The combination of lenalidomide and dexamethasone reduces bone resorption in responding patients with relapsed/refractory multiple myeloma but has no effect on bone formation: Final results on 205 patients of the Greek myeloma study group
This study is presented, in part, as an abstract at the 16th Annual Meeting of the European Hematology Association, London, UK, June 9–12, 2011
Conflict of interest: Nothing to report
Abstract
The combination of lenalidomide plus dexamethasone (RD) is very effective for patients with relapsed/refractory myeloma. However, the effect of RD on bone metabolism has not been previously evaluated in these patients. To address this issue, we initially performed a retrospective study in 106 consecutive patients with relapsed or refractory myeloma who received RD. We measured the following bone indices on Cycle 1/Day 1 and then on Cycles 3 and 6/Day 28: dickkopf-1 (Dkk-1), sRANKL, osteoprotegerin (OPG), bone resorption markers (C-telopeptide of collagen type-I, CTX and TRACP-5b) and bone formation markers (bone-specific alkaline phosphatase-bALP and osteocalcin). RD produced a reduction of CTX only in responders, with no effect on bone formation. To validate these results, we then evaluated prospectively 99 patients who received either RD (n = 50) or VRD (bortezomib + RD, n = 49). RD reduced CTX, mainly in responders but showed no effect on bone formation, confirming the result of the retrospective study. However, the addition of bortezomib to RD (VRD arm) reduced Dkk-1, sRANKL/OPG, and CTX, while it increased bALP and OC after six cycles of therapy. These changes were irrespective of treatment response, which was similar between treatment arms. No skeletal-related events were observed in the VRD arm while two, nonresponding patients treated with RD developed a vertebral fracture. We conclude that RD reduces bone resorption only in responding patients with relapsed/refractory myeloma but has no effect on bone formation. Combination with bortezomib, which enhances bone formation, seems to be preferred for the management of myeloma patients with osteolytic disease. Am. J. Hematol. 89:34–40, 2014. © 2013 Wiley Periodicals, Inc.
Introduction
Multiple myeloma (MM) is a plasma-cell malignancy and the second most common hematologic neoplasm after lymphoma 1. Although, MM is an incurable disease, novel treatments based on proteasome inhibitors and immunomodulatory drugs (IMiDs) have improved patients' survival during the recent years 2. Lenalidomide combined with dexamethasone (RD) is an effective treatment for patients with relapsed and/or refractory MM and provides an overall response (OR) rate of 60%, with a complete response (CR) rate of 15% 3, 4.
Bone disease is a hallmark of MM as it occurs in the majority of MM patients, with up to 80% of them developing osteolytic lesions 5. The characteristic lytic lesions are the result of excessive bone resorption due to increased osteoclast formation and activity and suppressed bone mineralization by osteoblasts. Cytokines produced locally by stromal or myeloma cells are responsible for the osteoclast activation 6, 7. The receptor activator of nuclear factor-κB (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) system has a major role in osteoclastogenesis, as RANKL directly induces osteoclast differentiation and proliferation and OPG is the soluble decoy receptor for RANKL that inhibits its function 8. In MM, the ratio of RANKL/OPG is increased due to an increase in RANKL production and a decrease in OPG production by stromal cells (BMSCs) 9-11. Furthermore, myeloma cells produce dickkopf-1 (DKK-1) protein and other suppressors of Wnt-signaling that inhibit osteoblast function; thus further supporting the development of bone lesions 12-14.
Data in the literature suggest that novel antimyeloma agents affect bone remodeling 15. Several studies have shown that bortezomib reduces osteoclast function and enhances osteoblast anabolic effects on MM patients 16-18. Regarding IMiDs, thalidomide and pomalidomide reduce osteoclast activity, but it seems that they have almost no effect on bone formation in MM 19-21. There are very limited data in the literature for the effect of lenalidomide on bone turnover of MM patients. The only published study is that of Breitkreutz et al. who have reported that lenalidomide reduces bone resorption both in vitro and in vivo along with a reduction of RANKL/OPG ratio 22, while there is only one case report suggesting that the combination of RD may increase bone formation in a patient with refractory MM 23.
