Endogenous/exogenous stimuli-responsive smart hydrogels for diabetic wound healing
Abstract
Diabetes significantly impairs the body's wound-healing capabilities, leading to chronic, infection-prone wounds. These wounds are characterized by hyperglycemia, inflammation, hypoxia, variable pH levels, increased matrix metalloproteinase activity, oxidative stress, and bacterial colonization. These complex conditions complicate effective wound management, prompting the development of advanced diabetic wound care strategies that exploit specific wound characteristics such as acidic pH, high glucose levels, and oxidative stress to trigger controlled drug release, thereby enhancing the therapeutic effects of the dressings. Among the solutions, hydrogels emerge as promising due to their stimuli-responsive nature, making them highly effective for managing these wounds. The latest advancements in mono/multi-stimuli-responsive smart hydrogels showcase their superiority and potential as healthcare materials, as highlighted by relevant case studies. However, traditional wound dressings fall short of meeting the nuanced needs of these wounds, such as adjustable adhesion, easy removal, real-time wound status monitoring, and dynamic drug release adjustment according to the wound's specific conditions. Responsive hydrogels represent a significant leap forward as advanced dressings proficient in sensing and responding to the wound environment, offering a more targeted approach to diabetic wound treatment. This review highlights recent advancements in smart hydrogels for wound dressing, monitoring, and drug delivery, emphasizing their role in improving diabetic wound healing. It addresses ongoing challenges and future directions, aiming to guide their clinical adoption.
1 INTRODUCTION
Alarming rates of diabetes, a metabolic condition characterized by elevated blood sugar levels due to inadequate insulin synthesis or impaired insulin efficiency, are occurring worldwide.[1, 2] According to the most recent International Diabetes Federation (IDF) estimate, 10% of people worldwide will have diabetes in 2021; by 2030 and 2045, that figure is predicted to rise to approximately 643 million and 783 million, respectively.[3, 4] Diabetic foot ulcers (DFU) stand out as one of the most serious and common chronic complications often associated with diabetes, highlighting a prevalent connection between diabetes and chronic wounds.[5-7] In Southeast Asia, the prevalence of DFU ranges between 10.0% and 30.0%, while in Brazil, it stands at 21.0%. Europe sees a variation between 1.0% and 17.0%; in the Middle East or North Africa, the prevalence ranges from 5.0% to 20.0%.[7] Individuals with diabetes often experience prolonged periods of elevated blood glucose, which can lead to specific harm to the blood vessels, nervous system, and immune system.[8, 9] Consequently, wounds in diabetic patients are prone to becoming persistently inflamed. Diabetic wounds are notoriously difficult to manage due to their complex origins and heightened risk of infection. These wounds take longer and are more likely to recur than ordinary injuries.[10, 11] For diabetic patients grappling with chronic wounds such as DFU, the risk of recurrent infection and amputation represents a grave concern. These complications can result in substantial healthcare costs and elevate morbidity or mortality rates worldwide.[12] Additionally, it is linked to hemostasis, inflammation, proliferation, and tissue remodeling, which are among the vital biological processes.[13-17] The wound-healing process for these wounds is more complicated, leading to overlapping phases and a longer inflammatory period.[18] Diabetic wounds so frequently heal incompletely and are likely to recur. Removal or casualty can occur as a result of a diabetic wound, even in the most extreme cases.[18] In short, healthcare systems across the globe are now swamped with the growing problem of wound healing.[7] The financial impact of these wounds is substantial, not only on families but also on societal healthcare systems, and they demand significant medical resources.[19-21] The clinical management of diabetic wounds heavily relies on wound dressing.[22] Despite their usefulness in halting bleeding, collecting exudates from wounds, and protecting the wound from infection, traditional wound dressings like gauze will not hasten the healing process.[23-25] Patient compliance will suffer due to the increased risk of subsequent or multiple injuries from frequent dressing replacement.[23, 26] Recent research guided by the moist wound healing theory indicates that the optimal dressing should maintain appropriate temperature, pH, and humidity levels. It should also be easily removable without harming skin tissue cells.[23, 27, 28] Wounds may benefit from dressing with superior hemostasis preservation qualities and anti-infection and pro-repair properties. Wet wound dressings sold commercially typically contain foam, film, and hydrogel.[29, 30] Hydrogels are superior to other dressings because they are transparent, biocompatible, and retain moisture well, allowing visual wound monitoring.[31, 32] Hyaluronic acid dressings, such as Intrasite Gel (Smith and Nephew) and Aquiform (Maersk Medical), as well as other hydrogel dressings, are capable of absorbing wound exudate, encouraging necrotic tissue autolysis and keeping the wound environment moist, have shown positive clinical outcomes in wound management. A greater risk for infection, insufficient angiogenesis, and delayed healing are additional difficulties associated with diabetes wounds. These challenges stem from the wound microenvironment's complexity, characterized by elevated blood glucose levels, low pH, increased reactive oxygen species (ROS), and abnormal levels of matrix metalloproteinases (MMPs).[33-36] Despite the potential for hydrogel wound dressings to be used alongside antibiotics or other medicinal treatments, their current clinical application falls short in addressing the specific characteristics of diabetes-related wounds. This limitation can lead to improper delivery of pharmaceuticals, potentially resulting in reduced efficacy or the development of drug resistance.[37] These concerns guide the development of diabetic wound dressings, introducing further challenges to their application. Thus, selecting proper treatment strategies to expedite the healing of wounds poses a critical challenge for healthcare providers worldwide.[30, 38, 39] Recent research presents a self-powered electronic bandage composed of soft, biodegradable materials that utilize dual electrostimulation pulsed electrostimulation for enhanced epithelial growth factor expression and d.c. electrostimulation for increased secretion of healing factors to significantly accelerate intestinal wound healing, demonstrating superior outcomes in vitro and in vivo compared to traditional suturing methods.[40, 41] A study presents a novel closed-loop system for diabetes management, integrating printable metallo-nucleotide hydrogels with optogenetic engineering for sustained, on-demand insulin expression. It represents a significant advance in personalized medicine, offering a promising platform for precision diabetes therapy and long-term glucose regulation.[42] Researchers introduce a novel insulin-loaded, silver-coated mesoporous nanoparticle (NP)-reinforced fibrous hydrogel with promising antibacterial, antioxidant, and regenerative properties, offering the potential for effective diabetic wound management.[43] Diabetic wound treatment is particularly challenging due to the complex wound environment characterized by infected biofilms, excessive inflammation, and impaired angiogenesis, with the critical role of the microenvironment often overlooked in therapeutic development. In a study, researchers introduce a microneedle (MN) bandage functionalized with dopamine-coated hybrid NPs containing selenium and chlorin e6 (SeC@PA), designed to regulate reactive species generation in response to the wound microenvironment, effectively enhancing anti-biofilm, anti-inflammatory, and wound healing processes through a self-enhanced, catabolic, and dynamic therapy approach.[44, 45] Research into smart/stimuli-responsive hydrogels continues to address the shortcomings of existing wound dressings in clinical practice. Depending on the exterior factors, such as temperature, light, or magnetism, or internal factors, such as overproduction of ROS, higher glucose level, low pH, and upregulated enzymes, these hydrogels are engineered to respond differently to diabetic wound settings.[46-48] One way to combat the rise of drug-resistant microbes brought on by antibiotic overuse is the creation of stimuli-responsive hydrogels that can regulate the release of drugs precisely and autonomously. Also, by providing a tailored approach to wound care, these cutting-edge hydrogels can soften the rough microenvironment in diabetic wound sites.[49] For example, DP7-ODex hydrogels (pH-responsive hydrogel) have been exploited, incorporating the antimicrobial peptide DP7 and ceftazidime. Ceftazidime's inhibitory dosage on multiple drug-resistant bacteria is considerably reduced when DP7 destroys bacterial cell membranes, while hydrogel degradation speeds up drug release in acidic settings. These clever, sensitive hydrogels, which were created with diabetic lesions characteristics in mind, may be better than current medical dressings and grip good ability for the dealing of diabetic chronic wounds.[50] Additionally, considering that most hydrogels are limited to a single function and are effective only during specific stages of wound healing, incorporating structures that respond to changes in the wound microenvironment can help create intelligent hydrogels. These intelligent hydrogels can be tailored to the unique characteristics of diabetic wounds, allowing for the controlled release of active substances to promote wound healing.[51] Currently, numerous reviews have explored the applications of hydrogels in diabetic wound healing.[52-55] However, there is limited discussion on the use of multifunctional hydrogels specifically designed based on the wound microenvironment for diabetic wounds. Additionally, the applications of these smart hydrogels in diabetic wound dressings have not been comprehensively explored, and a systematic analysis of the therapeutic strategies employed by these smart hydrogels for managing diabetic wounds remains largely unaddressed.
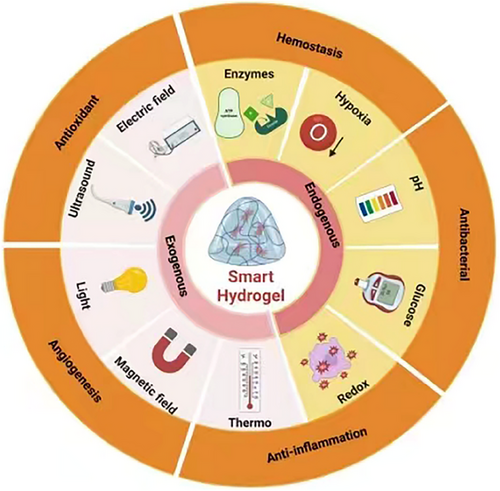
To treat diabetic lesions, we want to present an application-focused overview of the advantages and potential for future development of current types of innovative hydrogel dressing in this review study. The factors that impede the restoration of wounds and the environment surrounding them will be discussed in depth. The benefits of hydrogels for diabetic wound care will be discussed in this review study, emphasizing the part that stimuli-responsive hydrogels play in the recovery process (Figure 1). To describe it briefly, this review aims to support the ongoing advancements in hydrogel research and help them become the best dressing for healing diabetic wounds. If successful, this effort could expand hydrogels' usefulness in therapeutic settings. This study aims to provide guidance on the use of smart hydrogels in diabetic wound healing and to explore the role of these hydrogels in the treatment process.
2 PROCESSES AND CHALLENGES ASSOCIATED WITH NORMAL AND DIABETIC WOUND HEALING
2.1 The normal wound-healing process
The process of wound healing is intricate, promoting the restoration of injured skin tissue. Understanding the different stages of wound healing is essential for selecting the proper wound dressing to ensure effective wound care.[56, 57] In an ordinary healing process, there are four overlapping stages: hemostasis, inflammation, proliferation, and remodeling (Figure 2A).[27, 31, 43, 58-62] Numerous cells and constituents collaborate to regenerate damaged skin tissue and restore its integrity during these processes.[63] The generation and activity of neutrophils, platelets, and macrophages carry out the primary functions of the hemostatic and inflammatory phases: wound healing and pathogen resistance.[64] The hemostasis phase, the first line of defense against skin injuries, involves a quick contraction of capillaries and platelets, activating a coagulation cascade. This phase results in the reduction of blood flow and helps in sealing the wound.
