A Vanillin-Derived Inhibitor of Aggregates via Targeting Intrinsically Disordered Regions of Phytoviral Nucleocapsid Protein
Funding: The financial support from the National Natural Science Foundation of China (Nos. 32330087 and 32302388) and the Key Technologies R&D Program of Guizhou Province in China (No. 2017-5788-1) and the Scientific Research Innovation Team of Guizhou University (No. 202403).
ABSTRACT
Phase separation (PS) plays a fundamental role in organizing aggregates during the viral lifecycle, providing significant opportunities for in viral disease treatment by inhibiting PS. Intrinsically disordered regions (IDRs) have been extensively studied and found to be critical for PS. However, the discovery of small molecules that target residues within IDRs remains underexplored, particularly in the field of pesticides. Herein, we report a novel phytovirucide compound 29, which was screened from a series of vanillin derivatives designed with sulfonylpiperazine motifs. The inactivation efficacy of compound 29 against tomato spotted wilt virus (TSWV) was significantly superior to that of the control agents vanisulfane and ribavirin. Mechanistically, compound 29 binds to the TSWV nucleocapsid protein (NP) at residues Lys68 (K68), Thr92 (T92), and Arg94 (R94), with T92 and R94 located in the IDRs of NP. Mutations at these sites impair the ability to form aggregates. Furthermore, a host factor, GTP (Guanosine Triphosphate)-binding nuclear protein Ran-like (Niben101scf08341g01001, NbRANL), which interacts with NP and promotes its aggregation, was identified. Compound 29 also suppresses the expression of NbRANL, resulting in the dual inhibition of ribonucleoprotein complexes (RNPs) formation. This unique mechanism of action provides insights into IDRs-based virucide discovery.
1 Introduction
The tomato spotted wilt virus (TSWV) is the only member of the genus Orthotospovirus in the Tospoviridae family of Bunyavirales that infects plants, causing annual losses estimated at up to one billion dollars, second only to the tobacco mosaic virus [1-5]. TSWV is notable for its broad and wide host range, infecting more than 1000 species of vegetables, cash crops, and ornamental plants [2]. The primary vector responsible for transmission is the western flower thrip (Frankliniella occidentalis) [6, 7]. These small insects acquire the virus while feeding on infected plants and can spread it throughout their life cycle [8]. The agrochemical management of phytoviral diseases presents several challenges. First, thrip populations are prone to rapidly developing resistance to commonly used insecticides, diminishing the long-term efficacy of chemical control. This necessitates either increased dosages or the use of alternative insecticides, both of which can be more costly and pose environmental risks to nontarget organisms [9, 10]. Moreover, effective virucides remain scarce, with most current efforts focusing on managing thrip populations to curb virus spread [11, 12]. Therefore, the development of potent anti-TSWV agents is a crucial step in safeguarding food security.
Phase separation (PS) has emerged as an important mechanism in the life cycles of various viruses, although it is not universally essential [13-16]. In many cases, PS facilitates the formation of membraneless organelles or biomolecular aggregates within host cells, which can serve as hubs for viral replication, transcription, and assembly [17-20]. Recently, PS-driven aggregate formation has been shown to play a crucial role in infection by many plant viruses, including tomato yellow mottle associated virus (TYMaV), carnation Italian ringspot virus (CIRV), tomato bushy stunt virus (TBSV), and barley yellow striate mosaic virus (BYSMV) [21-23]. The replication critically depends on ribonucleoprotein complexes (RNPs), which consist of viral RNA encapsulated by the nucleocapsid protein (NP) [24, 25]. The RNPs of TSWV can be considered a type of aggregate in the context of PS, and their formation is vital for the viral lifecycle, including their ability to replicate within host cells and spread infection. Therefore, disrupting the formation or function of RNPs can be a powerful strategy for inhibiting TSWV replication and controlling the disease. Given that RNPs formation likely involves the PS of TSWV NP, the specific design of small-molecule PS inhibitors is expected to treat plant diseases caused by TSWV.
In recent years, numerous PS modulators have been developed (Figure 1a) [26]. These molecules do not universally inhibit PS; for instance, treatment with CLV218 and crystal violet has been shown to enhance PS, leading to larger condensates in their respective contexts [27, 28]. Conversely, 4,4′-Dianilino-1,1′-binaphthyl-5,5′-disulfonic acid dipotassium salt (bis-ANS), at high concentrations, has been found to inhibit droplet formation [29]. The irregular results among the existing PS regulators present challenges for the development of PS-based inhibitors. We are encouraged by our long-term research on phytovirucide discovery to focus on intrinsically disordered regions (IDRs) [30-33]. IDRs have been considered challenging targets for drug development because they lack stable structures, making it difficult to design small molecules that can specifically bind to them. This has led to a reduced focus on IDRs in the context of traditional drug development [34]. Nevertheless, several studies have shown that IDRs are crucially involved in the initiation of PS [35, 36]. Saito et al. were the first to demonstrate that acetylation/deacetylation modifications of IDR can regulate the PS and assembly of stress granules [37]. In addition, studies involving mutations in certain residues within IDRs have indicated the inhibition of PS [38]. Unfortunately, to the best of our knowledge, there is a lack of empirical evidence showing that small molecules can directly target the IDRs of PS proteins to disrupt PS (Figure 1b).