To evaluate the effect of the combination of RD on bone remodeling we studied 205 patients with refractory/relapsed MM. Initially markers of bone remodeling were evaluated retrospectively in 106 consecutive patients, who received the standard RD (lenalidomide + high-dose dexamethasone) regimen. To confirm these results, we then evaluated prospectively bone metabolism of 99 patients who received either RD or VRD (bortezomib, lenalidomide, and dexamethasone) in the context of a clinical study 24.
Patients and Methods
Patients of the retrospective RD study
Patients with relapsed or refractory myeloma after one or more prior therapies were treated with RD, regardless of their performance status, renal function, prior peripheral neuropathy, and prior treatment with high-dose dexamethasone, thalidomide, or bortezomib. Patients who were previously treated with lenalidomide were not included. Refractory disease was termed as progression during prior treatment or within 60 days of discontinuation of prior treatment while relapsed disease was termed as progression at least 60 days after completion of prior treatment. All patients received prophylaxis for deep vein thrombosis (DVT) with aspirin 100 mg/day unless they were already on coumadin or on low molecular weight heparin (LMWH) for pre-existing DVT or for other indications, usually atrial fibrillation. Response assessment was based on the International Myeloma Working Group (IMWG) criteria 25. The National Cancer Institute's Common Toxicity Criteria (version 3) were used to grade adverse effects for toxicity being related to treatment. Informed consent was obtained from all patients.
Lenalidomide was administered at a dose of 25 mg PO daily on Days 1–21 of a 28-day cycle if the baseline creatinine clearance (CrCl) was >50 m/min. For patients with lower CrCl the following adjustments were made: lenalidomide 10 mg/day for patients with CrCl ≥30 ml/min, 15 mg every other day for patients with CrCl <30 ml/min without need for dialysis and 15 mg three times a week on the day after dialysis for patients on dialysis. Dexamethasone was administered at a dose of 40 mg PO on Days 1–4 and 15–18 for the first four cycles and only on Days 1–4 thereafter. RD was repeated every 28 days. Patients who responded to treatment or had stable disease continued on treatment until disease progression or unacceptable toxicity. Patients received supportive treatment with erythropoietin or darbepoietin and G-CSF, as clinically indicated and zoledronic acid at a dose of 4 mg (or lower according to CrCl) every 28 days while on study.
Patients of the prospective RD vs. VRD evaluation
Consecutive patients with relapsed/refractory myeloma after at least one prior line of therapy who had prior neuropathy ≥Grade 2 received RD, while patients with prior neuropathy <Grade 2 received VRD 24. Patients who were treated with RD received the regimen as described for the retrospective study. The doses of VRD were as follows: bortezomib was given at 1 mg/m2 IV on Days 1, 4, 8, and 11, lenalidomide was given at a dose 15 mg PO daily on Days 1–14 (or at a lower dose if CrCl was <30 ml/min) and dexamethasone was administered at a dose of 40 mg PO on Days 1–4. VRD treatment was repeated every 21 days for eight courses and patients without progression continued treatment with lenalidomide, dosed according to CrCl, for 21 days and dexamethasone 40 mg PO Days 1–4 every 28 days, until disease progression or unacceptable toxicity.
Patients received supportive treatment with zoledronic acid (4 mg i.v. every 28 days), erythropoietin or darbepoietin and G-CSF, as clinically indicated. All patients received prophylaxis with valacyclovir and trimethoprime-sulfamethoxazole. Thromboprophylaxis was mandatory and consisted of low dose aspirin (100 mg PO daily) unless the patient was already on LMWH or coumadin for another indication.
Evaluation of bone disease
Evidence of bone involvement at the time of treatment, for both analyses, was documented using plain radiography within one month from treatment initiation. Patients were considered to have bone involvement if there were radiographic abnormalities consistent with MM bone disease, including osteoporosis, osteolytic lesions and fractures. A grading of bone morbidity into three stages according to the radiographic evaluation of the skeleton was made. Stage A included patients with no lytic lesions or osteoporosis alone; Stage B included patients with one to three osteolytic lesions, and Stage C included patients with more than three osteolytic lesions and/or a pathological fracture due to MM. We have used the <3/>3 as cut-off for bone lesions as advanced bone disease includes more than three lytic lesions in the Durie-Salmon staging system.
