Epstein-Barr virus infection manifested by hepatitis, infectious mononucleosis, and acute cerebellar ataxia: A case report and literature review
Funding information: Ditmanson Medical Foundation Chia-Yi Christian Hospital, Grant/Award Number: R102-10
Abstract
We described the case of an adult who presented with acute cerebellar ataxia (ACA) after Epstein-Barr virus (EBV) infection. We also reviewed the literature on the pathogenesis of ACA due to EBV infection using the Pubmed database. A 25-year-old man suffered from fever, sore throat, and poor appetite. Ataxia with nausea and vomiting developed in the following disease course. Laboratory tests showed atypical leukocytosis and hepatitis. The serological test confirmed a recent EBV infection. Analysis of cerebrospinal fluid (CSF) was unremarkable, so was brain magnetic resonance imaging (MRI). He was diagnosed with infectious mononucleosis and ACA. He fully recovered after conservative treatment. Possible pathogenetic mechanisms of EBV-associated central nervous system (CNS) manifestations could be categorized as a direct viral invasion of tissue and a postinfectious immune response. Most patients with ACA after EBV infection could be managed with supportive care. The diagnosis of ACA after EBV infection is typically made based on clinical symptoms and signs along with serological detection of EBV-specific antibodies.
1 INTRODUCTION
Acute cerebellar ataxia (ACA) is a rare manifestation of Epstein-Barr virus (EBV) infection.1 We present the case of a 25-year-old man who was diagnosed with infectious mononucleosis and acute cerebellitis. Conservative treatment led to a complete recovery. Possible pathogenesis of central nervous system (CNS) manifestations after EBV infection could be categorized as a direct viral invasion of tissue and a postinfectious immune response.
2 CASE REPORT
A 25-year-old man diagnosed with chronic kidney disease (stage 2) and hyperlipidemia presented at our outpatient clinic. He reported regular use of atorvastatin. He also reported being a nonsmoker and did not drink. Twelve days before he presented at our outpatient department, he reported experiencing fever (37.8°C) and chills. In the following days, he reported a sore throat and poor appetite. Blood tests at a local clinic a week after symptom onset revealed elevated alanine aminotransferase. Ten days after symptom onset, he developed unsteady gait and dizziness, accompanied by nausea and vomiting. No contact or travel history was reported.
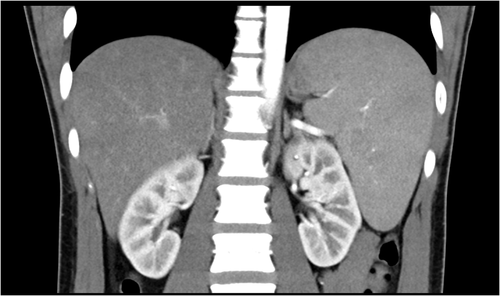
Upon hospital admission, the patient was afebrile. No abnormal findings were identified upon physical examination, except for exudative tonsillitis. Sensory and motor responses were normal. Both finger–nose–finger and heel–knee–shin tests revealed bilateral equivocal dysmetria. Romberg's test was positive. Laboratory tests revealed leukocytosis (white blood cell, 12,470/μl; lymphocyte, 62%) with atypical lymphocytes (7%). C-reactive protein was 0.377 mg/dl. The estimated glomerular filtration rate was 88.5 ml/min/1.73m2. Alanine aminotransferase was 706 U/L, and aspartate aminotransferase was 340 U/L. Alkaline phosphatase (1189 U/L) and γ-Glutamyl transpeptidase (604 U/L) were raised with normal bilirubin levels. Hepatitis A, B, and C serology tests were negative (Table 1). Abdominal computed tomography (CT) revealed splenomegaly (Figure 1). At this stage, infectious mononucleosis was impressed. Test for Cytomegalovirus immunoglobulin M (IgM) and immunoglobulin G (IgG) were negative. Treatment was initiated with empirical antibiotic cefotaxime (2 g, every 8 h).