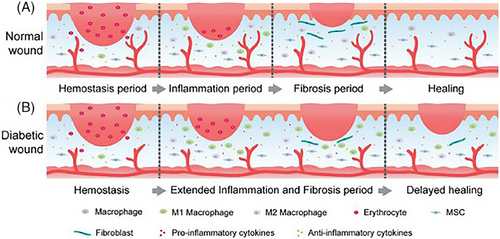
Additionally, platelets either directly recruit immune cells or emit chemokines to draw immune cells to the injury site, setting the stage for the upcoming inflammatory phase.[65] The endothelium, epidermis, and dermis cells congregate close to the wound site due to the release of cytokines and growth factors during the proliferation phase.[66] After the initial response, monocytes from the bloodstream migrate to the site and become activated M1 macrophages with an inflammatory phenotype. These M1 macrophages then release significant quantities of pro-inflammatory cytokines, aiding in eliminating pathogens and debris and promoting the proliferation of cells vital for the repair process.[67] Secretory factors also enhance vascular permeability and edema. Angiogenesis, which follows the proliferation phase, is characterized by a rise in fibroblasts and collagen synthesis, leading to granulation tissue creation.[68] Tissue remodeling occurs within one to three weeks as fibroblasts differentiate into myoblasts, increasing type I collagen and restoring the extracellular matrix (ECM).[69, 70] Scar tissue eventually forms when a newly created blood artery network swiftly matures into an established tissue framework with few residing cells and reduced structural strength.[71] These four phases of wound healing are also present in diseases like diabetes. Still, the complicated microenvironment of these conditions can impede healing and develop chronic wounds.[72]
2.2 Diabetic wound healing process
Diabetic wounds have more complex microenvironments than chronic and normal wound types (Figure 2B). For instance, DFU has a complicated etiology and is challenging to treat, making it one of the most severe consequences of diabetes.[73] The primary cause of this is the complex DFU microenvironment, which is marked by high blood sugar, low oxygen levels, excessive wound discharges, bacterial infections that recur, the buildup of ROS, aberrant cell factor, and growth factor expression, ongoing inflammation, elevated protease activity, tissue regeneration, and angiogenesis.[74] Any of these elements may slow down the healing process, extending one or more of the four phases that overlap when a wound heals,[75] making the treatment of diabetic wounds more challenging in the clinical setting.
2.2.1 Effect of diabetes on normal wound healing
Diabetes can interfere with the body's natural wound-healing process, often causing wounds to remain stuck at a particular stage for an extended period, which leads to delayed healing. Several pathophysiological factors associated with diabetes contribute to this impaired wound healing. Infections caused by microorganisms, oxidative stress, persistent inflammation, flawed mechanisms for tissue repair, such as inadequate blood vessel formation (angiogenesis), reduced oxygen supply (hypoxia), nerve damage (neuropathy), and issues with matrix deposition and the reformation of the skin layer (re-epithelialization).
2.2.2 Risk of bacterial infection
Studies reveal that microbial infections may worsen as many as 90% of chronic wounds, severely impeding healing.[76] Persistent bacterial infections can escalate to bacteremia and sepsis, posing life-threatening risks. Common bacteria in wounds, such as Staphylococcus aureus and Streptococcus, form biofilms. These biofilms shield bacteria from antibiotics, facilitating the emergence of drug-resistant strains.[77, 78] A critical indicator of a wound's infection state is its pH level. The pH should ideally be between 5.5 and 6.5 for normal wound healing. Chronic wounds that aren't infectious might have a pH between 7.15 and 8.9, which is alkaline and makes them a breeding ground for bacteria.[79] On the other hand, persistent wounds that have pus may exhibit a decreased pH level.[80] The pH falls to 5.5 or below in locations where methicillin-resistant S. aureus (MRSA) biofilms are present.[81] Moreover, the prolonged existence of microbes in wound areas can intensify inflammation and postpone the healing period.[82]
2.2.3 Injury due to oxidative stress
In the context of wounds in individuals with diabetes, inflammation for an extended period results in oxidative stress. This stress is characterized by an imbalance between substances that promote oxidation and the body's natural antioxidants. This imbalance is evident in the elevated levels of ROS.[83] ROS refers to oxygen-based chemical compounds generated through various biological processes, such as mitochondrial activities, peroxisomal actions, and about forty enzyme systems. These compounds include superoxide anions, hydrogen peroxide (H2O2), and hydroxyl radicals.[84] Usually, ROS serves as a defense mechanism against pathogens, maintaining low levels in the body.[85] The concentration of H2O2 in plasma ranges from 1 × 10−6 to 8 × 10−6 M (with an average of 3 × 10−6 M), and in healthy cells, the typical concentration of ROS is around 1 × 10−6 M.[86] However, in the context of diabetic wounds, persistent higher levels of blood sugar can cause ROS overproduction through various pathways, such as the polyol pathway, the formation and interaction of advanced glycation end-products (AGEs), and their receptors, the hexosamine pathway, and protein kinase C (PKC) activation, leading to ROS concentrations skyrocketing to 10–1000 × 10−6 M.[87, 88] Research has also highlighted a significant relationship between long noncoding RNA and ROS production in diabetes.[89] The excessive accumulation of ROS can cause considerable damage to numerous cell types, including keratinocytes, fibroblasts, and endothelial cells. This damage reduces their viability and hampers their ability to migrate and differentiate, further complicating the healing process.[90, 91]
2.2.4 Abnormal inflammation associated with diabetes
Chronic inflammation mimics diabetic wounds, with immune cells such as neutrophils, monocytes, and macrophages playing essential roles in healing.[92] However, in diabetic wound conditions, higher blood sugar levels impair the enzymatic activity of neutrophils and their bacterial killing capacity. This situation also leads to the phagocytic dysfunction of macrophages and their predisposition to adopt a senescent phenotype, diminishing their effectiveness in clearing inflammation and thereby prolonging chronic inflammation.[93, 94] Furthermore, the transition between macrophage phenotypes, from the inflammatory M1 to the healing-promoting M2, plays a vital role in wound repair.[95] These wounds tend to dominate pro-inflammatory M1 macrophages, with a scarcity or absence of anti-inflammatory M2 macrophages.[96] Usually, M1 macrophages switch to the M2 phenotype after pathogen elimination during the acute phase of inflammation. However, high glucose levels hinder this phagocytic activity of macrophages, leading to reduced inflammatory clearance and deferred transition from M1 to M2 macrophages.[67] This results in an accumulation of M1 macrophages, which release pro-inflammatory cytokines, attracting more monocytes that convert into M1 macrophages and creating a cycle of M1 polarization.[97-100] Such an imbalance between M1 and M2 macrophages keeps the inflammation in diabetic wounds chronic. This impedes the transition to the proliferation phase, which is essential for healing, preventing the wound from moving past the inflammatory phase.
2.2.5 Tissue damage
The diabetes-induced hostile environment can cause skin tissue damage and complicate healing processes, specifically through impediments in blood vessel formation (angiogenesis), reduced oxygen supply (hypoxia), nerve damage (neuropathy), and issues with tissue matrix formation and skin regeneration. The rapid development of new blood vessels is essential for the healing of wounds, as it enables the transportation of oxygen and nutrients to the injured tissue, hence aiding in the reduction of excessive inflammation.[101-105] Nonetheless, several factors in the diabetic microenvironment include a decrease in pro-angiogenic factors, an increase in anti-angiogenic factors, oxidative stress, and obstructing angiogenesis.[104, 106] Additionally, diabetic individuals often suffer from macrovascular disease, which diminishes blood flow. At the microvascular level, dysfunction occurs due to unbalanced regulation of capillaries and lower levels of nitric oxide synthase.[59, 104, 107, 108] This results in prolonged nutrient and oxygen scarcity in the wound area, further delaying blood vessel formation, skin cell proliferation, and the building of essential ECM components.[109, 110] Oxygen is critical in treating diabetic wounds, leading to therapies like hyperbaric oxygen treatment and localized oxygen delivery as supplementary treatments.[110, 111] Initially, inadequate blood supply due to microcirculatory issues and blocked angiogenesis results in insufficient oxygen delivery to the wound, heightening the oxygen requirement due to increased metabolic activity and thereby worsening cell hypoxia.[112, 113] Short-term hypoxia can activate hypoxia-inducible factor-1a and encourage angiogenesis; prolonged hypoxia disrupts blood vessel formation, energy production, and cell metabolism.[114-116] Addressing hypoxia is thus crucial in diabetic wound care.
Peripheral neuropathy, a common diabetes symptom, influences wound healing by dulling sensation, which diminishes protective responses, heightens injury risk, and reduces neuropeptide secretion, damaging tissue regeneration.[36, 117, 118] Neuropeptides are vital for skin cell communication and healing. Their diminished release can disrupt inflammation control, angiogenesis, matrix formation, and skin regeneration.[119, 120] The diabetic condition also interferes with the deposition of the tissue matrix and skin renewal due to high blood sugar levels. MMP, crucial for breaking down collagen and aiding skin cell movement, becomes unbalanced due to abnormal inflammation, leading to an overproduction of MMPs, particularly MMP-9.[121, 122] Hyperglycemia amplifies MMP activity directly or by oxidative stress (or AGEs), affecting the matrix around the wound.[123] Furthermore, the movement of skin-forming cells (keratinocytes) is hindered using excess tumor necrosis factor-alpha (TNF-α) in M1 macrophages. In contrast, the scarcity of M2 macrophages affects skin renewal by downregulating the levels of keratinocyte and epidermal growth factors (EGFs).