In the prospective VRD versus RD evaluation, skeletal-related events (SREs: pathological fractures, radiation therapy, surgery or spinal cord compression) were a secondary end point and were evaluated throughout the study period.
Measurement of markers of bone turnover
The following circulating molecules were evaluated in both studies: regulators of osteoclast function (sRANKL and OPG), osteoblast inhibitor DKK-1, markers of bone resorption [tartrate resistant acid phosphatase isoform type-5b (TRACP-5b), and carboxy-telopeptide fragments of collagen type-I α1 chains (CTX)], and markers of bone formation [bone alkaline phosphatase (bALP) and osteocalcin (OC)]. The above indices were measured at baseline, and then on Day 28 of the 3rd and 6th cycle of RD in the retrospective study, while in the prospective evaluation the bone markers were measured on Day 28 of the 3rd and 6th cycle of RD and on Day 21 of the 3rd and 6th cycle of VRD.
The above regulators of osteoclast/osteoblast function and markers of bone metabolism were also measured in 44 healthy individuals, age- and gender-matched to the patients (24 M/20 F, median age 67 years, range 45–81 years) who were served as controls for the retrospective RD study. Each control subject was examined to ensure that there was no evidence of bone disease (osteoporosis or osteoarthritis) and no receipt of medication that could alter the normal bone turnover during the last 6 months.
After veinpuncture serum was separated within 4 hr and stored at −70°C until the day of measurement. An enzyme-linked immunosorbent assay (ELISA) was used for the detection of serum: sRANKL (Biomedica Medizinprodukte, No. BI-20422H, Gesellschaft GmbH & Co KG, Wien, Austria), OPG (Biomedica Medizinprodukte, Gesellschaft GmbH & Co KG, Wien, Austria), DKK-1 (Biomedica Medizinprodukte, Gesellschaft GmbH & Co KG, Wien, Austria), TRACP-5b (BoneTRAP, Immunodiagnostic Systems, Boldon, Tyne & Wear, UK), CTX (Serum CrossLaps, Immunodiagnostic Systems, Boldon, Tyne & Wear, UK), bALP (Metra BAP, Quidel Corporation, San Diego, CA), and OC (N/MID Osteocalcin, Nordic Bioscience Diagnostics A/S, Herlev, Denmark), according to manufacturers' instructions. All samples from the same patient were measured on the same ELISA plate. Both studies were conducted after approval by the Institutional Ethics Committee and under the guidelines of the Declaration of Helsinki.
Statistical analysis
Differences between patients and controls were evaluated using the Mann-Whitney test. Differences between baseline and 3rd- and 6th-cycle values of the studied parameters were evaluated using the Wilcoxon signed rank test. Associations between bone disease status and biochemical markers were examined by the Kruskal–Wallis test, whilst the Spearman rank correlation test was employed to examine relationships between various parameters and clinical patient characteristics. Survival probabilities were calculated by the Kaplan–Meier method and comparisons made using the log-rank test to identify potential prognostic factors. All P values are two sided and confidence intervals refer to 95% boundaries.
Results
Results of the retrospective RD study
Patients
One hundred and six consecutive patients (54 M/52 F) who were treated in six centers of the Greek Myeloma Study Group and had serum available were included in this study. The characteristics of the patients are depicted in Table 1. The median age of patients was 68 years (range: 43–87 years). Before the initiation of treatment 34 (32%) patients had Stage I disease, 37 (35%) patients had Stage II and 35 (33%) had Stage III disease according to the International Staging System (ISS). Prior to lenalidomide/dexamethasone patients had received a median of three lines of treatment (range: 1–7). Fifty-two percent of the patients were refractory to last therapy. Before the start of treatment 18 (17%) patients had grade A bone disease, while 35 (32%) patients had grade B, and 53 (50%) patients had grade C bone disease.
