increased infection risk reported in the previously published series including younger patients with few comorbidities 14, 16. However, the infection risk in real-life practice has not yet been accurately assessed 17, 24. We found that hospitalization for pneumonia occurred in 12% of the exposed patients. In the review by Arnold et al. in 2007 of 306 published rituximab-treated adult patients, one patient died of pneumonia 13 weeks after rituximab infusion, and another died of acute respiratory failure 6 days after rituximab. Both patients were elderly (respectively, 73 and 71 years old) and had chronic lung disease. The vaccination rate was lower among rituximab-treated patients than among splenectomized patients (32.4 versus 77.5%) in our cohort 25. This may explain in part a relatively high frequency of serious lung infections. Although the clinical efficacy of anti-pneumococcal vaccine in ITP patients has not yet been confirmed, our results strongly encourage vaccination of ITP patients as soon as possible when the eventuality of treatment with rituximab or by splenectomy becomes probable 3.
The frequency of local complications after splenectomy in this series (11%) was similar to that observed in other cohorts. As expected, it was lower after laparoscopic surgery 4, 26-28. Incidence of symptomatic portal vein thrombosis was slightly higher than expected 24, 29, 30.
This work has some limitations. It is a retrospective study exposed to a selection bias: this is a university hospital cohort that may be not representative of all patients suffering ITP in the corresponding area. Selection omissions in the PMSI and the pharmacy dispensing list cannot be excluded. Recall bias has been minimized by the choice of robust outcomes. We cannot exclude an information bias for some baseline variables such as history of mucosal bleeding (missing information in medical charts and recall bias) but there is no reason for a differential bias between the two groups. As a result, this should not have heavily biased the comparisons. The lack of power prevented us from assessing the proper role of each covariate in mortality. Lastly, this study was not designed to study late adverse events such as cardiovascular events. In previous studies, both venous thromboembolism and arterial thrombosis were more frequent in ITP patients than among controls (adjusted incidence rate ratio of vascular events of any type: 1.70; 95% CI [1.41-2.05]) but myocardial infarction or death from cardiovascular cause were not 31-34. Risk factors for these events have not yet been described in ITP patients.
This study deserves to be repeated in larger cohorts. At stake is the respective place of splenectomy and rituximab in second-line ITP treatment. Of note, little is known about both strategies in the elderly. Patients aged over 70 years have a higher bleeding score compared to younger ITP patients for a given platelet count 35, and splenectomy may be less effective in the elderly 36. No patient over 65 years was exposed to splenectomy in our cohort, but serious infections and bleeding in older patients exposed to rituximab were not rare. Only comorbidities and not age or treatment allocation were associated with outcomes. We found no data in the literature and no guidelines indicating that age per se should contraindicate splenectomy. Therefore, the benefit-to-risk ratio of splenectomy in patients over 65 with few comorbidities should be prospectively assessed.
Acknowledgments
The authors thank Anaïs Grand, Pharm.D. (Pharmacy Department of Toulouse University Hospital) who provided the list of rituximab-treated patients during the study period.