3 FACTORS AFFECTING WOUND HEALING PROCESS
3.1 Glucose level
Hyperglycemia, defined as the ongoing contact of body cells with high amounts of glucose, hampers wound healing by modifying the processes via which cells transport glucose in response to higher glucose levels outside the cells. This leads to increased glucose within the cells.[74, 124] The activation of multiple metabolic processes, including the hexosamine, polyol, and PKC pathways, causes a significant rise in ROS levels in this state. At the same time, the activity of glutathione and other antioxidant enzymes is also declining.[125] High blood glucose levels, in combination with oxidative stress and precursors to AGEs, lead to the formation of AGEs. The accumulation of these AGEs in the ECM contributes to vascular stiffness.[126]
Furthermore, the activation of the nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) can be triggered when AGEs bind to the receptor for AGEs (RAGE). This activation exacerbates inflammation and oxidative stress, raising the transcription of genes linked to pro-inflammatory compounds. RAGE activation harms the movement and reproduction of fibroblasts, which play a crucial role in normal wound healing.[127] In addition, keratin and laminin, two important protein markers for keratinocytes, are downregulated in hyperglycemia, which hinders keratinocyte differentiation and re-epithelialization.[128]
Clinical evidence shows that high blood glucose levels at the injury site cause cell damage and vascular lesions, significantly increasing the chance of bacterial infection. For instance, AGE development can directly induce ROS production at high levels in the case of immune cells.[66] A worsening of metabolic problems in the wound region may ensue from increased oxidative stress, which upsets the cellular redox equilibrium.[129] Moreover, AGEs can prevent macrophages from changing from conventionally activated macrophages (M1), which have pro-inflammatory properties, into preferentially activated macrophages (M2), which can heal tissue and minimize inflammation.[130] The persistent inflammation at the site of the injury results from the ongoing accumulation and stimulation of pro-inflammatory cells, which is triggered by an excessive influx of M1 macrophages.[131]
Additionally, the constriction of blood vessels and the stiffening of cell membranes brought on by elevated blood sugar levels impede wound healing by reducing blood flow and cutting off oxygen and nutrients from the affected region.[131] A bacterial illness that recurs can result from elevated blood glucose levels because it can supply more nutrients for the growth and multiplication of bacteria.[132] Consequently, the most significant barrier to healing in diabetic wound settings is the high level of blood glucose, a hallmark characteristic. Therefore, controlling blood sugar levels becomes the primary prerequisite in treating wounds.
3.2 pH level
The skin's pH is acidic, with a range of 4–6, but the pH of other tissues in the body is maintained at a pH of 7–9. This discrepancy, amounting to a 2–3 pH unit difference between the skin's outermost layer (stratum corneum) and the deeper layers (epidermis and dermis), is believed to serve as a protective barrier against microbial invasion.[128] In the case of acute wounds, the pH is initially neutral but tends to decrease during the healing process, reflecting a physiological shift conducive to recovery.[133] Conversely, chronic diabetic wounds are characterized by a persistent alkaline state, with pH values between 7.15 and 8.90, which hampers the healing process.[134] The release of ammonia can further increase this wound's alkalinity due to pathogenic bacteria in these wounds.[135] An alkaline pH impairs wound oxygenation and encourages the infiltration and proliferation of biofilm-forming bacteria. This perpetuates a cycle of chronicity, making the healing of diabetic wounds particularly challenging.[136] While a wound is healing, several factors can impact its pH. These include microbial activity, enzyme activity, oxygen availability, and cell development. The skin's surface typically has a pH of 4–6, which is somewhat acidic to prevent microbial invasion.[80] Weak alkalinity is seen when skin injury exposes the underlying tissue, which has an internal pH milieu of 7.4. The pH of many wounds changes from alkaline to acidic during healing, but chronic and infected wounds remain alkaline for longer because of ongoing inflammation.[137] An evaluation of the pH levels in chronic wounds of 39 patients for various reasons revealed a range between pH 5.45 and 8.65. Diabetic wounds typically begin with an alkaline pH level, eventually shifting towards neutrality and acidity, similar to the progression observed in acute wounds.[138] On the other hand, prolonged inflammation often causes the pH at the wound bed to drop.[48] The initial pH of the microenvironment enclosing chronic wounds, which is higher relative to healthy skin, encourages bacterial growth and reproduction. As a result, the possibility of sustained bacterial infection is increased.[139] Analysis of numerous clinical trials indicated that chronic and infected wounds tend to persist in an alkaline pH environment.[80] Still, most hydrogel wound dressings that respond to pH have been engineered to break down at lower pH levels. The fact that diabetic wound-associated bacteria may convert glucose into lactic acid, yet another way to reduce the pH, likely inspired this design decision.[140] Furthermore, this disparity could result from differences between diabetic individuals and animal models. When the environment is acidic, diabetic wounds in experiment animals heal more quickly and may still be in the acute stage. This situation is not the same as the environment (pH > 7.3) in clinical diabetic patients during the chronic stage.[80] Not to add, a wound's pH changes with its stage and healing process; chronic wounds in the healing phase, for instance, will likewise have an acidic pH. In conclusion, there are significant pH variations in diabetic wound sites. These variations can be attributed to a variety of factors, including the phase of the healing process and microbial colonization. As a result, creating coverings for wounds with precise pH control could significantly accelerate the treatment activity in diabetic wounds.
3.3 Concentration of ROS
ROS are highly reactive forms of oxygen, including superoxide (O−2), H2O2, and hydroxyl radicals, primarily produced as byproducts of the mitochondrial electron transport chain during oxidative phosphorylation.[141] Superoxide dismutase and catalase are ROS-scavenging enzymes that carefully regulate ROS levels during routine wound healing, ensuring that the harmful effects of ROS are minimized and that they fulfill their role in signaling and defense against pathogens.[91] However, in conditions of hyperglycemia, the scenario changes significantly. The production and accumulation of ROS increase due to several mechanisms, including the formation of AGEs, glucose autoxidation, and polyol pathway activation.[142] The interaction between AGEs and RAGE can further boost the production of superoxide and H2O2, aggravating oxidative stress.
Additionally, the upregulation of NADPH oxidase enzymes and the activation of the PKC pathway, combined with a reduction in glutathione and other antioxidant defenses, contribute to an excessive generation of ROS. This heightened state of oxidative stress undermines cellular functions. It can significantly impair wound healing, making ROS management critical in chronic conditions like diabetes.[143, 144]
Angiogenesis is effectively promoted, and normal levels of ROS (secondary messenger of numerous immunological and non-lymphocyte cells) resist bacterial infections.[66] On the other hand, persistently high ROS levels can lead to continuous inflammation and ultimately cause irreversible damage to the cells in the surrounding tissue, which weakens the wound and prevents endogenous stem cells and macrophages from doing their jobs, impeding wound healing.[88] Oxidative stress-induced inflammation in diabetic wounds results in the breakdown of collagen and ECM and impairs angiogenesis.[145] Furthermore, elevated ROS levels generated by immune cells within the wound trigger the activation of NF-κB, higher levels of interleukin (IL)-6 (an inflammatory mediator), and TNF-α, ultimately resulting in persistent inflammation and deceleration of wound healing. Consequently, maintaining the proper level of ROS at the wound site may aid in promoting wound healing.
3.4 Hypoxia
Hypoxia, characterized by reduced oxygen levels in tissues, results from a mismatch between the oxygen supply and its consumption.[146] This state is particularly prevalent in diabetic wounds, where various microvascular complications such as vessel damage, fibrosis, or sclerosis, hindered angiogenesis, or increased oxygen demand contribute to reduced oxygen availability.[112, 147] The elevated pH levels (7.1–8.9) found in chronic wounds further complicate this issue by inhibiting the release of oxygen from oxyhemoglobin molecules, thereby limiting oxygen supply to wound tissues.[148] Additionally, the signaling pathway of HIF-1, which typically adapts to hypoxic conditions by promoting angiogenesis and fibroblast migration and proliferation, is disrupted in diabetic wounds.[149] Chronic hypoxia adversely impacts the healing process of diabetic wounds because aerobic respiration, a major source of ATP for critical physiological processes, including cell proliferation and signaling, is compromised.[124] Furthermore, hypoxia can suppress collagen production and ECM formation by affecting fibroblast function. It can weaken the immune response mediated by neutrophils, exacerbating the wound-healing challenges.[150, 151]
According to clinical research, oxygen plays a crucial role in wound healing and has been used as a therapeutic strategy to promote and expedite wound healing. At different stages of wound healing, the regulation of oxidative stress, energy metabolism, and resistance of bacteria to the drugs are crucial and are influenced by the microenvironment's oxygen level.[152] Capillary injury and reduced angiogenesis in diabetic trauma might result in insufficient oxygen delivery and a weakened immune system, which worsen local inflammation and bacterial infection. When appropriate, hyperbaric oxygen therapy is applied in clinical settings to treat diabetic foot patients, accelerating wound healing and lowering the risk of amputation.[153, 154] It is important to note that hyperbaric oxygen therapy is still a pricy treatment. Consequently, it could be beneficial to develop easy wound dressings while considering their potential effects on the oxygen content of the wound.
3.5 MMPs level
MMPs are a group of zinc-dependent endopeptidases comprising up to 28 enzymes known for their diverse substrate specificity.[155] Key members include the collagenases (MMP-1 and MMP-8) and gelatinases (MMP-2 and MMP-9), pivotal in wound healing. They facilitate this by breaking down the ECM and the capillary basement membrane, essential for wound contraction and remodeling.[123] Their activity is naturally regulated by tissue inhibitors of MMPs (TIMPs-1,2,3,4), which bind to MMPs to prevent excessive ECM degradation.[156] In diabetes, the regulatory balance between MMPs and TIMPs is altered, leading to elevated levels of MMPs (specifically MMPs 1, 2, 8, 9, 14, and 26) and reduced TIMP expression.[123] This imbalance disrupts normal wound healing processes, as evidenced by a high MMP9/TIMP1 ratio associated with poor wound healing outcomes.[157] The resultant pathological condition is marked by excessive ECM degradation, destruction of growth factors, and diminished collagen deposition during the remodeling phase, thereby increasing the risk of chronic wounds. This highlights the critical role of the MMP/TIMP balance in wound healing and how its disturbance can significantly impact the healing trajectory in diabetic conditions.[158]
MMPs, particularly the gelatin enzyme variety, play a dual role in diabetic wound healing. Produced in the dermis after an injury, MMPs are essential for tissue recombination and the breakdown of the ECM during the healing process.[159] MMP overexpression may eliminate growth factors needed for wound healing and prevent initial connective granulation tissue development. Because of its overexpression, wound closure is impeded, and the possibility of bacterial infection and persistent inflammation increases. Additionally, the excessive presence of ECM protease in the wound area further aggravates the problem by inhibiting the ECM accumulation in deep fascial units (DFUs).[160] Research presents a complex picture of MMPs in diabetic wound healing. Some studies suggest that certain MMPs, like MMP-8, are more beneficial for the healing process, while others indicate that the overexpression of MMP-2 and MMP-9 can impede the healing process in wounds.[161, 162] Thus, the secret to diabetic wound healing is to suppress matrix protease action selectively. Compared to the diabetic mice used in the study, the wounds from diabetes humans may have a considerably lower concentration of active MMPs.[157, 161, 162]
3.6 Persistent infection
An increased susceptibility to bacterial infections and biofilm formation worsens the prolonged phase of inflammation seen in diabetic wound healing. Studies have indicated that infections in DFUs may result in lower limb amputations in about 60% of the patients, substantially elevating the risk of morbidity and mortality.[163] Bacteria such as Staphylococcus, Pseudomonas, and Corynebacterium are known for their role in biofilm formation, indicating a heightened risk for wound-delayed healing.[164-166] These elements combine with a pro-inflammatory environment to further delay the healing process, compounding the challenges faced in managing diabetic wounds effectively.[167, 168]
3.7 Inflammation
Diabetic wounds often suffer from prolonged healing times, primarily due to an extended inflammatory phase characterized by continuous and extended infiltration of immune cells.[108, 169, 170] This condition is further aggravated by elevated blood glucose levels and AGEs, which intensify the inflammatory state.[171-173] Typically, in wound healing, M1 macrophages, which are pro-inflammatory, dominate the wound site initially. As healing progresses, these cells transition to an M2 phenotype, which exhibits anti-inflammatory properties.[130] In diabetic wounds, the transition from pro-inflammatory M1 to anti-inflammatory M2 macrophages is impaired, leading to the persistence of M1 macrophages. These macrophages continuously produce inflammatory mediators, including nitric oxide (NO), ROS, ILs (IL-1 and IL-6), and TNF-α. They also secrete MMPs such as MMP-2 and MMP-9, which contribute to the breakdown of the ECM, further complicating the healing process. This ongoing inflammatory response hinders the formation of granulation tissue and disrupts the migration and proliferation of keratinocytes and fibroblasts, crucial for wound healing. Consequently, this disruption leads to delayed wound healing in individuals with diabetes, highlighting the critical need for targeted interventions to modulate the inflammatory response and promote efficient healing.[67, 130, 174-176]
Consequently, research findings also imply that while designing sensitive hydrogel wound dressings, consideration should be given to the distinctions between humans and experimental animals. The complex microenvironment of diabetic chronic wounds is summarized as follows: high levels of ROS,[177] hypoxia,[178] bacterial infection,[179] excess glucose,[180] excessive exudate,[181] overexpression of MMPs.[182] Each of them is a difficulty that needs to be addressed in clinical treatment and can obstruct clinical recovery in diabetic wounds.
