Long-term follow-up of European APL 2000 trial, evaluating the role of cytarabine combined with ATRA and Daunorubicin in the treatment of nonelderly APL patients
Conflict of interest: Nothing to report.
Abstract
All-trans retinoic acid (ATRA) combined to anthracycline-based chemotherapy is the reference treatment of acute promyelocytic leukemia (APL). Whereas, in high-risk patients, cytarabine (AraC) is often considered useful in combination with anthracycline to prevent relapse, its usefulness in standard-risk APL is uncertain. In APL 2000 trial, patients with standard-risk APL [i.e., with baseline white blood cell (WBC) count <10,000/mm3] were randomized between treatment with ATRA with Daunorubicin (DNR) and AraC (AraC group) and ATRA with DNR but without AraC (no AraC group). All patients subsequently received combined maintenance treatment. The trial had been prematurely terminated due to significantly more relapses in the no AraC group (J Clin Oncol, (24) 2006, 5703–10), but follow-up was still relatively short. With long-term follow-up (median 103 months), the 7-year cumulative incidence of relapses was 28.6% in the no AraC group, compared to 12.9% in the AraC group (P = 0.0065). In standard-risk APL, at least when the anthracycline used is DNR, avoiding AraC may lead to an increased risk of relapse suggesting that the need for AraC is regimen-dependent. Am. J. Hematol. 88:556–559, 2013. © 2013 Wiley Periodicals, Inc.
Introduction
In spite of recent promising results obtained with arsenic trioxide (ATO) in this situation 1, 2 the combination of all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy (CT) remains the reference first-line treatment of acute promyelocytic leukemia (APL), which leads to complete remission in 90–95% and prolonged calorie restriction (CR) in 75–80% of the patients 3. Reducing the amount of CT in APL patients has been investigated in order to limit its toxicity. The PETHEMA and GIMEMA groups, in particular, reported very few relapses and deaths in CR with regimens combining ATRA and intercalating agents (idarubicin and mitoxantrone) without AraC, suggesting that AraC can be omitted from the treatment of APL, at least in standard-risk patients [i.e., with white blood cell (WBC) count <10,000/mm3] 4, 5.
On the other hand, our APL 2000 trial randomizing AraC during induction and consolidation treatment, in patients aged ≤60 years with WBC count <10 G/l, was closed after the first interim analysis, made after a median follow-up of 24 months, due to significantly higher 2 year cumulative incidence of relapse (CIR) in the group without AraC 6, 7.
We present here long-term results of this trial, 7 years after the last patient inclusion, with a median follow-up of 103 months.
Patients and Methods
Study design
From June 2000 to February 2004, patients with newly diagnosed APL from 63 centers in France, Switzerland, Germany, and Belgium were included in APL 2000 trial, approved by local ethical committees (NCT00591526).
Inclusion criteria were (1) morphological diagnosis of APL (which had to be subsequently genetically confirmed), (2) no contraindication to intensive CT, and (3) written informed consent.
Induction and consolidation treatment were stratified on age and initial WBC count. Treatment schedule was previously published 6, 7 and is summarized in Fig. 1. Patients older than 60 years are not reported here. Briefly, patients aged 60 years or less with baseline WBC count <10,000/mm3 were randomized to receive either the reference treatment of our previous APL 93 trial (ATRA 45 mg/m2/d and CT with Daunorubicin (DNR) 60 mg/m2/d × 3 and AraC 200 mg/m2/d × 7 (AraC group) or the same treatment but without AraC (No AraC group) 6. Consolidation courses consisted of two DNR–AraC courses in the AraC group and two DNR courses in no AraC group. Patients aged 60 years or less with baseline high WBC count (>10,000/mm3) were not randomized and received the same treatment as those included in the AraC group, with a higher dose of AraC during the second consolidation course, and addition of CNS prophylaxis (consisting of 5 triple intrathecal injections of MTX 15 mg, AraC 50 mg, and steroids).