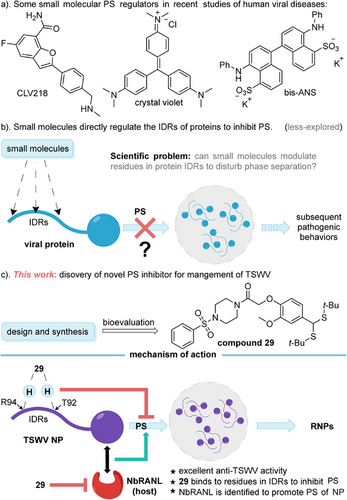
Herein, we present a newly discovered phytovirucide, 29, which was screened from a group of vanillin derivatives specifically constructed with sulfonylpiperazine moieties. Compound 29 (EC50 = 154.8 µg/mL) demonstrates significantly greater efficacy in inactivating TSWV compared to the control agents vanisulfane (EC50 = 281.8 µg/mL) and ribavirin (EC50 = 701.9 µg/mL). Mechanistically, molecule 29 bound to TSWV NP at specific amino acid residues, namely, Lys 68 (K68), Thr 92 (T92), and Arg 94 (R94), with T92 and R94 specifically located within the IDRs of the NP. Mutations at these sites abnormalize the ability of NPs to induce PS, resulting in a significant reduction in the number of droplets. Notably, a host pathogenic factor, the NbRANL, was first identified using immunoprecipitation (IP) mass spectrum, yeast two-hybrid (Y2H), bimolecular fluorescence complementation (BiFC), and luciferase complementation assay (LCA). The interaction between NbRANL and NP positively enhanced the generation of droplets, while NbRANL gene knockout does not affect the growth of test plants. Compound 29 also reduced the expression of NbRANL, which simultaneously suppressed RNP production and substantially weakened the pathogenicity of TSWV (Figure 1c). This study reveals a unique mode of action for a new PS inhibitor, providing valuable insights for the future development of PS-based virucides that specifically target IDRs.
2 Results and Discussion
2.1 The Synthesis of Target Compounds and Anti-TSWV Activity
In recent years, vanillin, a natural product, has garnered attention for its potential applications beyond its traditional uses in medicine and as a food additive, particularly in the development of molecules with antiplant virus activity [30-33]. These compounds have been shown to either directly target the functional coat proteins of plant viruses, thereby inactivating their pathogenicity, or act as immune activators, enhancing the expression of plant-related disease resistance pathways. To identify small-molecule inhibitors that can bind to TSWV NP, a series of vanillin-based analogs (1–33) were designed by structurally incorporating the antiviral moieties sulfonylpiperazine and dithioacetal, based on prior studies [39, 40]. The synthesis of these target molecules typically involves five chemical reactions: common nucleophilic substitution, addition–elimination, deprotection of N-protecting groups, and thioacetalization. All reagents required for these reactions are commercially available, and the overall process can be conducted under mild conditions, without the need for excessive temperature or high pressure (Schemes S1 and S2). The bioactivity of the synthesized compounds 1–33 against TSWV was evaluated using the half-leaf method [41-43]. As shown in Table S1, these compounds exhibited varying degrees of curative, protective, and inactivating effects against TSWV. Significantly, compounds 8, 13, 15, 16, and 29 exhibited protective activity values of 51.2%, 51.1%, 51.2%, 52.4%, and 55.5%, respectively, which exceeded those of the control agents vanisulfane (44.2%) [44] and ribavirin (40.9%). The enhanced protective action of these compounds may be attributed to the stimulation of associated plant immunological pathways, including abscisic acid signaling, salicylic acid signaling, and oxidative phosphorylation, or their capacity to decrease the expression of certain host pathogenic factors [45, 46]. It is worth mentioning that the inactivation activity of drugs provides a more direct reflection of their interaction with viruses, aligning with our purpose. To accurately assess the inactivation effect of small molecules, we calculated the corresponding concentration for 50% of the maximal effect (EC50) based on the percentages of inactivation activities. As indicated in Table 1, several of title compounds had eminent inactivating activity against TSWV, the EC50 of compounds 13, 20, and 29 were 154.0, 121.6, and 154.8 µg/mL, respectively, which stood in contrast to vanisulfane (281.8 µg/mL) and ribavirin (701.9 µg/mL). A comprehensive analysis of the antiviral performance of all the compounds revealed that derivative 29 exhibited versatile properties in treatment, protection, and inactivation, making it a promising candidate for subsequent mechanism of action studies.