4 TRADITIONAL DRESSING VERSUS HYDROGEL DRESSING
Traditional wound dressings like gauze, foam, films, and specific medicated options such as iodine and silver have been staples in wound care for their protective capabilities and ability to absorb exudates. Nonetheless, a notable limitation of these conventional dressings, highlighted frequently, is their insufficient exudate absorption capacity.[183, 184] This deficiency necessitates regular dressing changes, and the removal of these absorbent materials, once saturated, can stick to the wound, risking secondary damage and potentially extending the healing process. Hydrogels present a significant advancement in this area. Their unique three-dimensional, cross-linked polymer networks can hold considerable water within their porous structures, enhancing exudate absorption and preventing wound maceration. Furthermore, these hydrogels maintain a high moisture level, essential for a healing-friendly environment that avoids undue dryness.[185-187]
One of the challenges with standard dressings is their prefabricated shapes, which may not conform well to the diverse shapes and sizes of wounds, covering only portions of the wound site. This lack of customization can complicate dressing changes and affect the patient's daily activities. Additionally, the design of traditional dressings often obstructs the view of the wound, limiting the patient and healthcare provider's ability to monitor the healing progress visually, thus hindering timely and informed medical assessments. Hydrogels can become fluidic thanks to their self-healing or thermally responsive properties, enabling them to fill wounds of varied shapes and depths effectively. This makes them exceptionally suited for managing long-term, non-healing diabetic wounds.[188] Notably, they can incorporate optical or electronic indicators to monitor the wound's condition, such as bacterial infections, the stage of healing, or the need for dressing changes. This capability significantly enhances medical decision-making by providing healthcare professionals with precise information, moving beyond guesswork.[189]
The ideal wound dressing should prevent infection and foster the healing process. While the market has seen the introduction of advanced dressings capable of releasing therapeutic molecules, these typically do so passively, potentially leading to localized toxicity and other issues.[190] In contrast, hydrogels are designed to adapt to the evolving needs of a wound proactively, delivering drugs as required for targeted therapy. This approach enables more accurate treatment, enhancing outcomes and minimizing side effects.[60]
Hydrogel dressings comprise up to 90% water within a gel made from water-loving polymers that expand when they come into contact with water.[191] Their moisture-rich composition facilitates the gentle removal of dead or infected tissue, encourages tissue growth, and accelerates healing.[192] Suitable for lightly to moderately leaking wounds because of their significant water content and semi-absorbent nature, these dressings stand out. In contrast, though highly absorbent, traditional dressings like gauze, foam, and cotton balls can cause discomfort during changes due to their frequent application needs. They also lack adhesive solid properties and sufficient drainage for wounds. Addressing the healing of chronic wounds promptly is crucial for public health.[193]
Modern technologies for smart dressing, including hydrogels, nanofibers, films, and NP sprays, aim to overcome the limitations of traditional dressings, albeit facing their challenges. Nanofiber dressings, for example, promote cell movement due to their large surface area but may cost more and prove tricky to apply on large or irregularly shaped wounds.[194] Typically made from polyurethane or silicone, film dressings are thin, waterproof, and breathable but lack moisture absorption, making them less suitable for heavily exuding wounds. Removing them can also be painful and may harm the wound. Nanoparticle sprays, similarly, fall short in moisture retention and protection and are generally pricier.[195] Hydrogels, however, with their advantageous degradation characteristics, stand apart. They are especially beneficial for delivering biological agents directly to wounds.[193] Hydrogel dressings outperform traditional ones by enhancing moisture at the wound surface, allowing gas exchange, absorbing exudate, and not sticking to the wound, thereby aiding autolytic debridement. They also match human skin in terms of compressibility, stretchability, adhesive strength, and the ability to recover from deformation rapidly. This makes hydrogels a distinct option among modern wound dressings, offering a versatile platform for delivering medications, growth factors, and other therapeutic agents directly to the wound site.[196, 197] Hydrogels can be seamlessly integrated with mesenchymal stem cells and their derivatives, further enhancing their therapeutic potential. Ultimately, the array of benefits provided by hydrogel dressings positions them as superior alternatives for managing various wound types.
In summary, the essential features of effective wound dressing include the ability to absorb exudates, maintain a moist environment to avert wound drying, be non-toxic and compatible with bodily tissues, conform to various wound configurations, provide secure adhesion without damaging new tissue, enable the monitoring of wound progression, and facilitating the controlled release of medications in response to the wound's needs. Some smart hydrogels already embody these characteristics, illustrating a forward leap in wound care technology, see Table 1.
| Traditional dressing | Hydrogel dressing | Features |
|---|---|---|
| Basic protection | Provide protection | Wound protection |
| Can adhere to the wound | Non-adherent, easy removal | Adherence to wound |
| Limited | Excellent moisture retention | Moisture retention |
| Often painful | Minimal pain | Pain during removal |
| Required frequent changes | Less frequent changes required | Frequency of changes |
| Less conducive, may dry out the wound | Optimal moist environment | Healing environment |
| Limited/none | Can be enhanced with antimicrobial agents | Antimicrobial properties |
| Not available | Allows controlled drug delivery | Drug delivery capability |
| Basic biocompatibility | High biocompatibility | Biocompatibility |
| Limited customization | Highly customizable and adaptable | Customization and adaptability |
5 ADVANTAGES OF HYDROGEL DRESSING IN DIABETIC WOUND HEALING
An essential component of diabetic wound care is wound dressing, which should have the dual properties of efficiently absorbing wound exudate and creating a microenvironment beneficial for the healing process.[69, 198, 199] Conventional wound dressings, such as bandages, gauze, and other inert materials, are commonly used due to their low cost and ease of production. They can safely be coupled with antibiotics, absorb exudate, and protect the wound. Conventional wound coverings, however, don't encourage wound healing. Furthermore, conventional dressings frequently cause a dry crust around the wound, which can further harm the area when removed and cause pain and other discomforts for the patient.[200, 201] Based on the idea that healing occurs best in moist conditions, various wound dressings, including films, foams, hydrocolloids, alginates, and hydrogels, have recently been created.[198, 202-205] Studies have shown that wound repair is enhanced in moist environments.
Film dressings are an excellent option for superficial wounds with slight exudation because they are highly breathable and isolate fluids and bacteria effectively. Foam dressings, such as Cavicare and Allevyn from Smith & Nephew, maintain the wound moist and warm while preventing secondary wound injury when removed. Although they are widely used and have good exudate absorption qualities, hydrophilic adhesive, and sodium alginate (SA) dressings are debatable when treating infected wounds.[7] Various hydrogel dressings, such as Intrasite Gel (Smith and Nephew) and Aquaform (Maersk Medical), are also sold. Hydrogel dressings are considered suitable to deliver drugs and bioactive compounds because of their bioadhesive qualities,[206, 207] outstanding water absorption qualities,[208, 209] three-dimensional porous structure,[210] and customizable disintegration rate.[211] Over the past few decades, hydrogel dressings have been the subject of substantial research in diabetic wound healing.[212] Hydrogel dressings offer several benefits over conventional wound dressings like bandages, including biodegradability. Composed of natural or synthetic polymers, using hydrogel dressings could alleviate environmental pressure associated with many individuals facing diabetic foot complications. Additionally, the main constituents of commercially available self-adhesive wound dressings include a silicone layer for wound contact, a cover made of polyethylene film, a backing of nonwoven fabric, and a bonding agent made from polypropylene. On the other hand, simpler and more biocompatible components make up hydrogel dressings with adhesion, eliminating the need for additional adhesives. For diabetic wounds, a long-term platform exists, and there is an opportunity to develop and conduct an in-depth study of responsive hydrogel dressings. These dressings boast unique properties, such as enhanced bio-adhesive capabilities, controlled drug delivery, and adaptable environmental settings.[213] This section describes the advantages of stimuli-responsive hydrogels for treating diabetic wounds.