| Characteristic | No. patients | % |
|---|---|---|
| Total no. of patients | 106 | |
| Gender | ||
| Male | 54 | 52 |
| Female | 52 | 48 |
| Age—median (range) | 68 (43–87) | |
| Type of MM (IgG/IgA/IgD/LC/NS) | 56/29/3/15/3 | |
| Stage at diagnosis (ISS) | ||
| I | 34 | 32 |
| II | 37 | 35 |
| III | 35 | 33 |
| Bone disease status | ||
| Grade A | 18 | 17 |
| Grade B | 35 | 32 |
| Grade C | 53 | 50 |
| Previous treatments—median (range) | 3 (1–7) | |
| Status before treatment | ||
| Relapse after last treatment | 52 | 48 |
| Refractory to last treatment | 54 | 52 |
| Prior Thalidomide | 76 | 72 |
| Prior Bortezomib | 65 | 61 |
| Prior HDM | 37 | 35 |
| Creatinine clearance <50 ml/min | 17 | 16 |
- Abbreviations: MM, multiple myeloma; Ig, immunoglobulin; LC, light chain only; NS, nonsecretory; ISS, International Staging System; HDM, high-dose melphalan.
Response to treatment and side-effects
The median cycles of RD given in our patients were 8 (range: 3–31 cycles). According to IMWG criteria, 13 patients (12%) achieved a complete response (CR), 12 patients (11%) a very good partial response (VGPR), and 34 patients (32%) partial response (PR). Ten patients (9%) experienced progressive disease (PD) while on treatment and discontinued the study while 37 patients (36%) had stable disease (SD). The overall response rate (CR + VGPR + PR) was 55%. Median time to first response was 8 weeks (range: 3–112 weeks).
Most common side-effects included neutropenia 55% (Grade 3 or more, 36%), fatigue 54% (Grade 3, 12%), thrombocytopenia 46% (Grade 3 or more, 20%) and infections 32% (Grade 3 or more, 19%). Deep venous thrombosis (DVT) occurred in three patients (3%). All of them continued on treatment with the addition of low molecular weight heparin (LMWH). Among the patients treated, 38% had at least one dose reduction of lenalidomide and 22% had at least one dose reduction of dexamethasone.
Markers of bone remodeling and osteoclast function at baseline
Patients with relapsed/refractory myeloma before treatment had elevated median values of serum sRANKL (P = 0.002), DKK-1 (P = 0.004), TRACP-5b (P = 0.025), CTX (P = 0.027) compared with controls, while serum levels of bALP and OC were lower than in controls (P = 0.03 and P < 0.001, respectively). There was no difference in terms of OPG between patients and controls and the respective ratio of sRANKL/OPG was significantly higher in myeloma patients before the administration of RD compared with the control group (P = 0.01) (Table 2). The levels of the measured indices were not predictive of response to RD.
| Patients at baseline median (range) (N = 106) | Controls median (range) (N = 44) | p-value (baseline vs. controls) | Patients after three cycles median (range) (N = 103) | p-value (baseline vs. after three cycles) | Patients after six cycles median (range) (N = 92) | p-value (baseline vs. after six cycles) | |
|---|---|---|---|---|---|---|---|
| Osteoclast regulators | |||||||
| sRANKL (pmol/l) | 0.15 (ND–2.7) | 0.03 (ND–0.84) | 0.002 | 0.17 (ND–0.28) | 0.13 | 0.16 (ND–2.52) | 0.41 |
| OPG (pmol/l) | 5.2 (1.3–24.6) | 4.8 (2.1–9.4) | 0.247 | 5.6 (1.8–16) | 0.59 | 5.6 (1.5–23) | 0.94 |
| sRANKL/OPG | 0.02 (0–0.4) | 0.004 (0–0.16) | 0.01 | 0.04 (0–0.7) | 0.04 | 0.02 (0–0.6) | 0.712 |