| Variable | Reference range | On initial presentation | Two weeks after presentation |
|---|---|---|---|
| Hemoglobin (g/dl) | 14–18 | 15.7 | 16.4 |
| White cell count (per μl) | 3500–9900 | 12,470 | 6820 |
| Differential count (%) | |||
| Neutrophils | 45–75 | 25 | 47.4 |
| Lymphocytes | 15–47 | 62 | 38.6 |
| Monocytes | 3–10 | 3 | 10.3 |
| Eosinophils | 0–6 | 0 | 3.1 |
| Basophils | 1 | 0–2 | 0.6 |
| Atypical lymphocyte | <0 | 7 | <0 |
| Platelet count (per μl) | 130,000–400,000 | 190,000 | 215,000 |
| Aspartate aminotransferase (U/L) | 8–38 | 340 | 57 |
| Alanine aminotransferase (U/L) | 4–44 | 706 | 180 |
| Total bilirubin (mg/dl) | 0.2–1.2 | 0.7 | 0.82 |
| Direct bilirubin (mg/dl) | 0–0.4 | 0.26 | NA |
| Alkaline phosphatase (U/L) | 104–338 | 1189 | NA |
| γ-Glutamyl transpeptidase (U/L) | 0–73 | 604 | NA |
| HBsAg | Nonreactive | Nonreactive | NA |
| Anti-HAV IgM | Nonreactive | Nonreactive | NA |
| Anti-HCV | Nonreactive | Nonreactive | NA |
- Abbreviations: HAV, Hepatitis A virus; HBsAg, the surface antigen of the hepatitis B virus; HCV, Hepatitis C virus; IgM, Immunoglobulin M.
No fever was noted during the following days; however, the patient's condition deteriorated 3 days after hospital admission. He was able to sit up but could barely walk. A CNS infection was suspected. Analysis of cerebrospinal fluid (CSF) revealed no infection. Stains and bacterial cultures did not identify any pathogens. No antigens were identified (including Group B Streptococcus, Haemophilus influenzae, Streptococcus pneumoniae, and Cryptococcus). Treatment was modified with guidance from an infectious disease specialist from Cefotaxime to Ceftriaxone (2 g, every 8 h), Vancomycin (500 mg, every 12 h), and Ampicillin (2 g, every 4 h). His IgG index was 0.41, with no oligoclonal bands. Brain CT, contrast-enhanced magnetic resonance imaging (MRI), and brainstem auditory evoked potential were unremarkable.
The human immunodeficiency virus was nonreactive. Both herpes simplex virus type 1 and type 2 were negative. The serological test for Varicella Zoster revealed a past infection. However, EBV viral capsid antigen antibody IgM and IgG were positive. All antibiotics were discontinued on day seven following admission as the patient was afebrile with stable vital signs. A diagnosis of EBV hepatitis accompanied by ACA was made. The patient was treated with supportive care for the remainder of the hospital stay. He did not receive any corticosteroid or anti-viral therapy. Liver function tests gradually improved after the fourth day following hospital admission. After 2 weeks of hospitalization, the patient was discharged with a quad cane to support ambulation. The patient fully recovered 4 to 5 weeks after the onset of illness.
3 DISCUSSION
The pathogenesis of EBV-related ACA is unclear. A literature review was performed using Pubmed database (Table 2). Several hypotheses have been suggested, including direct viral invasion of tissue and a postinfectious immune response. The rise of antibodies against EBV has been detected in the CSF of young adults who suffered from ataxia.2 Positive polymerase chain reaction (PCR) for EBV deoxyribonucleic acid results from CSF has been suggested to be indicative of direct viral invasion of the cerebellum.3 Studies have also reported that neurological manifestations may precede systemic features of EBV infection, which may also indicate a direct viral effect on neurons and supporting cells.1 However, in most cases, no pathogen isolated from the CSF. When considering the postinfectious immune response, secretion of anti-neuronal antibodies, or a cross-reaction between anti-EBV antibodies and neuronal antigens are possible mechanisms. Serum IgM and IgG autoantibodies against cerebellar Purkinje cells and other neural cells have been found in a patient with recent EBV infection.4 Another study reported that the titer of antibody against triosephosphate isomerase (TPI) increased before ACA developed in 8 out of 23 patients with acute EBV infection. Cerebellar tissue possesses the highest TPI antigenicity in humans.5 Intravenous immunoglobulin (IVIg) and plasmapheresis have been reported to be effective treatments, which implies that the immune response is responsible for ACA.6, 7 Previously published studies have reported that most patients have recovered within months without sequelae.1 For those with a prolonged disease course or severe symptoms, acyclovir and corticosteroids may be beneficial treatments.3