5.1 Controllable drug delivery
A key factor in the success of wound healing is the efficient administration of medications or other tiny molecules in dressings. Because biomaterials have strong bio-adhesive qualities, hydrogels can respond to stimuli like MMPs, ROS, pH, and exogenous light to produce regulated release and effective distribution of active components.[214] Wound healing with exosomes (EXO) has been extensively studied. EXO transportation and release are limited by the diabetic wound milieu when using standard hydrogel dressings, reducing their therapeutic efficacy.[215] Recently, innovative hydrogel dressings have been developed to influence the response to MMP enzymes, aiming to improve the delivery of EXO. These hydrogels can supply and control the release of adipose-derived stem cell EXO (ADSC-exo) for 20 days at 90%. This approach significantly enhances the therapeutic potential of ADSC-exo.[216] Low pH conditions characterize diabetic wounds. Developing polymeric hydrogels for controlled drug release triggered by pH changes often utilizes Schiff-base cross-linking as the conventional reaction mechanism.[217, 218] To address chronically infected wounds' acidic environment and high ROS levels, researchers created a pH-responsive hydrogel patch that activates at pH 8.5. Drug distribution and controlled release methods are both made more accessible by this design. The hydrogel is made from aldehyde (ALD)-rich oxidized dextran (ODex) and ethylenediamine-modified gelatin. Building on the concept of dual-responsive hydrogels, this creation leverages pH and ROS sensitivity to achieve precise and timely release of various medications, showcasing an innovative approach to spatiotemporal drug delivery.[219]
Recently, there has been significant interest in developing innovative hydrogel dressings responsive to exogenous stimuli for the regulated release of medications.[220] Researchers developed a graphene oxide-based responsive hydrogel dressing with exceptional mechanical characteristics. The light-responsive properties enable this improved dressing to use cyanoacetate dextran (with histidine) and benzaldehyde.[72] In a rifampicin hydrogel dressing, green indocyanine (ICG) and fatty acids packed natural halloysite clay nanotubes made this dressing hollow, biocompatible, and light-responsive.[221] Upon exposure to NIR laser light, the ICG within the hydrogel facilitates the gradual release of rifampicin into the wound. This is achieved by locally heating the encapsulating fatty acid through thermal conversion, reaching the melting point, and allowing controlled drug release. The hydrogel's robust mechanical and exceptional photothermal properties enable the management of the drug's sustained release. Given the intricate nature of diabetic wound healing, where dressings and medications are frequently employed in tandem, this approach to controlled and efficient drug delivery is essential.
5.2 Adjustable environmental adaptability
It is common for the healing process to be slowed down because current hydrogel dressings only contain one mechanical characteristic and can't adjust to different environmental stimuli.[101] A smart, multipurpose hydrogel dressing can adapt to environmental conditions, moisturize, and maintain antibacterial qualities. Using SA and poly[2-(methacryloyloxy)-N, N, N-trimethylethylammonium chloride], Dong et al. created a wound dressing with over 90% water retention for seven days and outstanding anti-freezing capabilities. Below −20°C, this dressing has good electrical conductivity and mechanical strength, proving its environmental adaptability and stability. The delivery of medications requiring cryopreservation thus offers a promising alternative.[181] In addition, the hydrogel dressing based on SA and [2-(methacryloyloxy ethyl) dimethyl-(3-sulfopropyl)] that the team developed exhibited remarkable antibacterial adhesion and stability, and it works well even when the temperature drops.[222] Researchers have created a hydrogel with strong adhesion (up to 0.28 MPa on glass), conductivity (17.1 mS/cm), stretchability (up to 2167%), and self-healing properties (recovery rates of 90%). This hydrogel can be modified to detect changes in wound dynamics, and it works wonderfully as a biosensor to detect human activity.[223] Moreover, hydrogel wound dressings responsive to ROS and glucose can adapt to the wound environment, consuming ROS and glucose. This action helps improve the wound site's microenvironment, facilitating healing.[224, 225]
6 APPLICATION OF ENDOGENOUS/EXOGENOUS STIMULI-RESPONSIVE SMART HYDROGELS IN DIABETIC WOUND HEALING
In hydrogel-based medical treatments, active agents are dispensed via compression, expansion, molecular dispersion, and hydrogel breakdown processes. Hydrogels that can alter their attributes in reaction to environmental changes are termed stimuli-responsive hydrogels.[226, 227] These advanced innovative hydrogels can swiftly enact their therapeutic action within pathological environments, aiding cellular and tissue health maintenance.[227-229] Drawing on these creative designs, biofunctional materials equipped with sophisticated, responsive mechanisms for therapy and repair have been engineered. They can adapt to both external physical triggers, including ultrasound, illumination, electrical and magnetic influences, as well as internal disease signals, such as heightened levels of ROS, pH shifts, specific ionic distinctions, enzyme outputs, or certain immune conditions, through either reversible or irreversible modifications in their structure and physical characteristics.[230-235]
Clinical trials for several gel products are either finished or ongoing for treating diabetic wounds, Table 2. Thus, more research may be done to determine the hydrogel dressings' potential for treating diabetic wounds. Regarding medication release and degradation, hydrogel dressings are constant in clinical settings. Considering the variation in individuals' conditions and wound states, therapeutic outcomes can differ. To address this, numerous studies have integrated features like hydrophobic interactions and reversible covalent chemical bonds, including Diels-Alder reactions, disulfide bonds, Schiff-base bonds, borate ester bonds, and acyl hydrazone bonds into hydrogels. These innovations have paved the way for developing injectable hydrogel dressings responsive to stimuli such as temperature, glucose, ROS, and enzymes. Such hydrogel wound dressings, capable of adapting to environmental changes, offer precise control over drug release and can regulate behaviors like self-degradation. This adaptability underpins the creation of personalized treatment approaches. Identifying each patient's wound environment's unique characteristics is vital for effective treatment. Various innovative, smart, and responsive hydrogel wound dressings are highlighted, underscoring their potential application in treating diabetic wounds.[4, 236-248] In biomaterials, stimuli-responsive hydrogels have shown promise in improving diabetic wound healing. This review organizes and summarizes recent advancements in stimuli-responsive hydrogel dressings for diabetic wound management, categorizing them based on different stimulus triggers.
| Product name/hydrogel dressing | Delivery system | Cargo | Administration route | Clinical phases | Status | Clinical trials number | Date | Marketed |
|---|---|---|---|---|---|---|---|---|
| AmeriGel | NA | NA | Local | Phase 4 | Terminated | NCT01350102 | 2012.04–2014.03 | No |
| Fitostimoline hydrogel | NA | NA | Local | Phase 4 | Completed | NCT05661474 | 2021.02–2022.12 | Yes |
| SANTYL | NA | NA | Local | Phase 4 | Completed | NCT02111291 | 2014.04–2015.12 | Yes |
| Woulgan gel | NA | NA | Local | Phase 4 | Completed | NCT02631512 | 2015.10–2019.04 | Yes |
| ALLO-ASC-DFU (hydrogel sheet with allogenic mesenchymal stem cells) | Hydrogel sheet with allogenic mesenchymal stem cells | Mesenchymal stem cells | Local | Phase 1 | Completed | NCT03183726 | 2016.01–2017.07 | No |
| Hydrogel Purilon | NA | NA | Local | Phase 2 | Completed | NCT03700580 | 2012.08–2016.10 | No |
| IZN-6D4 Gel | NA | NA | Local | Phase 2 | Completed | NCT01427569 | 2012.03–2015.08 | No |
| Lavior diabetic wound gel | NA | NA | Local | Phase 2 | In progress | NCT05607979 | 2022.12–2023.06 | No |
| NanoDOX hydrogel | NA | NA | Local | Phase 2 | Completed | NCT00764361 | 2009.01–2010.08 | No |
| TWB-103 (mixture of TWB-102 cells and TWB-103 hydrogel) | Mixture of TWB-102 cells and TWB-103 hydrogel | Cells and hydrogel mixture | Local | Phase 1/2 | Unknown | NCT03624023 | 2019.12–2021.07 | No |
| Hydrogel with 3% sodium pentaborate pentahydrate | Hydrogel with sodium pentaborate pentahydrate | Anti-inflammatory agent | Local | Phase 1 | In progress | NCT02241811 | 2014.09–2023.12 | No |
| RMD-G1 (hydrogel with erythropoietin) | Hydrogel with erythropoietin | Erythropoietin | Local | Phase 1 | Completed | NCT02361931 | 2016.03–2018.06 | No |
| Cadexomer iodine gel | NA | NA | Local | Not applicable | Terminated | NCT02181621 | 2014.08–2015.10 | No |
| Hydrogel/nano silver-based dressing | Nano silver-based hydrogel | Nanosilver particles | Local | Not applicable | Completed | NCT04834245 | 2019.01–2019.12 | No |
| Regranex | NA | NA | Local | Not applicable | Unknown | NCT00446472 | 2007.04–2010.09 | No |
| Solosite gel | NA | NA | Local | Not applicable | Terminated | NCT02181621 | 2014.08–2015.10 | No |
| ConvaTec DuoDERM hydroactive gel | NA | NA | Local | Not applicable | Completed | NCT00971048 | 2009.09–2011.01 | No |
| Antibacterial-antioxidant hydrogel | Chitosan-silver nanoparticles | ROS scavenger, antibacterial agents | Local | Phase II | Ongoing | NCT04512398 | 2012.04–2014.03 | No |
| Hydrogel with growth factors | Recombinant human EGF | Growth factor (rhEGF) | Local | Phase III | Completed | NCT03122811 | 2014.04–2015.12 | Yes |
| Temperature-responsive hydrogel | PNIPAM-based hydrogel | Curcumin | Local | Phase I | Recruiting | NCT04678921 | 2021.02–2022.12 | No |
| Glycyrrhizic acid (GA)-based hydrogel | GA-Zinc Ion (Zn2⁺) hydrogel | Anti-inflammatory, immunomodulation | Local | Preclinical | Preclinical | Not Applicable | 2015.10–2019.04 | No |
| Stem cell-containing hybrid hydrogel | Alginate-mesenchymal stem cells | Mesenchymal stem cells | Local | Phase II | Ongoing | NCT04322197 | 2016.01–2017.07 | No |
| Hyaluronic acid-based hydrogel | Hyaluronic acid nanoparticles | ROS scavenger, antimicrobial peptide | Local | Phase I | Ongoing | NCT03537275 | 2012.08–2016.10 | No |
| Collagen-based hydrogel with copper ions | Collagen-copper nanoparticles | Copper ions (angiogenesis enhancer) | Local | Phase II | Recruiting | NCT04295845 | 2022.12–2023.06 | No |
| Nano-composite hydrogel with antimicrobial peptides | Nanoparticle hydrogel matrix | Antimicrobial peptides (AMPs) | Local | Phase I | Recruiting | NCT03941673 | 2009.01–2010.08 | No |
| Hydrogel with polyvinyl alcohol (PVA) | PVA-based hydrogel | Anti-inflammatory agents | Local | Phase I/II | Recruiting | NCT04150033 | 2019.12–2021.07 | No |
| Hydrogel with chitosan and silver nanoparticles | Chitosan-silver nanoparticles | Antibacterial, ROS scavenging agents | Local | Phase II | Ongoing | NCT04523456 | 2014.09–2023.12 | No |