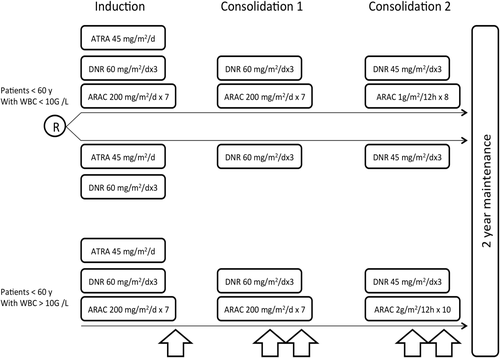
All patients received, after consolidation treatment, maintenance therapy combining continuous low-dose CT (6-mercaptopurine 90 mg/m2/day orally + methotrexate 15 mg/m2/week orally) and intermittent ATRA (ATRA 45 mg/m2/day, 15 days every 3 months), scheduled for 2 years, as this maintenance treatment was shown to significantly reduce the relapse rate in our previous APL 93 trial 8, 9.
Outcome
The main end point was the CIR (including hematological, extramedullary, and molecular relapses). Secondary end points included CR rate, survival, and event-free survival (EFS). EFS was measured from the date of randomization, with CR achievement, treatment failure, death in first CR, and relapse included as events.
Statistical analysis
Statistical analysis was made on a modified intent-to-treat principle, excluding only diagnostic errors and consent withdrawals. Baseline characteristics were compared by Kruskal–Wallis test or Fisher's exact test, respectively. CR rates were compared using Fisher's exact test. Censored end points were estimated by the nonparametric Kaplan–Meier method, and compared by the log-rank test, after checking for proportional hazards 10. In estimating relapses, we took into account for competing risks deaths in first CR using the cumulative incidence curves, and then compared by the Gray test 11, 12. The protocol scheduled three interim analyses, each performed at the nominal value of 0.01 to address an overall type I error rate of 0.05. Upon first interim analysis, made at the reference date of June 1, 2004 on the patients included before February 1, 2004, P-value for testing equality of CIR in randomized groups was below 0.01, leading to stop accrual in the no AraC group. The second-interim analysis, performed at the reference date of May 1, 2005, with a median follow-up of 35 months, was previously published 6.
We present here the third analysis performed at the reference date of January 1, 2011, based on all patients included in the trial before February 1, 2004.
Results
Initial patient characteristics
Overall, 286 patients aged 60 years or less had entered the trial. Diagnosis of APL was genetically confirmed in 272 of them. The remaining 14 patients were excluded as diagnostic error, and two other patients withdrew their consent.
The remaining 270 patients had a median age of 47 years. Seventy-four had high WBC count (>10,000/mm3). The remaining 196 patients were randomized between the AraC (95 patients) and no AraC (101 patients) groups. No significant differences in baseline characteristics between the AraC and no AraC groups were seen 6.
Long-term treatment results
Median follow-up was 103 months [interquartile range (IQR): 94.6–113.3]. In the AraC group, 94 patients (99%) achieved CR compared to 95 (94.1%) CR in the no AraC group (P = 0.12). Failure to reach CR was due to early death in one patient in the AraC arm, and to early death in four patients and resistant leukemia in two patients in the no AraC arm.
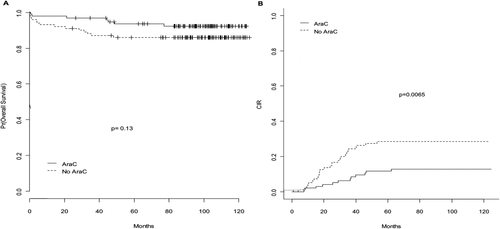
The 7-year CIR was 28.6% in the no AraC group compared to 12.9% in the AraC group (P = 0.0065) and the 7-year EFS was 65.2% in the no AraC group compared to 82.8% in the AraC group (P = 0.0029; Fig. 2). On the other hand, overall survival did not significantly differ between the no AraC group and the AraC group (with 7-year estimate at 86.1% vs. 92.4%; P = 0.13). Of the 29 relapses observed in the no AraC group, 25 were hematological relapses and 4 were molecular relapses, compared to 10 and 2, respectively, for the 12 relapses in the AraC group. Among the molecular relapses, one extra hematological relapse [involving the central nervous system (CNS)] was seen, in the no AraC group. After relapse, the 2-year estimated survival was 81.8% in the no AraC arm and 75.9% in the AraC arm (P = 0.82).
Of note, in younger patients with high-WBC count (who all received AraC), the hematological CR rate was 97.3%. The 7-year CIR, 7-year EFS, and 7-year OS were 7.1%, 82.2%, and 87.6%, respectively, an outcome that appeared slightly better than that of standard-risk patients treated without AraC.