| Compound | Inactivating activity (µg/mL)a | Compound | Inactivating activity (µg/mL)a |
|---|---|---|---|
| 1 | 233.7 ± 13.5 | 18 | 413.9 ± 9.3 |
| 2 | 1277.2 ± 9.5 | 19 | 1284.8 ± 12.7 |
| 3 | 824.1 ± 59 | 20 | 121.6 ± 6.1 |
| 4 | 186.9 ± 5.1 | 21 | 429.9 ± 11.2 |
| 5 | 240.7 ± 3.8 | 22 | 254.6 ± 11.6 |
| 6 | 409.0 ± 14.8 | 23 | 1186.9 ± 6.5 |
| 7 | 460.8 ± 2.5 | 24 | 163.9 ± 6.2 |
| 8 | 252.0 ± 13.7 | 25 | 828.9 ± 14.5 |
| 9 | 1436.8 ± 8.4 | 26 | 210.9 ± 7.5 |
| 10 | 223.9 ± 5.7 | 27 | 407.5 ± 2.9 |
| 11 | 814.0 ± 14.7 | 28 | 452.8 ± 2.9 |
| 12 | 890.6 ± 12.9 | 29 | 154.8 ± 4.4 |
| 13 | 154.0 ± 4.0 | 30 | 415.7 ± 13.6 |
| 14 | 436.2 ± 13.5 | 31 | 684.4 ± 8.9 |
| 15 | 275.7 ± 9.6 | 32 | 171.5 ± 13.1 |
| 16 | 832.8 ± 5.7 | 33 | 874.4 ± 10.3 |
| 17 | 831.2 ± 14.1 | Ribavirinb | 701.9 ± 8.0 |
| Vanisulfanec | 281.8 ± 9.9 |
- Note: aAverage of three replicates. b,cThe ribavirin and vanisulfane were used for comparison of activity.
2.2 The Binding Sites of Compound 29 With TSWV NP
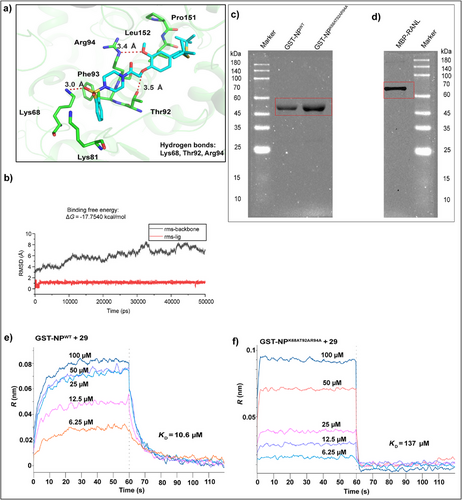
TSWV NP is a major structural component that assembles and converts genomic RNA into RNPs, which serve as templates for viral genome replication and gene transcription. TSWV NP also interact with NSm intercellular motor proteins, which play a critical role in the cell-to-cell spread of viral genomic RNA [24, 25, 47–50]. To investigate the anti-TSWV mechanism of compound 29, we targeted the NP as a potential binding partner [40]. First, molecular docking between compound 29 and TSWV NP (Code: 5ip1) [50] was performed using the AutoDock 4.2 software (Figure 2a). The root mean square deviation (RMSD) was calculated to ensure the complex stability. The binding free energy was determined through molecular mechanics/Poisson Boltzmann (or generalized boron) surface area calculations using 100 snapshots from the last 1 ns of the molecular dynamics simulation. The ΔG for compound 29 was −17.7540 kcal/mol (Figure 2b), indicating a strong binding affinity for TSWV NP. Compound 29 formed hydrogen bonds with amino acid residues K68, T92, and R94 of TSWV NP, along with hydrophobic interactions involving Lys81, Leu152, Pro151, and Phe93. The subsequent step was to assess the binding constant of 29 with either wild-type NP or the NPK68AT92AR94A mutant. Plasmids for GST-NPWT and GST-NPK68AT92AR94A were constructed and purified by prokaryotic induction, and their identities were confirmed by sequencing and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Figure 2c). The molecular weight range of GST-NPWT and GST-NPK68AT92AR94A was 45–60 kDa, which was consistent with the expected size of ∼55 kDa. Bio-Layer Interferometry (BLI) was then employed to determine the binding affinities, revealing dissociation constants (KD) of 10.6 µM and 137 µM for the interaction of compound 29 with GST-NPWT and GST-NPK68AT92AR94A, respectively. These results suggested that K68, T92, and R94 are likely key amino acid sites involved in the binding of compound 29 to NP (Figure 2e,f) [39].