| Alginate-based hydrogel with curcumin | Alginate-curcumin hybrid | Curcumin (anti-inflammatory) | Local | Preclinical | Preclinical | NCT03874578 | 2016.03–2018.06 | No |
| Electrospun hydrogel with bioactive glass | Electrospun hydrogel with bioactive glass | Bioactive glass particles | Local | Preclinical | Preclinical | NCT04182768 | 2014.08–2015.10 | No |
| PEGylated hydrogel with ZnO nanoparticles | PEGylated hydrogel with ZnO nanoparticles | ZnO nanoparticles (ROS scavenger) | Local | Preclinical | Preclinical | NCT04457795 | 2019.01–2019.12 | No |
| Hydrogel Loaded with insulin-like growth factor-1 (IGF-1) | Insulin-like growth factor-1 (IGF-1) | Growth factor for enhanced healing | Local | Phase I | Recruiting | NCT04938245 | 2007.04–2010.09 | No |
6.1 Endogenous stimuli-responsive hydrogels
6.1.1 Glucose-responsive hydrogel
In a hyperglycemic state, heightened glucose levels trigger molecular alterations by promoting AGE accumulation. This process begins with the interaction between the ALD group of glucose and the lysine residues in proteins, initiating a two-step reaction that results in the formation of AGEs. These AGEs bind to their receptors (RAGE), triggering oxidative stress by increasing the production of ROS. Additionally, the accumulation of AGEs in the ECM leads to glycosylation, further complicating the wound-healing process. Consequently, researchers have explored elevated glucose levels to regulate drug release, with three potential methods for creating glucose-responsive hydrogels: utilizing glucose oxidase (GOx), concanavalin A (Con A), or phenylboronic acid (PBA).[249] A groundbreaking glucose-responsive antioxidant hybrid hydrogel was engineered in a separate study to enhance diabetic wound repair. This was achieved by integrating PBA-modified hyaluronic acid (HA) and myricetin into a polyethylene glycol diacrylate (PEG-DA) hydrogel matrix. The hybrid hydrogel showcased a glucose-triggered antioxidant release, efficient ROS scavenging, and modulation of the wound microenvironment. The promising in vitro and in vivo outcomes indicate the superiority of this hybrid hydrogel in diabetic wound healing compared to non-responsive hydrogel platforms.[244] Chen et al. have pioneered the development of an intelligent antioxidant hydrogel scaffold using reversible boronic bonds. By modifying gelatin methacryloyl (GelMA) with 4-carboxyphenylboronic acid (CPBA) to create GelMA-CPBA and subsequently cross-linking it with (−)-epigallocatechin-3-gallate (EGCG), they formulated the GelMA-CPBA/EGCG (GMPE) hydrogel. This hydrogel intelligently responds to glucose fluctuations, enhancing the release of EGCG as glucose concentrations increase, a reaction facilitated by the dissociation of boronic ester bonds. Demonstrating excellent biocompatibility, biodegradability, and mechanical similarities to skin tissue, the GMPE hydrogel has proven through both in vitro and in vivo research to effectively neutralize ROS, alleviate inflammation, stimulate angiogenesis, and facilitate tissue remodeling, thereby advancing diabetic wound healing. This innovative strategy sheds light on glucose-responsive scaffolds, showcasing substantial promise for managing chronic diabetic wounds.[250] A study introduced a novel hydrogel for diabetic wound repair featuring the regulation of hyperglycemia through glucose responsiveness and antioxidant capabilities. Gallic acid (GA) was grafted onto chitosan (CS) chains and combined with a PEG-DA hydrogel matrix to form an antioxidant hybrid hydrogel (PEG-DA/CS-GA). Furthermore, insulin-loaded polyethyleneimine NPs (PEI-PBA/insulin NPs) with glucose-sensitive PBA were integrated into the PEG-DA/CS-GA hybrid hydrogel by forming dynamic borate bonds. The resulting PPIC hydrogel showcased remarkable biocompatibility, pronounced antioxidant effects, and efficient insulin release in response to glucose concentrations. Both in vitro and in vivo evaluations confirmed its efficacy in promoting angiogenesis, regulating the inflammatory microenvironment, and facilitating wound closure within 20 days, highlighting its effectiveness as a therapeutic platform for diabetic wound management.[251] A glucose-responsive hydrogel dressing system (CGH) with dual functionality was developed to treat MRSA-infected wounds in rats and promote wound healing effectively. This hydrogel comprises copper nanoclusters (CuNCs) cross-linked with oxidized HA (HA-ALD) and is applied directly at the wound site. Equipped with GOx, the hydrogel specifically targets and enzymatically degrades excess glucose, enhancing the healing process. This breakdown produces gluconic acid and H2O2, optimizing conditions for the Fenton reaction, which is key in eliminating drug-resistant bacteria by catalyzing ROS production. Additionally, the CuNCs provide the hydrogel with excellent conductivity, facilitating electrical stimulation (ES) that promotes blood vessel formation and supports tissue repair near the wound. This innovative multifunctional wound healing system adapts effortlessly to the contours of irregular wounds, lowers glucose levels at the wound site, maintains a continuous antimicrobial environment, and promotes blood vessel formation through ES. It offers a comprehensive and promising approach to treating complex diabetic wounds.[252] In a recent study, researchers delved into the role of folliculin-interacting protein 1 (FNIP1), revealing its capacity to modify mitochondrial morphology and decrease oxidative phosphorylation, thereby safeguarding against the accumulation of ROS. Through in vitro experiments, FNIP1 was shown to effectively mitigate oxidative stress and rejuvenate compromised angiogenesis in high-glucose conditions. To facilitate targeted FNIP1 delivery to diabetic wound sites, a novel glucose-responsive HA-PBA-FA/EN106 hydrogel was developed. This hydrogel employs a dynamic phenylboronate ester mechanism for glucose-sensitive drug release, while fulvic acid (FA) acts as a crosslinking agent endowed with antibacterial and anti-inflammatory effects. The introduction of the FEM1b-FNIP1 axis inhibitor, EN106, within the hydrogel further alleviates oxidative stress and encourages angiogenesis. The promising outcomes from both in vivo and in vitro studies underscore the HA-PBA-FA/EN106 hydrogel's effectiveness in speeding up the repair of diabetic wounds, highlighting its potential as an innovative approach for treating chronic diabetic wounds, (Figure 3A).[253]

Sophisticated hydrogel dressing, composed of 3,3′,5,5′-tetramethylbenzidine/ferrous ion/Pluronic F-127/GOx (TMB/Fe2+/PF127/GOx), has been developed to lower blood glucose levels and enhance wound healing by generating antibacterial agents directly at the wound site. This hydrogel utilizes GOx to break down blood glucose into H2O2 and gluconic acid, facilitating a Fe2+-dependent Fenton reaction that produces hydroxyl radicals (·OH) to oxidize TMB. This process allows for the visual tracking of blood glucose concentrations, shifting from colorless to green for levels between 1 and 10 mM. It activates chemodynamic therapy (CDT) by producing ·OH radicals for bacterial elimination. Additionally, the oxidation of TMB enables strong near-infrared (NIR) absorption, converting NIR light into heat for targeted photothermal therapy (PTT). This dual approach of PTT and CDT effectively eradicates S. aureus and Escherichia coli, ensuring the comprehensive repair of infected wounds, highlighted by decreased IL-6 and increased vascular endothelial growth factor (VEGF) and MMP-2 levels. The hydrogel's thermosensitive feature supports its injectability, self-healing ability, and easy removal, minimizing additional harm. With its combination of hypoglycemic, chemodynamic, photothermal, antibacterial, and thermosensitive properties, this multifaceted hydrogel offers significant promise for treating diabetic wounds.[254] The self-assembly behaviors and bioactivities of two naturally occurring ellagitannins, 1,2,3,4,6-penta-O-galloyl-α-D-glucose (α-D-PGG) and 1,2,3,4,6-penta-O-galloyl-β-D-glucose (β-D-PGG), which are plentiful in plants, were investigated. Remarkably, β-D-PGG formed a hydrogel without any modifications, a phenomenon attributed to its balanced distribution of phenolic hydroxyl groups and aromatic rings. This balance facilitates self-assembly into nanofibers through π–π stacking and hydrogen bonding. Conversely, the self-assembly of α-D-PGG was impeded by steric effects. Importantly, the β-D-PGG hydrogel demonstrated superior antibacterial properties and the ability to direct macrophage polarization towards the M2 phenotype, alleviating the inflammation. This investigation sheds light on the distinct self-assembly properties of these glucose derivatives and underscores their potential in biomedical applications, especially in wound healing.[255] The phenylborate-ester-cross-linked hydrogel MN patch (MNP) holds potential for diabetic treatment with its glucose-responsive insulin delivery and straightforward production process. However, its efficacy in blood glucose regulation has been constrained by the suboptimal design of the MN network. In research, insulin-loaded MNPs featuring polyzwitterionic characteristics were crafted using modified ε-polylysine and PVA. The study clarified the link between the MNP network's charging dynamics and the release of insulin by fine-tuning the amount of post-protonated amino groups with a positive charge on them. The refined MNP showcased enhanced in vivo glucose-responsive insulin delivery, adeptly managing blood glucose levels in a simulated three meals per day scenario. Additionally, insulin's bioactivity within the MNP remained stable for two weeks at 25°C. This investigation offers a promising approach to improving the glucose-responsive phenylborate-ester-cross-linked MNP, pushing it closer to clinical application in diabetes management.[256] Guo et al. engineered hydrogels featuring distinctive glucose-responsive phenyl borate groups through gelatin methacrylate, the glucose-responsive monomer 4-(2-acrylamide ethyl amino formyl)-3-fluorobenzene boric acid, and gluconic insulin in-situ copolymerization. This innovative hydrogel-based MN dressing released insulin in response to glucose concentration, effectively improving the hyperglycemic conditions in diabetic wounds. It also decreased inflammation and accelerated the process of healing in type I diabetic mice (C57BL/6) induced with streptozocin, showcasing its potential in enhancing diabetic wound care.[243] While PBA-mediated glucose-responsive hydrogels maintain a stable composition, PBA exhibits lower sensitivity to glucose compared to endogenous proteins such as GOx and Con A.[257] GOx can catalyze the conversion of glucose into glucuronic acid and H2O2, resulting in a reduction of pH and an enhancement of antibacterial activity. An injectable drug-loaded, glucose-responsive, “self-healing” metal-organic hydrogel containing zinc ions, for example, has been reported by Yang et al. (Figure 3B)[225] to fight antibiotic-resistant microorganisms. More intriguingly, this hydrogel reduced excess free radicals in wounds and promoted blood vessel growth by combining the synergistic antibacterial action of GOx-catalyzed H2O2 with the medication's loading capacity [deferoxamine (DFO)]. The pH of the solution decreased noticeably as the glucose concentration increased, and the DFO release in the DG@Gel group accelerated.