Discussion
The second interim analysis of APL 2000 trial had been made with a median follow-up of 35 months 6, but 11 relapses (i.e., 27% of the observed 41 relapses) occurred in the trial after the first 35 months of follow-up, justifying the present long-term follow-up analysis. We confirmed here, with a median follow-up of 103 months, a significantly higher incidence of relapse in the no AraC group. This follow-up appears sufficient, as very few relapses are seen in APL beyond 7 years 13, and no relapse was seen beyond 7 years in APL 2000 trial. The present long follow-up showed, in particular, that the addition of AraC did not just delay, but that it reduced the incidence of relapses.
The poorer results of the no AraC group were seen in spite of maintenance treatment with continuous 6MP and MTX and intermittent ATRA, a maintenance treatment reducing the relapse risk after ATRA combined to anthracycline-based CTs in our experience and that of several other groups 4, 8. Paradoxically, high-risk patients aged 60 years or less, who received AraC, had a 7-year CIR of only 7.1%, which appeared lower than the 28.6 % observed in the no AraC group, although the latter group included standard-risk APL patients. Also of note, in the no AraC group, was that two patients had resistant leukemia, a distinctly rare event in APL in the ATRA era (found in 1/413 patients in our APL 93 trial 9 and 0/95 patients in the AraC group of APL 2000 trial), possibly suggesting a suboptimal effect of ATRA and 3 days of DNR for induction treatment of APL 14.
Following results of the second-interim analysis, we had compared results of APL 2000 trial (limited to treatment groups allocated to AraC) with those of the Spanish PETHEMA LPA 99 trial (where patients received no AraC) 7. While, in patients with WBC count >10,000/mm3, fewer relapses were seen with APL 2000 trial (suggesting a benefit for AraC in those patients), on the contrary fewer relapses were seen with the PETHEMA trial in standard-risk patients. This advantage of the PETHEMA trial over APL 2000 trial in standard-risk patients was tentatively attributed to the use of idarubicin and mitoxantrone in the former trial, versus DNR in the latter trial or to a difference in the intensity of anthracycline therapy used. Indeed, in APL 2000 patients, a total of 495 mg/m2 of DNR was used. By contrast, standard-risk patients in the PETHEMA study received 80–100 mg/m2 of idarubicin and 50 mg/m2 of mitoxantrone. Using the anthracycline-equivalence algorithm proposed by Sanz et al. 15 where 50 mg DNR is equivalent to 10 mg of idarubicin and 12 mg of mitoxantrone, PETHEMA LPA 99 patients received a DNR-equivalent dose of 608–708 mg/m2, that is, substantially more than the APL 2000 patients. On the other hand, using such a high-cumulative dose of DNR may carry a risk of cardiac toxicity.
We had concluded from the comparative analysis that AraC was probably not required in standard-risk APL, if the anthracycline used was idarubicin (and mitoxantrone) at the cumulative doses reported by Sanz et al. 7. Long-term results of APL 2000 trial presented here confirm that, on the contrary, AraC may not be dispensable in standard-risk APL if DNR is the anthracycline used (at least at the usually recommended cumulative doses, i.e., <500 mg/m2). Therefore, attempts to transpose PETHEMA results by substituting DNR for idarubicin may result in poorer outcome. The same may occur if the prolonged maintenance treatment used in APL 2000 and PETHEMA trials is omitted.
Current efforts to reduce the amount of CT in the first-line treatment of APL should therefore be careful, and possibly be combined with the introduction of ATO, which is highly effective and nonmyelosuppressive in APL 2, 16, 17. Recent results suggest that combinations of arsenic trioxide (ATO) and ATRA may even allow treatment of standard-risk APL without CT [1, 18-20). On the other hand, ATO is currently not approved for the first-line treatment of APL in most countries, and optimal ATRA CT combinations still required in those patients.
Acknowledgments
LA, SC, CC, HD, LD, and PF designed the study. LA, ER, AGB, AP, NV, TL, FH, AV, JFL, BL, SdB, ED, AF, XT, BQ, HD, and PF collected and analyzed the data. LA, SC, and PF drafted the manuscript. BC and CC did MRD monitoring. SC did statistical analysis. All authors reviewed and approved the manuscript.
Funding sources for the manuscript
This study was supported by the programme hospitalier de recherche clinique and the centre hospitalier universitaire (CHU) of Lille-France, the Association pour la Recherche sur le Cancer (ARC) and the Ligue contre le cancer (Comité du Nord).