2.3 The Binding Sites of Compound 29 With TSWV NP Affect the Proliferation of TSWV
After preliminarily identifying the pivotal residues targeted by compound 29, we explored the functions of these sites in the infectivity in vivo. To verify the effects of mutations at amino acid sites K68, T92, and R94 of NP on TSWV infection in Nicotiana benthamiana (N. benthamiana), we constructed a series of plasmids (SR(+)eGFPK68A, SR(+)eGFPT92A, SR(+)eGFPR94A, SR(+)eGFPK68AT92AR94A, SR(+)eGFPK68GT92GR94G) using SR(+)eGFP as a template [51]. All plasmids were confirmed by sequencing, followed by infection of N. benthamiana via Agrobacterium-mediated transformation. Real-time quantitative polymerase chain reaction (RT-qPCR) and western blotting were performed to quantify NP gene and protein expression in systemically infected leaves at 14 and 17 days postinfection. Compared to the wild-type virus TSWVWT, infections with mutant strains TSWVK68A, TSWVT92A, TSWVR94A, and TSWVK68AT92AR94A showed delayed and attenuated symptoms. The RT-qPCR results (Figure 3b) indicated that the relative expression level of NP gene was significantly lower in mutant infections (TSWVK68A, TSWVT92A, TSWVR94A, and TSWVK68AT92AR94A) than in TSWVWT. Western blotting (Figure 3c,d) further confirmed that the NP levels of TSWVK68A, TSWVT92A, TSWVR94A, and TSWVK68AT92AR94A were markedly reduced compared to TSWVWT in the systemic leaves. Based on these data, we concluded that alanine mutations at K68, T92, and R94 have a significant negative impact on the proliferation of TSWV in N. benthamiana. After demonstrating that the simultaneous mutation of K68, T92, and R94 to alanine had the most pronounced impact on TSWV infection, we proceeded to determine whether other amino acid substitutions at these positions also affected TSWV infectivity. Glycine substitutions were introduced at specific sites (K68G, T92G, and R94G). Additionally, we evaluated the effect of compound 29 (TSWVWT + 29), applied 24 h postinfection with TSWVWT. Based on the western blot (WB) analysis (Figure 3e,f), it was observed that the green fluorescent protein (GFP) levels in leaves infected with TSWVK68GT92GR94G and TSWVWT + 29 were lower at 3, 5, 7, and 9 days postinfection compared to TSWVWT. Notably, GFP protein levels in TSWVK68AT92AR94A-infected leaves were significantly lower than in TSWVWT-infected leaves. In addition, WB analysis of NP levels in systemically infected leaves at 14 and 17 days (Figure 3g,h) confirmed that the NP content in TSWVK68GT92GR94G and TSWVK68AT92AR94A was substantially reduced compared to that in TSWVWT, with TSWVK68AT92AR94A showing the most pronounced effect. These findings indicate that mutations at K68, T92, and R94 of TSWV NP impair the replication and transcription of the virus, with alanine substitutions having a stronger effect than glycine substitutions, ultimately reducing TSWV proliferation in N. benthamiana.

2.4 TSWV NP Interacts With the Host Protein NbRANL
2.4.1 The Interaction Between NbRANL and NP in N. benthamiana Was Screened by CO-IP Mass Spectrometry
Previous studies have established a strong link between TSWV infection and host proteins, with TSWV NP positively regulating infection by interacting with Bobber1 (NbBobber1), a small heat shock protein in N. benthamiana. Additionally, the interaction between NP and the B3 domain protein (NbB3) has been shown to negatively affect TSWV infection [52]. However, the identification of other host proteins that interact with TSWV NP remains an area of interest. Understanding these interactions is crucial for gaining deeper insights into host–virus dynamics, which could form the basis for developing targeted pest management strategies. To explore potential host proteins interacting with TSWV NP, we employed IP coupled with mass spectrometry. WB analysis of the immunoprecipitate (Figure S1a) revealed specific bands in the IP group that were absent in the input group. Silver staining further highlighted distinct bands in the IP group (Figure S1b). Mass spectrometry analysis identified 1144 proteins in N. benthamiana infected with pCambia2300-Flag-NP (Flag-NP), excluding the background IgG group. Of these, 456 proteins were uniquely identified in the IP group (Figure S1c and Excel S1).