| Bone resorption markers | |||||||
| CTX (ng/ml) | 0.57 (ND–3.7) | 0.45 (0.02–0.9) | 0.027 | 0.42 (ND–5.7) | 0.172 | 0.51 (ND–2.7) | 0.539 |
| TRACP-5b (U/l | 1.6 (ND–5.4) | 1 (0.12–3.4) | 0.025 | 2.1 (ND–5.9) | 0.006 | 1.7 (ND–4.7) | 0.07 |
| Osteoblast inhibitors | |||||||
| Dkk-1 (pmol/l) | 90.9 (13–382) | 43.0 (18.8–76) | 0.004 | 89.3 (13.9–189) | 0.476 | 90.6 (16.4–206) | 0.967 |
| Bone formation markers | |||||||
| bALP (U/l) | 18.6 (7.6–48.2) | 21 (10.5–58.3) | 0.03 | 17 (8.1–59) | 0.393 | 18.5 (7.5–88.6) | 0.433 |
| OC (ng/ml) | 7.7 (ND–87.7) | 18 (5.9–52.1) | <0.001 | 6.3 (ND–28) | 0.514 | 6.3 (ND–47.2) | 0.377 |
There was a significant correlation between bone disease status at baseline and sRANKL/OPG ratio as well as DKK-1. Patients with osteolytic bone disease (Stages B + C) had elevated levels of sRANKL/OPG ratio compared with patients with no osteolysis [median and range was: 0.03 (0–0.28) vs. 0.003 (0–0.4), respectively; P = 0.032]. Furthermore, patients with Stage C bone disease had increased serum DKK-1 levels compared to patients with Stages A + B bone disease before initiation of RD [median and range was: 113.5 ng/mL (19.5–382 ng/mL) vs. 69.6 ng/mL (13–183 ng/mL), respectively; P = 0.01]. No other significant correlation was observed between the different markers of bone remodeling or osteoclast function and the extent of bone disease before treatment.
The pretreatment values of sRANKL correlated with CTX and DKK-1 (r = 0.266, P = 0.007 and r = 0.200, P = 0.047, respectively). DKK-1 serum levels also showed a strong association with TRACP-5b values (r = 0.403, P < 0.001). Furthermore OC levels correlated with bALP (r = 0.398, P < 0.001).
We also studied possible correlations between pretreatment bone markers values and pretreatment β2-microglobulin, albumin, creatinine, calcium, hemoglobin and LDH. There was only a weak positive correlation between CTX and β2-microglobulin (r = 0.256, P = 0.022).
Effect of the RD on markers of bone remodeling/osteoclast function and myeloma bone disease
The RD combination produced borderline increase of sRANKL/OPG ratio and an increase of TRACP-5b after three cycles of treatment compared to baseline (P = 0.04 and P = 0.006, respectively) that were not continued after six cycles of therapy while the levels of CTX, DKK-1 and bone formation markers had no significant alterations during the study period.
However, in patients who responded to therapy there was a reduction of DKK-1 after six cycles of treatment (median, range: from 92 pmol/L, 18–382 pmol/L at baseline to 77 pmol/L, 28–249 pmol/L; P = 0.035), and a mild reduction of CTX compared to baseline values (median change: −4% ranging from −100% to +209%). In contrast, in patients who did not respond to therapy, DKK-1 was increased (median 51%, ranging from +12% to +192%) and there was also an increase in CTX values (median 17% ranging from −97% to +391%; P = 0.04; Fig. 1) and in TRACP-5b serum levels (median 21% ranging from +9% to +113%; P = 0.046) after six cycles of treatment compared to baseline values. There was no difference regarding changes in bone formation markers (bALP, OC) between responders and nonresponders. All changes of the studied parameters after RD therapy are depicted in Table 2.