Consequently, DG@Gel may enhance the hyperglycemic wound microenvironment and facilitate the healing of diabetic wounds in type I diabetic mice (BALB/c) through its anti-inflammatory, antibacterial, and pro-angiogenic properties. Notably, while pH plays a crucial role in wound healing, GOx-containing glucose-responsive hydrogel dressings utilize glucose to generate gluconic acid, potentially causing a significant shift in pH at the wound site. Therefore, the clinical application of GOx-based hydrogel dressings may necessitate continuous wound pH monitoring or the implementation of pH-mitigating strategies. Con A-based glucose-responsive hydrogels have been extensively studied to regulate insulin release, with most research being in vitro and few exploring diabetes wound models. The development potential of Con A is hindered by its competitive glucose binding mechanism and limited biocompatibility.[229-231]
Additionally, its stability leaves much to be desired. The effectiveness of hydrogel dressings available clinically is limited, as the treatment of diabetic wounds in a hyperglycemic context presents considerable challenges. These challenges stem from the dressings' inadequate physiological responses, which struggle to accommodate the wound's intricate microenvironment. Glucose-responsive hydrogel dressings have the inherent capacity to carry drugs and enhance the wound microenvironment, facilitating synergistic therapeutic outcomes. Nonetheless, targeted modifications are essential to create hydrogels that respond to glucose levels. For example, PBA-based hydrogels require improvements in glucose selectivity, while protein-based hydrogels (involving GOx and Con A) need enhancements in protein stability.
6.1.2 pH-responsive hydrogels
Medication, such as insulin and antibiotics, can be administered on-demand using pH-responsive hydrogel dressings. This may reduce adverse effects, maintain drug concentrations, and enhance therapeutic effectiveness.[79] pH is a natural signal for the release of drugs because changes in pH during wound healing may be related to the healing process.[122, 258, 259] HA, a naturally occurring polysaccharide, is frequently utilized in wound therapy due to its ability to enhance signaling and cell motility associated with healing. A study by Lee et al. on treating DFUs with HA dressings (Healoderm) demonstrated that pure HA accelerates the healing of wounds without adverse side effects.[34] A proposed innovative dressing offers advanced features, including rapid moisture-draining, non-stickiness, pH sensitivity, and antibacterial effectiveness, which is ideal for managing wound exudates and monitoring healing. This dual-layered Janus dressing combines a hydrophilic, pH-sensitive, antioxidant cellulose layer with a hydrophobic, antibacterial polycaprolactone (PCL) layer in direct contact with the wound, allowing unidirectional exudate drainage and minimal wet adhesion. It changes colors in response to pH shifts from 5 to 9, facilitating in situ tracking of healing progress. Superior to conventional gauze in promoting healing, collagen synthesis, and angiogenesis, its efficacy is validated through in vivo tests and histopathology, with real-time monitoring enabled by a smartphone application utilizing Python-RGB programming, making it an innovative solution for diabetic wound care in hyperglycemic conditions.[117] A cutting-edge hydrogel incorporating quaternized carboxymethyl CS (QCMCS), tannic acid (TA), oxidized SA (OSA), and carbon quantum dots (CQD) (QCMCS/TA/OSA@CQD), has been created to enhance diabetic wound healing and enable real-time monitoring. Exhibiting remarkable self-healing, antibacterial, antioxidant properties, and superior biocompatibility, these hydrogels also effectively manage bleeding in a mouse liver injury model and significantly speed up the healing process in diabetic wound models. Additionally, they facilitate the reliable and timely acquisition of diabetic wound pH data through imaging signals, supporting monitoring of the wound's healing progression. Thus, the pH-responsive TA/QCMCS/OSA@CQD hydrogels emerge as promising candidates for wound dressings that foster diabetic wound healing and provide real-time monitoring capabilities.[260]
A groundbreaking adhesive hydrogel has been crafted through dual dynamic covalent cross-linking Schiff base and borate ester bonds combining carboxymethyl CS (CMCS), 2-formylphenylboronic acid (2-FPBA), and EGCG to serve as a multifaceted dressing for diabetic wound healing. The CMCS/2-FPBA/EGCG (CFE) adhesive hydrogels are distinguished by their excellent mechanical qualities (adhesion, injectability, moldability, and self-healing capabilities), pH-responsive EGCG release, and photothermal features. They also showcase outstanding antimicrobial, antioxidant, anti-inflammatory, and angiogenic properties. Notably, the CFE adhesive hydrogels can be detached on-demand using natural organic acids (such as citric acid, salicylic acid, and vitamin C), minimizing the risk of secondary injuries during dressing changes. This innovation presents a comprehensive and promising approach to advancing diabetic wound care.[261] Developed through a Schiff base reaction between gelatin and benzaldehyde-grafted Pluronic F127 drug-loaded micelles (FCHO), a novel pH-responsive, injectable hydrogel dubbed GCM micromotors offers self-healing, injectable, and pH-responsive traits for targeted drug delivery to wound sites. Curcumin (CUR), encapsulated within the hydrogel via micellization (CUR-FCHO micelles), brings antioxidative, anti-inflammatory, and antibacterial benefits to the formulation. Additionally, magnesium-based micromotors (Mg micromotors) embedded within the hydrogel release active hydrogen (H), tackling ROS and reducing inflammation. Demonstrating strong antibacterial, antioxidative properties and high biocompatibility, the GCM hydrogel effectively modifies the inflammatory wound environment, promotes vascularization and collagen formation, and supports wound healing and tissue regeneration in diabetic mice, showcasing its potential as a therapeutic solution for chronic diabetic wounds, (Figure 4A).[262] This research unveils a cutting-edge method to manage the pH microenvironment at diabetic wound sites through a glycopeptide-based hydrogel derived from modified HA and poly (6-aminocaproic acid). Formed by Schiff base interactions and metal complexation, this hydrogel provides anti-inflammatory benefits and boosts angiogenesis, which is essential for healing. It stands out for its impressive mechanical durability, self-healing properties, and ability to adhere to tissues, further aiding wound repair by promoting macrophage shift towards the M2 phenotype. As demonstrated through in vivo studies, hydrogel significantly improves diabetic wound healing and skin regeneration by quickly reducing inflammation and enhancing blood vessel growth, marking a significant advancement in diabetic wound care strategies.[263] A bilayer pad was designed to improve chronic wound healing, consisting of an outer layer of PCL nanofibers loaded with tetracycline hydrochloride (PCL-TH) and an inner layer of poly(vinyl alcohol) (PVA) hydrogel containing SA microspheres encapsulating curcumin (PVA-Alg@CUR). This pad features a porous structure that releases CUR in response to the pH of chronic wounds, offering suitable water vapor transmission, absorption capacity, and mechanical properties that align with the skin's natural movements. Its antimicrobial and antioxidant properties were validated through inhibition zone assays, co-culture methods, and in vitro free radical scavenging tests. In vivo studies further confirmed the pad's efficacy in reducing bacterial infection and promoting the healing of chronic diabetic wounds. These findings suggest that this antibacterial and antioxidant bilayer dressing, with its pH-responsive release mechanism, has significant potential for clinical application (Figure 4B).[264]
Li et al. synthesized a multifunctional, drug-loaded hydrogel dressing by integrating N- CMC (N-chitosan) and adipic acid dihydrazide with in situ cross-linked HA-ALD. Insulin glargine was incorporated into the hydrogel, allowing for pH-responsive and sustained release for up to 14 days. It lowers the pH from 7.4 to 6.5 and enhances insulin release from the hydrogel, facilitating its breakdown. Applied to diabetic rats, this hydrogel dressing shortened the inflammation period post-wound therapy, expedited the healing process, and alleviated symptoms of peripheral neuropathy.[236] Silver NPs (AgNPs) are extensively utilized in clinical antibacterial hydrogel dressings, often in conjunction with medications, to combat infections in diabetic wounds. For instance, the clinical application of Aquacel Ag dressing, which contains 1.2% silver combined with Aquacel hydrofiber, boosts its bactericidal effectiveness. This is achieved by facilitating the controlled release of silver at the wound site, maintaining its antimicrobial action for up to two weeks.[265] However, it is impossible to modify the hydrogel's drug-release behavior to accommodate various wound conditions. In response, AgNPs and the angiogenic drug DFO have been encapsulated within a double-cross-linked, pH-responsive hydrogel (hydrogel@AgNPs&DFO), aiming to address this limitation.[237] This hydrogel exhibits both angiogenic and antibacterial capabilities, enabling the targeted release of treatments at the wound site under controlled conditions. The combination of double Schiff-base breaking, the bactericidal action of CS quaternary ammonium salt, and AgNPs swiftly eliminate bacteria, reducing irritation at the site of diabetic wounds.
Meanwhile, DFO fosters angiogenesis. In a study with type 2 diabetic rats induced by streptozotocin, those with S. aureus-infected wounds treated with the hydrogel entirely healed in 10 days, compared to 17 days for the control group. The rate of release from the hydrogel increases in more acidic conditions (pH 5.0) compared to neutral (pH 7.4), underscoring the critical impact of responsive drug delivery in wound management. This approach facilitates precise drug release based on the wound environment's pH changes, reducing the need for frequent drug application and mitigating the risk of developing drug resistance, yet poses challenges in designing dressings that neither harm the wound nor compromise on delivering consistent medication release.
6.1.3 ROS-responsive hydrogels
Elevated levels of ROS significantly impede the healing process of diabetic wounds, making them a focal point in the study of responsive hydrogels. An oxygen-releasing temperature-sensitive hydrogel composite loaded with dihydromyricetin (DHM-OTH), which includes an oxygen-producing matrix of CaO2 NPs, was designed to address this issue. In vitro experiments showed that DHM-OTH generated an optimal oxygen environment for cells around the wound and displayed excellent biocompatibility and a range of biological activities. Applied to a type 2 diabetes (T2D) wound model, DHM-OTH was found to diminish the presence of inflammatory cells, boost collagen synthesis, encourage blood vessel formation, and stimulate cellular growth, thereby expediting the healing of chronic diabetic skin wounds. These promising results from both in vitro and in vivo studies position DHM-OTH as an innovative method for effective oxygen delivery to wound sites, presenting a viable avenue to improve diabetic wound healing outcomes.[266] The matrix for synthesizing an OGLP-CMC/SA hydrogel with a double network structure was oxidized Ganoderma lucidum polysaccharide (OGLP) superimposed with SA and CMC. The hydrogel demonstrated biocompatibility, oxidation resistance, tissue adhesion, and excellent mechanical properties.