2.4.2 Y2H, BiFC, and LCA Verify the Interaction of NP With NbRANL
Among the identified proteins, NbRANL was identified as a potential interacting partner of TSWV NP. Ran is a small soluble GTP-binding protein essential for nuclear transport, and a homologous comparison suggested 98.64% similarity between NbRANL and RAN2 (P38547.1.A). Previous studies have shown that Ran1 overexpression in wheat and rice alters mitotic processes and auxin sensitivity [53, 54]. Likewise, overexpression of OsRan2 in tobacco epidermal cells interferes with nuclear import of maize leaf color transcription factors. Silencing of Ran in rice plants, mediated by RNA interference, results in male sterility and pleiotropy [55]. Thus, to verify the interaction between TSWV NP and NbRANL, Y2H, BiFC, and LCA experiments were used to verify the interaction between NP and NbRANL proteins. The Y2H results (Figure 4a) showed that all treatment groups grew on yeast dioxy (SD-Trp/Leu) plates. Only the combination of pGADT7-NP (AD-NP) + BD-RANL and AD-NPK68AT92AR94A + BD-RANL in the experimental group, as well as AD-NP + pGBKT7-N (BD-NP) and pGADT7-T (AD-T) + pGBKT7-53 (BD-53) in the positive control group, was grow on SD-Trp/Leu/His and SD-Trp/Leu/His/Ade plates containing 3 mM 3-amino-1,2,4-triazole (3-AT). Conversely, no growth was observed in the negative control group, confirming the interactions of TSWV NP and TSWV NPK68AT92AR94A with NbRANL. The BiFC assay (Figure 4b) revealed GFP fluorescence in the combinations nYFP-NP + cYFP-NP, nYFP-NP + cYFP-RANL, and nYFP-NPK68AT92AR94A + cYFP-RANL. However, no GFP fluorescence was detected in the negative control group, providing further evidence of this interaction. Similarly, LCA verification results (Figure 4c) indicated the presence of fluorescence in both nLUC-NP + cLUC-RANL and nLUC-NPK68AT92AR94A + cLUC-RANL samples, whereas no fluorescence was observed in the negative controls. In summary, the findings from all three experimental approaches prove that both TSWV NP and the TSWV NPK68AT92AR94A mutant interact with NbRANL.
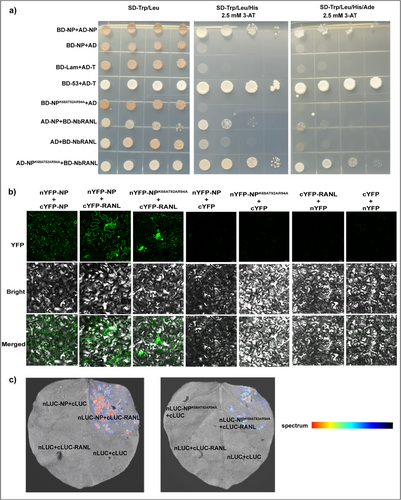
2.4.3 NP Binds to NbRANL
To confirm the binding interaction between TSWV NP, its mutant TSWV NPK68AT92AR94A, and NbRANL, we acquired the crystal structure of NbRANL (Code: AF-P38547-F1) from the PDB database via a homology comparison. Docking was performed using GRAMM (http://gramm.compbio.ku.edu/). The molecular docking results (Figure S4a) revealed that the interaction surface area between TSWV NP and NbRANL is 1840.5 Å2, with a free energy of −6.7 kcal/mol. The interface exhibited 10 potential hydrogen bonds and three potential salt bridges. Although the key interaction regions of TSWV NP were located at residues 20–31, 81–94, 160–173, 180–187, 189–194, 196–204, while those of NbRANL were concentrated at residues 57–59, 142–150, 156–180, 187–212. But strong hydrogen bond interactions were identified between CYS194, THR195, LYS198, ASP171, LYS182, and MSE204 of TSWV NP, and ALA175, TYR150, GLU161, LYS170, ARG143, and ASN58 of NbRANL, with bond lengths of 2.81 Å, 3.35 Å, 2.36 Å, 2.98 Å, 2.25 Å, and 3.15 Å, respectively. In addition, LYS198 and ASP171 formed salt bridges with GLU161 and LYS170, respectively. K68, T92, and R94 of TSWV NP are not included in the amino acid residues that form strong hydrogen bond interactions and salt bridges, so mutations at these three amino acid sites may not affect the interaction of TSWV NP and NPK68AT92AR94A with NbRANL. To evaluate the binding affinity between TSWV NP and NbRANL, we expressed MBP (Maltose-binding protein)-tagged NbRANL in prokaryotic cells (Figure S1b) and used BLI to measure the affinity. The KD value of TSWV NP with NbRANL was determined to be 0.21 nM (Figure S4b), while the KD result for TSWV NPK68AT92AR94A with MBP-NbRANL was 0.45 nM (Figure S4c). These results suggest that both TSWV NP and its mutant, TSWV NPK68AT92AR94A exhibit a strong binding affinity for NbRANL.