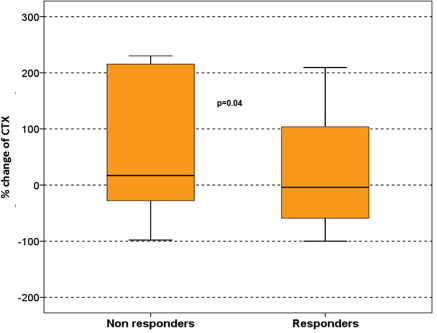
Survival
The median follow-up for all patients in the retrospective study was 24.5 months (range: 9–42 months). The median overall survival (OS) of all patients from the start of RD was 18 months (95% CI: 11.7–24.3), while the median progression free survival (PFS) was 11 months (95% CI: 8.9–13). Elevated LDH at baseline (HR: 8.2, 95% CI: 4.2–16.4, P < 0.001), response to therapy (HR: 5.8, 95% CI: 3.5–10.1, P < 0.01) and ISS at diagnosis (HR: 2.3, 95% CI: 1.6–5.5, P = 0.02) were the only factors that independently correlated with OS. No biochemical marker of bone remodeling at baseline was prognostic for overall or progression-free survival.
Results of the prospective RD versus VRD treatment
The characteristics of the patients, data of response to therapy and survival data have been previously presented 24. Regarding treatment response, the objective response rate (≥PR) was 61% for RD and 63% for VRD. Time to best response was not statistically different between the two arms (median: 1.6 vs. 2.2 months for VRD and RD, respectively; P = 0.198). The alterations of markers of bone turnover after 3 and 6 months of RD or VRD therapy are depicted in Table 3. Patients who received RD showed an increase of Dkk-1 after 6 months of therapy but responding patients had only a borderline increase (median of 9%) compared to nonresponders (median increase of 91%; P = 0.01; Fig. 2A). Furthermore, responding patients had a significant reduction of the levels of CTX (median change: −56%, range from −80% to +23%) compared to nonresponding patients (median: −12%, ranging from −68% to +130%; P < 0.01 for responders vs. non responders; Fig. 2B). There were no other significant alterations of the other bone indices with RD.
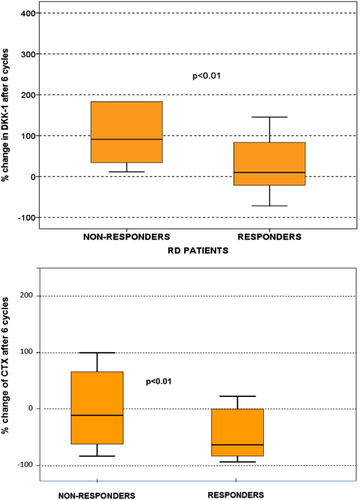
| RD patients | VRD patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients at baseline median (range) ( N = 50) | Patients after three cycles median (range) (N = 48) | p-value (baseline vs. after three cycles) | Patients after six cycles median (range) (N = 43) | p-value (baseline vs. after six cycles) | Patients at baseline median (range) (N = 49) | Patients after three cycles median (range) (N = 46) | p-value (baseline vs. after three cycles) | Patients after six cycles median (range) (N = 44) | p-value (baseline vs. after six cycles) | |
| Osteoclast regulators | ||||||||||
| sRANKL (pmol/l) | 0.22 (ND–2.8) | 0.19 (ND–1.9) | 0.736 | 0.22 (ND–2.25) | 0.572 | 0.33 (ND–2.6) | 0.34 (ND–1.8) | 0.145 | 0.1 (ND–0.67) | 0.024 |
| OPG (pmol/l) | 6.6 (3.5–24.6) | 7.5 (3.6–15.9) | 0.555 | 6.7 (2.5–22.9) | 0.776 | 7.1 (2.2–27.3) | 6.5 (3–19.7) | 0.643 | 6.3 (2.3–27.8) | 0.534 |
| sRANKL/OPG | 0.02 (0–0.48) | 0.03 (0–0.35) | 0.652 | 0.03 (0–0.28) | 0.545 | 0.05 (0–0.49) | 0.02 (0–0.46) | 0.027 | 0.01 (0–0.25) | 0.045 |
| Bone resorption markers | ||||||||||
| CTX (ng/ml) | 0.65 (0.04–3.6) | 0.42 (ND–3.8) | 0.07 | 0.38 (ND–2.7) | 0.05 | 0.97 (0.02–3.2) | 0.29 (0.09–4.3) | 0.01 | 0.28 (0.05–3.1) | <0.01 |
| TRACP-5b (U/l) | 2.4 (0.8–5.5) | 2.3 (1.2–5.5) | 0.379 | 2 (1.3–5.2) | 0.258 | 2.5 (0.9–6.8) | 2.1 (1.1–6.2) | 0.157 | 2.0 (1.3–3.7) | 0.174 |
| Osteoblast inhibitors | ||||||||||
| Dkk-1 (pmol/l) | 115.7 (28.4–226) | 116.9 (14.9–249) | 0.904 | 165.5 (49.3–306) | 0.018 | 121.6 (22.1–250) | 67.9 (19.5–277) | 0.01 | 50.2 (32.2–232) | <0.01 |
| Bone formation markers | ||||||||||
| bALP (U/l) | 21.3 (10.7–48.1) | 20.1 (11.7–59.5) | 0.334 | 19.6 (11.1–88.6) | 0.585 | 21.9 (10.6–93.5) | 27.8 (9.2–130) | 0.175 | 33.4 (11.2–106) | 0.01 |
| OC (ng/ml) | 6.9 (ND–90.2) | 5.9 (ND–28.2) | 0.287 | 7.6 (0.19–47.2) | 0.429 | 7.6 (ND–83.4) | 10.8 (2.7–70.1) | 0.031 | 15.6 (7.3–84.5) | 0.01 |