| Title | CSF PCR | CSF EBV Ab | Image finding | Remark | References |
|---|---|---|---|---|---|
| Direct viral invasion | |||||
| Acute cerebellar ataxia due to EBV | Negative | Negative | Normal MRI | Neurological symptoms preceded the systemic features | McCarthy et al1 |
| Infectious mononucleosis presenting as acute cerebellar syndrome | N/R | Positive | N/R | Lascelles et al2 | |
| Immune response (immunotoxicity, autoimmune, postinfectious demyelination, para infectious molecular mimicry) | |||||
| Autoantibodies in postinfectious acute cerebellar ataxia | N/R | N/R | N/R | Anti-TPI IgM antibody in serum | Uchibori et al5 |
| EBV infections of the central nervous system | N/R | Negative | N/R | Response to corticosteroid treatment | Fujimoto et al3 |
| Brain SPECT imaging and treatment with IVIg in acute postinfectious cerebellar ataxia: case report | N/R | N/R | Normal CT and MRI Abnormal SPECT |
IVIg shortened the course of the disease | Daaboul et al6 |
| Antineuronal antibodies in acute cerebellar ataxia following EBV infection | N/R | N/R | Normal MRI | Serum antibodies reacted with Purkinje cells | Ito et al4 |
| Acute cerebellar ataxia and hearing loss as initial symptoms of infectious mononucleosis | N/R | Negative | Normal CT | Erzurum et al9 | |
- Note: The cells colored in black means that it was not done or specifically mentioned.
- Abbreviations: Ab, antibodies; CSF, cerebrospinal fluid; CT, computed tomography; EBV, Epstein-Barr virus; IgM, immunoglobulin M; IVIg, intravenous immunoglobulin; MRI, magnetic resonance imaging; N/R, not reported; PCR, polymerase chain reaction; SPECT, single photon emission computed tomography; TPI, triosephosphate isomerase.
In patients with EBV encephalomyelitis, white matter involvement per MRI suggests a postviral response, while gray matter involvement implies direct viral infection.8 However, in most cases with EBV-associated cerebellitis, brain CT and contrast-enhanced MRI were normal. The immune response could be nonsignificant in the absence of an oligoclonal band in CSF and based on evidence from a T2-weighted MRI.6, 9 A uniform increase in cerebellar perfusion was identified on a brain single-photon emission computerized tomography (SPECT) study, suggesting that neurological dysfunction may correlate with vasodilatation.6
EBV infection is a primary cause of infectious mononucleosis. The most common manifestations in digestive system are acute hepatitis and splenic rupture. The hepatic change is usually transient and self-limiting; however, decompensated liver failure was ever reported. Splenic rupture is probably the most dreadful complication of infectious mononucleosis. It should be suspected in any infectious mononucleosis (IM) patients presenting with acute abdominal pain or chest pain. Antiviral medications have been used to treat IM, but the use of antivirals for IM is controversial. The effectiveness of antiviral agents (acyclovir, valomaciclovir, and valacyclovir) in acute IM is uncertain.10, 11
This article's limitation lacks sequential serology data of EB virus, nor the measurement of spleen size and confirmation of anti-hepatitis C virus (HCV) serology appearance. Due to the limitations of our laboratory, we could not directly confirm EBV in the CSF, using either PCR or antibodies. We favored direct viral invasion or nonsignificant immune response to be the possible pathogenesis in our patient. The oligoclonal band was negative. MRI also revealed no remarkable findings. Furthermore, the duration of neurological symptoms was relatively short and self-limited.
In conclusion, patients with IM due to EBV infection usually require symptomatic treatment. In more complicated cases such as acute hepatitis and/or ACA, the evidence for specific treatment is controversial. A diagnosis is typically made based on clinical symptoms and signs along with serological detection of EBV-specific antibodies. Brain images provide little information, but SPECT could be a valuable tool. The prognosis of patients with ACA from EBV is generally good. However, for those patients with prolonged or severe symptoms, acyclovir, corticosteroids, IVIg, and plasmapheresis should be considered.
ACKNOWLEDGMENTS
This study was supported in part by the Ditmanson Medical Foundation Chia-Yi Christian Hospital (R102-10).
CONFLICT OF INTEREST
The authors declare no conflict of interest.