2.5 NbRANL Positively Regulates the Infection of TSWV
2.5.1 Transcriptomics Verified the Change of NbRANL Content in TSWV Infection
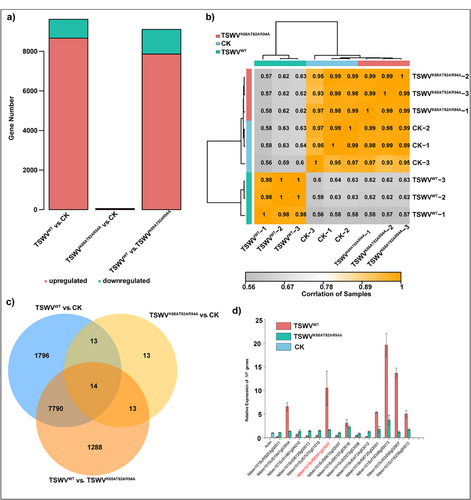
Based on our understanding of the interaction between TSWV NP and NbRANL, we investigated the potential effect of NbRANL on TSWV infection. To assess this, we performed a transcriptomic analysis of systemic leaves at 14 days postinfection. The three treatment groups were healthy N. benthamiana (CK), TSWVWT, and TSWVK68AT92AR94A. A total of 9613 differentially expressed genes (DEGs) were identified in the TSWVWT versus CK comparison (Excel S2), with 8663 upregulated and 950 downregulated genes. In the TSWVWT versus TSWVK68AT92AR94A group, 9105 DEGs were identified, including 7858 upregulated and 1247 downregulated genes (Excel S3). When comparing TSWVK68AT92AR94A and CK (Excel S4), only 53 DEGs were identified, comprising 42 upregulated and 11 downregulated genes. Downregulated and upregulated gene expression patterns, sample correlations, and Venn diagrams are shown in Figure 5a–c, respectively. A combined analysis of the DEGs across the three groups revealed notable variations between TSWVWT, TSWVK68AT92AR94A, and CK, whereas no significant differences were observed between TSWVK68AT92AR94A and CK, suggesting that TSWVK68AT92AR94A had disease susceptibility similar to that of the healthy group. To validate the transcriptomic data, we randomly selected 14 DEGs from the three comparison groups for RT-qPCR analysis. The results (Figure 5d) showed that the expression patterns of these genes were consistent with the transcriptomic data. Notably, the NbRANL gene was upregulated in TSWVWT-infected N. benthamiana, indicating that NbRANL modulated the susceptibility to TSWV infection and acted as a susceptibility-related host factor.
2.5.2 Effects of Overexpression and Gene knockout of NbRANL on TSWV Infection
To explore the role of NbRANL in TSWV infection, we constructed the plasmid p2300-Flag-RANL (Flag-RANL) for transient overexpression and NbRANL gene was knocked out by CRISPR-Cas9 and stable genetic transgenic plants were obtained. NbRANL was transiently expressed in N. benthamiana and subsequently infected with TSWV agrobacterium. The experimental groups included FLAG-RANL + TSWVWT and FLAG-RANL + TSWVK68AT92AR94A, whereas the negative control groups included p2300 + TSWVWT and p2300 + TSWVK68AT92AR94A. Sixty hours postinfection, WB analysis was performed on the infected leaves. Hybridization results with the Flag mouse monoclonal antibody (Figure S3a) confirmed the successful transient expression of Flag-RANL. Furthermore, GFP antibody detection of the enhanced green fluorescent protein (eGFP) reporter gene in the SR(+)eGFP plants indicated that transient NbRANL expression in N. benthamiana promotes TSWV infection (Figure S3b). Next, the knockout of NbRANL gene was confirmed by sequencing in the NbRANL knockout transgenic plants, NbRANL gene knockout did not affect the normal growth of N. benthamiana (Figure S3c), and the sequencing result of knockout was in Figure S3d. Ten days after infected by infectious cloning TSWVWT, WB were used for detect the content of NP (Figure S3f). This finding suggests that knockout the NbRANL gene reduces TSWV infection symptoms (Figure S3e), indicating that NbRANL positively regulates TSWV infections.
2.6 The Compound 29 Inhibits the Expression of NbRANL
To investigate the relationship between NbRANL and 29, a molecular docking analysis was performed, which showed that NbRANL could strongly bind to 29. Hydrogen bonds were formed between 29 and amino acids Asn12, Phe93, Ile152 Phe160 of NbRANL (Figure S4a), with a binding free energy of −85.6851 kcal/mol (Figure S4b). The BLI analysis revealed a binding constant of 11.4 µM 29 and NbRANL (Figure S4c). RT-qPCR results further indicated that 29 inhibits the expression of NbRANL (Figure S4d). These findings demonstrate that compound 29 binds to the NbRANL protein and suppresses NbRANL gene expression.