On the contrary, VRD reduced dramatically sRANKL/OPG, DKK-1 and CTX and increased bALP and OC after three and six cycles of treatment and these changes were irrespective of treatment response. In VRD arm, the reduction of DKK-1 was strongly correlated with an increase in bone formation markers (bALP) (r = −0.425, P < 0.01). Compared to RD, VRD produced significant reductions of sRANKL and DKK-1 and strong increases of both bone formation markers that were not altered with RD (Fig. 3). No SREs were observed in the VRD arm while two patients treated with RD, who had not responded to therapy, developed a vertebral pathological fracture during the duration of the study.
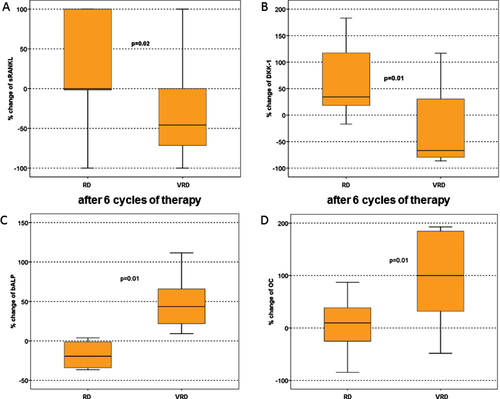
Discussion
Lenalidomide is a potent antimyeloma agent, which is used in both relapsed/refractory 3, 4 and newly-diagnosed myeloma patients 26, 27. The effect of lenalidomide on bone remodeling of myeloma patients is currently unknown. The only reported data to-date support an in vitro antiresorptive effect of lenalidomide along with a reduction of serum sRANKL/OPG ratio in myeloma patients who receive lenalidomide monotherapy after 2 months of treatment 22. Regarding bone formation, there is only one case report suggesting a possible bone formation effect of the RD combination 23. The aim of our study was to evaluate the effect of the standard RD combination on bone metabolism of patients with relapsed/refractory myeloma, as the use of lenalidomide as a single agent in relapsed/refractory MM is extremely rare. Therefore, we first contacted a retrospective analysis of 106 consecutive patients who were treated in six Greek centers that had available serum samples for the measurement of a series of bone-cell regulators and markers of bone resorption and formation. This study confirmed that there is altered bone remodeling in patients with relapsed/refractory myeloma who have increased bone resorption and reduced bone formation that led to the presence of lytic lesions in 82% of them. The combination of lenalidomide with high-dose dexamethasone had similar efficacy and safety with previously published data. Regarding bone turnover, the only important observation of our retrospective analysis was that the RD regimen produced a reduction of bone resorption marker CTX only in responding patients. Our group has previously shown that the combination of another IMiD, thalidomide with dexamethasone (TD) reduced bone resorption markers and sRANKL/OPG ratio in 35 patients with relapsed/refractory myeloma after three and six cycles of therapy, but had no effect on bone formation markers 20. In our study, RD had also no effect on bone formation. Furthermore, RD did not reduce sRANKL/OPG ratio as it was described for lenalidomide monotherapy and for TD. To confirm these results, we prospectively evaluated the effect of either RD or VRD on bone remodeling of 99 patients with relapsed/refractory myeloma who received one of the two regimens based on the presence of peripheral neuropathy. According to our knowledge, this is the first prospective study which evaluated the alterations of bone turnover by the use of RD. In that study, we confirmed that RD reduced bone resorption marker CTX, mainly in responders, and that RD had no effect on sRANKL/OPG ratio after 6 months of therapy. The inhibitory effect of lenalidomide on bone resorption may be due to the inhibition of osteoclast by lenalidomide 22, due to the reduction of myeloma burden or due to both. However, the failure of RD to reduce sRANKL/OPG ratio after 3 and 6 months of therapy, even in responders, is in contrast to what Breitkreutz et al. has