2.7 The Binding of Compound 29 to the IDRs of NP Affects the Formation of Aggregates
Thus far, we have confirmed that molecule 29 targets TSWV NP by acting on residues K68, T92, and R94, effectively inactivating its pathogenicity. Additionally, we identified NbRANL as a potential host factor interacting with NP in plants. However, the precise mechanism by which molecule 29 or the mutation of these three residues inhibits viral pathogenicity remains unclear. In particular, the pathway through which NbRANL promotes viral infection requires further investigation. PS has been reported to be closely associated with the onset of various diseases [13-20], and there is growing evidence linking PS to plant viruses. For example, membraneless inclusion bodies (IBs) induced by TYMaV are biomolecular aggregates produced via PS [21]. Additionally, p33 of TBSV and p36 of CIRV have been shown to form droplets driven by IDRs in vitro [22]. Similarly, the phosphoprotein BYSMV drives biomolecular condensation via PS to promote viral replication [23]. TSWV NP can form particles that move along the actin/endoplasmic reticulum network [49]. Processing bodies (PBs), which result from biomolecular aggregation, include core proteins that can undergo PS in vitro, indicating that nucleation may be involved in PB biogenesis in vivo. DECAPPING PROTEIN 5 (DCP5) is a component of PB [56-58]. In order to explore the PS potential of NP, we conducted subcellular colocalization of DCP5 and NP in N. benthamiana. The results (Figure S5a) showed that NP particles colocalized with DCP5, suggesting that NP and its particles may share similar biophysical properties with PBs. This implies that NP may also have the potential for PS, allowing it to spontaneously aggregate into granular structures under specific cellular conditions. Abundant evidence supports the crucial role of IDRs in driving PS, particularly in the formation of membraneless organelles. These regions lack a fixed three-dimensional structure that provides flexibility and unique biophysical properties conducive to PS [34-36]. Unfortunately, studies involving small molecules that act directly on the IDRs of proteins to regulate PS are lacking. Therefore, we predicted the IDRs of the NP using PONDR VL-XT (Figure 6a). Unexpectedly, we found that T92 and R94 are located in the IDRs, which encouraged us to further investigate the relationship between the PS of TSWV NP and its pathogenicity. We constructed plasmid p2300-NPK68AT92AR94A-YFP (NPK68AT92AR94A-YFP) to compare the distribution of p2300-NP-YFP (NP-YFP) and NPK68AT92AR94A-YFP in N. benthamiana following instantaneous expression. After transient expression of NP-YFP in N. benthamiana for 60 h, confocal microscopy revealed that the NP underwent PS and condensation (Figure S5b). Treatment with compound 29 reduced NP aggregation and almost no aggregation was observed in the mutant NPK68AT92AR94A-YFP. Fission (Figure 6b and Video S1), fusion (Figure 6c and Video S1), fluorescence recovery after photobleaching (FRAP) experiments (Figure 6e, and Video S2), and quantification of NP-YFP condensates by FRAP (Figure 6d) were further used to demonstrate the PS of NP. The fluorescence intensity was 100%, and the LSM900 confocal bleaching function was adopted. In order to further confirm the formation of NP condensates, N. benthamiana was injected with 10% 1,6-hexadiol and 10 µM thioflavin T (ThT) for 48 h after instantaneous expression of NP-YFP, and was observed by confocal 30 min later. Treatment with 1,6-hexanediol was found to reduce the number of condensates, but did not completely disappear (Figure 6g). After ThT treatment, it was found that some condensates could be labeled by ThT, while some could not (Figure 6h). The results of 1,6-hexanediol and ThT indicate that the amorphous and amyloid may coexist in NP aggregates.
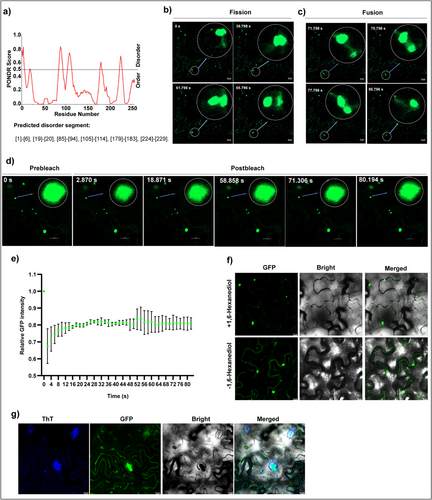
2.8 NbRANL Promotes the Formation of NP Aggregates
NbRANL has been previously identified as a host factor that interacts with TSWV NP, although its role in TSWV infection remains unknown. To investigate the relationship between NbRANL and NP PS, we constructed plasmid p2300-RANL-YFP (RANL-YFP). The results (Figure S6) demonstrated that overexpression of the NbRANL protein promoted the aggregation of NP-YFP, but failed to enhance the condensation of the mutant NPK68AT92AR94A-YFP. Consequently, we propose that NbRANL enhances TSWV pathogenicity by promoting NP aggregation in the host. Although the NbRANL NPK68AT92AR94A protein can still interact with NPK68AT92AR94A, it does not promote aggregation, leading us to conclude that drug 29 or the mutations at K68, T92, and R94 may reduce TSWV proliferation by inhibiting the PS of NP in the host. In contrast, 29 can also decrease the expression of NbRANL which positively promotes the PS of NP, thereby jointly achieving the weakening viral pathogenicity (Figure 7).
3 Conclusion
PS plays a crucial role in organizing viral aggregates during the viral lifecycle, unlocking a variety of pathogenic downstream behaviors. Ongoing research in this field has identified compound 29 as a promising and straightforward candidate to inhibit the PS of TSWV NP. This study is also the first to identify the host pathogenic factor NbRANL, which promotes the formation of NP aggregates. The underlying inhibitory mechanism was uncovered: compound 29 binds to the amino acid residues K68, T92, and R94 of TSWV NP, with disorder prediction indicating that T92 and R94 are located within the IDR of NP. Mutations at K68, T92, and R94 significantly reduce the pathogenicity of TSWV, and these mutations also prevent NP from forming aggregates. Notably, our study also identified NbRANL, a host factor that interacts with NP, capable of interacting with both wild-type NP (NPWT) and the mutant NPK68A/T92A/R94A. NbRANL promotes the formation of NP aggregates but fails to promote aggregates of the NPK68A/T92A/R94A mutant protein. After injecting compound 29 into N. benthamiana, NbRANL gene expression was significantly inhibited, resulting in a double inhibitory effect on RNP formation. Compound 29, identified in this study, inhibited NP aggregation by binding to the K68, T92, and R94 residues of NP, providing insights into how small molecules can bind to IDRs to inhibit the formation of plant virus aggregates. This research lays a foundation for the development of IDR-targeting PS inhibitors, and the identified NbRANL gene holds potential for future use in breeding plants with enhanced resistance to viral infections.
4 Experimental Section
4.1 Materials and Virus Sources
Seeds of Nicotiana glutinosa (N. glutinosa) and N. benthamiana were preserved in our laboratory. All plants were cultured in an artificial climate chamber (25°C during the day, 50% relative humidity/23°C at night, 40% relative humidity). The plasmids of full-length infectious clone L(+)opt, M(−)opt, SR(+)eGFP, SR(+)eGFPK68AT92AR94A, SR(+)eGFPK68GT92GR94G, and P19-Hcpro-γb were provided by Prof. Xiaorong Tao and Dr. Mingfeng Feng at Nanjing Agricultural University. TSWV lettuce isolate was maintained in N. benthamiana and the infected leaves were maintained in a −80°C refrigerator.
4.2 Chemistry and Instruments
The chemicals used in this study were purchased from commercial suppliers and were not specifically treated. The degree of reaction was observed under ultraviolet light and detected by thin layer chromatography (TLC) using a silica gel plate GF254. The nuclear magnetic resonance carbon spectrum (13C NMR) and hydrogen spectrum (1H NMR) of the compounds were obtained using an ECX-500 (JEOL, Tokyo, Japan) or Ascend-400 spectrometer (Bruker, Germany) with deuterated chloroform (CDCl3) or dimethyl sulfoxide (DMSO-d6) as the solvent and tetramethylsilane as the internal standard. The melting point and mass spectrum of high resolution (HRMS) of the target compounds were determined by X-4B instrument (Shanghai Yidian Physical Optical Instrument Co., Ltd., China; uncorrected) and Orbitrap LC–MS instrument (Thermo Scientific TM, Q Active, USA), respectively.
4.3 Evaluation of Anti-TSWV Activity
Curative, protective, and anti-TSWV activities of the target compounds were evaluated according to previously reported methods [41-43]. Ribavirin and vanisulfane were used as the controls.
The general procedure for the synthesis of intermediates and target compounds 1–33 (refer to Supporting Information P5) involved the expression and purification of TSWV NP, with slight modifications based on previous literature [40] (see Supporting Information P4). The induced expression and purification of MBP-RANL followed a similar approach (see Supporting Information P5). The affinity of compound 29 for TSWV NP and MBP-RANL was evaluated (see Supporting Information P5), along with molecular docking (see Supporting Information P6). The construction of plasmids (see Supporting Information P6), Agrobacterium-mediated infection of N. benthamiana (see Supporting Information P7), and laser scanning confocal microscopy for observing green fluorescence (see Supporting Information P8) were part of the experimental setup. The extraction of total RNA and synthesis of cDNA from N. benthamiana (see Supporting Information P8) preceded various PCR techniques, including ordinary PCR (see Supporting Information P9), high-fidelity enzyme PCR (see Supporting Information P9), and RT-qPCR (see Supporting Information P9). Further experiments included WB analysis (see Supporting Information P9), IP mass spectrometry (see Supporting Information P10), Y2H (see Supporting Information P10), BiFC (see Supporting Information P11), and LCA (see Supporting Information P12). Additionally, NbRANL gene knockout (see Supporting Information P12) and transient overexpression of NbRANL and TSWV infectious cloning were used to infect N. benthamiana (see Supporting Information P12).
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China (Nos. 32330087 and 32302388) and the Key Technologies R&D Program of Guizhou Province in China (No. 2017-5788-1) and the Scientific Research Innovation Team of Guizhou University (No. 202403). We thank Prof. Xiaorong Tao and Dr. Mingfeng Feng (Nanjing Agricultural University) for their assistance with plasmid construction and their mechanism of action.
Conflicts of Interest
The authors declare no conflicts of interest.