described for lenalidomide monotherapy. This is possibly because of the different study population but also because of the use of high-dose dexamethasone in our study, which is a well-known stimulator of RANKL production 28. Furthermore, our patients were also receiving zoledronic acid, which reduces bone resorption but enhances RANKL and OPG gene expression 29, 30. Zoledronic acid may also contribute to the reduced CTX levels in our cohort of RD patients, despite its effect on the RANKL/OPG system. Thus it is difficult to know if the reduction of CTX was due to RD alone, to zoledronic acid alone or more possibly due to their combination. At this point, we have to stress that in the study of Breitkreutz et al., there was no measurement of any bone resorption or bone formation marker, or sRANKL/OPG measurement at another time point after the 2-month evaluation. Thus, it is not possible to conclude if the observed reduction of sRANKL/OPG after 2 months of lenalidomide monotherapy was continued after that period of time and if this reduction could lead to any significant changes in bone resorption or formation.
Our prospective study, also confirmed that RD has no effect on bone formation. In that study even responding patients showed a mild increase of the bone formation inhibitor DKK-1, while nonresponders had a dramatic increase of DKK-1. In the retrospective analysis, DKK-1 was not altered in the whole cohort of patients, while responders showed a median reduction of 17% and nonresponders a median increase of 51%. The Arkansas group has reported that both thalidomide and lenalidomide enhance the expression of DKK-1 mRNA and DKK-1 protein in vitro 31, 32. Dexamethasone also increases the production of DKK-1 33. Thus, the effect of the RD combination on DKK-1 seems to contribute to the failure of RD to enhance bone formation, despite the reduction of tumor burden. Furthermore, two patients who did not respond to RD developed vertebral fractures. On the contrary, when bortezomib was combined with RD there was a strong bone anabolic effect. In our study, the VRD combination increased bALP by 50% compared to baseline values and OC by almost 100%, irrespective of antimyeloma response, which was similar to that of RD. This is attributed to bortezomib, which has produced such increases in bone formation when it was given either alone or in combination with dexamethasone 15-18, 34. Furthermore, the combination of VRD significantly reduced sRANKL/OPG ratio, CTX and DKK-1, supporting a beneficial effect of this regimen in both bone formation and resorption. In our cohort of 49 patients who were treated with VRD, we had no SRE during the study period, even in nonresponders or in patients who progressed while on treatment. The shorter time to best response with VRD compared to RD, although not statistically significant, may also contribute to the absence of SREs in the VRD arm. However, the low number of patients who developed SREs during the study does not allow to draw final conclusions for SREs rate under treatment with VRD or VD.
In conclusion, our studies show that the combination of lenalidomide with high-dose dexamethasone, although it is very effective for the treatment of relapsed/refractory MM, reduces bone resorption only marginally and mainly in responders, and has no effect on bone formation. Furthermore, the coadministration of zoledronic acid may also contribute to the observed reduction of bone resorption markers. Our studies support the combination of lenalidomide with agents with known anabolic effect on bone, such as bortezomib, for the treatment of relapsed/refractory myeloma patients with extensive bone disease.