Additionally, it effectively enhanced fibroblast proliferation and migration, along with antibacterial properties. Furthermore, the hydrogel promoted M1 macrophage polarization towards M2, reduced intracellular ROS levels, mitigated inflammatory responses, and facilitated epidermal growth, skin appendage development, and collagen deposition in wounds, expediting diabetic wound healing. Repairing chronic diabetic wounds with this biologically active hydrogel network that uses OGLP is an exciting new therapeutic prospect.[267] A nanocomposite (Mo, Fe/Cu, I-Ag@GOx) exhibiting multi-zyme-like activities was ingeniously embedded within a multifunctional fluorescent hydrogel. This enriched nanozyme hydrogel, containing GOx, mimics the natural activities of several enzymes, including GOx, peroxidase, oxidase, catalase, and superoxide dismutase, facilitating a glucose-triggered, pH-switchable cascade reaction aimed at diabetic wound healing. The hydrogel initiates a two-step cascade reaction; first, converting glucose and oxygen into gluconic acid and H2O2 to generate radicals for bacterial destruction, and second, mimicking the action of SOD and CAT to reduce oxidative stress and hypoxia as the wound environment shifts to alkalinity. Crafted with calcium ion-cross-linked SA and CS, this hydrogel stands out for its injectability, adhesion, fluorescence, and biocompatibility, offering a promising approach for the treatment and monitoring of bacteria-infected diabetic wounds without causing secondary injury, Figure 5A.[268] A ROS-responsive and scavenging supramolecular hydrogel was ingeniously crafted using the hexapeptide sequence Glu-Phe-Met-Phe-Met-Glu (EFM). Remarkably, this hydrogelator is constructed entirely from canonical amino acids and lacks specific ROS-sensitive motifs, yet swiftly transitions to a hydrogel state upon sonication. Hydrogel disassembly and payload release are facilitated by interaction with ROS, which oxidizes Met residues into methionine sulfoxide. Biocompatibility and the promotion of cell proliferation and migration were validated through cellular assays. The hydrogel demonstrated remarkable wound healing capabilities in diabetic mice during in vivo studies, demonstrating its dual activity as a ROS-scavenger and a drug delivery system. This hydrogel can treat various health concerns through its simple and effective biological applications (Figure 5B).[269]
A novel dual-layered hydrogel has been created to control ROS in wounds, particularly beneficial for healing diabetic wounds. The inner layer contains GOx, ferrocene-modified quaternary ammonium CS, and poly(β-cyclodextrin), which produce hydroxyl radicals (•OH) for antimicrobial effects during wound recovery. This layer is designed to break down progressively. The external layer is made of gelatin and dopamine, which contribute to the elimination of ROS during the latter stages of wound repair. The hydrogel's ability to regulate ROS in a two-phased approach for programmed diabetic wound healing was validated through antibacterial, ROS scavenging, and wound healing tests. This hydrogel dressing shows exceptional potential for the advanced treatment of diabetic wounds.[270] A versatile hydrogel known as HA@Cur@Ag has been devised, showcasing combined antioxidant, antimicrobial, and anti-inflammatory features. It is synthesized by cross-linking thiolated HA with disulfide-containing hyperbranched PEG via Michael addition and by embedding CUR liposomes and AgNPs. This hydrogel is distinguished by its excellent biocompatibility, degradability, and injectable nature. Both laboratory and animal studies confirm that hydrogel efficiently carries and releases CUR liposomes and silver ions. It aids diabetic wound closure through a suite of actions: neutralizing ROS, eliminating bacteria, reducing inflammation, and encouraging new blood vessel formation. Detailed gene sequencing has shown that the HA@Cur@Ag hydrogel suppresses the tumor necrosis factor/nuclear factor κB signaling pathway, reducing oxidative stress and inflammation in diabetic wounds. The evidence points to this ROS-sensitive, multifunctional, injectable hydrogel as a promising strategy for expedited healing of diabetic wounds by effectively orchestrating the complex biological and molecular activities involved in the healing process.[271] Zhao and colleagues created a hydrogel tailored to manage high ROS levels commonly present in wound environments. This hydrogel is compounded with antibiotics and granulocyte-macrophage colony-stimulating factor, a growth factor that encourages tissue regeneration. The hydrogel's capabilities include substantial reduction of intracellular ROS, minimization of pro-inflammatory factor release, guidance of macrophage phenotype shifts, stimulation of new blood vessels, and collagen formation, significantly enhancing wound healing.[272] Additionally, quaternized CS, a positively charged derivative of CS known for its innate antimicrobial properties, is frequently employed in the formulation of antibacterial materials.[273] Tannin, a bioactive compound known for its clot-promoting properties, is a small natural molecule rich in phenolic hydroxyl groups that can scavenge ROS. Pan et al. developed an injectable hydrogel, QT, prepared from quaternized CS and TA. This hydrogel displayed strong hemostatic abilities, effective antibacterial properties, and efficient ROS scavenging capabilities. It notably successfully reduced blood loss in rats that underwent surgical tail resection. A characteristic of this hydrogel is the oxidation of TA in the presence of H2O2, which causes the hydrogel to transition to a liquid state after 12 h. This transformation facilitates the release of active substances contained within the hydrogel network. Additionally, when used in diabetic rat skin models, the QT hydrogel treatment resulted in faster collagen deposition and improved skin tissue regeneration.[274] Ni et al. harnessed the antioxidant capabilities of TA in combination with PBA-modified polyphosphazonitrilene and PVA to create a hydrogel known as PPBA-TA-PVA. This hydrogel could respond to ROS and exert anti-inflammatory effects. Thanks to the dynamic phenyl borate bonds, the PPBA-TA-PVA hydrogels possess injectable and self-healing properties, which can be particularly valuable for fitting into and healing irregular or deep wounds, including those at joints subject to constant movement. In vivo, testing demonstrated that this hydrogel significantly reduced the duration of inflammation in diabetic rat wounds induced by streptozotocin compared to standard Tegaderm films, and it also accelerated the healing process.[224] The careful management of wound microenvironments is critical in treating diabetic wounds that are slow to heal, where excess levels of ROS play a substantial role in delaying the repair process and may lead to infection. ROS-responsive hydrogels like PPBA-TA-PVA have the potential to facilitate a controlled drug release and minimize ROS at the site of injury, which enhances the wound environment. However, it is important to balance ROS levels, as appropriate levels are necessary for antibacterial action and supporting the wound-healing process.
6.1.4 Enzymes responsive hydrogels
Diabetic wounds overexpress MMPs compared to normal wounds, delaying the healing process. Clinical wound healing may be accelerated with an MMP-responsive hydrogel dressing, which can either cause MMPs to become inactive or shield cells by competing for their substrate. In their research, Li et al. developed an HA-based hydrogel for diabetic wound healing that is responsive to MMPs and loaded with DFO, an agent beneficial for wound repair but limited by its toxicity and short half-life. By modifying HA with maleimide and attaching an MMP-cleavable peptide, they engineered an enzyme-responsive hydrogel crosslinked with varying amounts of ODex to form the final gel. This hydrogel's application to wounds in diabetic rats significantly improved the rate of wound closure, the process of new skin formation (epithelialization), and the development of new blood vessels (angiogenesis) compared to a control hydrogel incorporating a non-cleavable peptide. This finding highlights the efficacy of MMP-responsive hydrogels in delivering potent therapeutic substances like DFO directly to diabetic wounds, circumventing issues related to their toxicity and instability.[180] A groundbreaking hydrogel, termed CBP/GMs@Cel&INS, has been formulated to be responsive to both glucose and the enzyme MMP-9 and is temperature-sensitive, allowing it to adapt its consistency. This innovative hydrogel combines insulin and celecoxib-loaded gelatin microspheres (GMs) within a composite matrix made from PVA and CS modified with phenylboric acid. Remarkably, it transitions to a fluid-like state at body temperature (37°C), enabling it to conform quickly to the contours of deep wounds.
In comparison, at room temperature (25°C), it gains a solid-like elasticity that shields the wound from external pressures. It is precisely engineered to release insulin and celecoxib in response to increased glucose concentrations and the enzyme MMP-9. The CBP/GMs@Cel&INS hydrogel also boasts features like reconfigurability, self-healing, superior adhesion to biological tissues, suppression of MMP-9, and the facilitation of cell growth, movement, and glucose uptake. In models of diabetic skin defects that penetrate the full thickness of the skin, this hydrogel notably diminishes inflammation, effectively balances local glucose and MMP-9 concentrations, and expedites wound healing by exploiting its temperature-responsive adaptive characteristics and dual-responsive release system synergistically, Figure 6.[121]
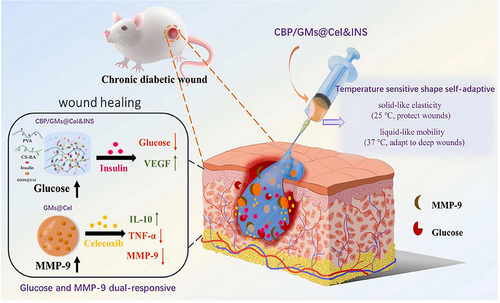
Liu et al. developed a novel approach for diabetic wound healing by encapsulating CUR NPs (CNPs) within GMs to create CNPS@GMs. These were then incorporated into a temperature-sensitive hydrogel (CNPS@GMs/hydrogel) for application on diabetic skin wounds. The hydrogel exhibited responsiveness to MMP-9 in the wounds, releasing Cur accordingly. In vitro experiments showed that MMP-9 concentration correlated with Cur release, confirming the specificity of the response. In a diabetic wound healing model, the CNPS@GMs exhibited notable enhancement in the healing process and excellent compatibility with biological tissues.[182]
Similarly, Shao et al. developed a microsphere-based MMP-9-responsive hydrogel for diabetic wound management, leveraging boronate bonds to respond to high glucose and H2O2 concentrations, enhancing therapeutic efficacy. This hydrogel demonstrated excellent responsiveness to MMP-9, glucose, and biocompatibility, improving diabetic wound healing.[101] Sonamuthu et al. crafted a natural protein hydrogel for wound dressing that is responsive to the enzyme MMP-9, utilizing the induction of the dipeptide L-carnosine. L-carnosine contains a histidine residue with a powerful affinity for zinc ions, effectively inactivating MMP-9 by forming complexes with the zinc ions at the enzyme's active site. Thus, hydrogels based on L-carnosine can significantly aid in healing diabetic wounds by inhibiting the activity of MMP-9, an enzyme commonly associated with chronic wounds due to its role in degrading the ECM and disrupting normal healing processes, Figure 7.[242]
Zn2+ has been shown to influence the activity of MMP-9 positively.[242, 275] Developing a composite hydrogel (L-car@cur/SF) composed of CUR and L-carnosine loaded into silk fibroin harnesses the wound-healing properties of both components. L-carnosine inhibits MMP-9 activity by chelating zinc in the enzyme's active core, which is critical for its function. Together with CUR, known for its anti-inflammatory effects, this combination enhances wound healing in diabetic models. EXO secreted by various cell types, such as immune cells, fibroblasts, and mesenchymal stem cells, can also play a pivotal role in facilitating wound repair. For instance, EXO from adipose-derived stem cells has been shown to activate the PI3K/Akt signaling pathway, which regulates fibroblast proliferation and ECM production, accelerating the healing process in diabetic wounds.[276] Diabetic wound healing presents a global challenge due to high MMP9 levels and inflammation impeding recovery. The CUR analog H8 shows promise in anti-inflammatory diabetic wound care, but its systemic distribution to organs like the liver and kidney necessitates a targeted delivery system. The study explores macrophage membrane-derived nanovesicles (H8NVs) within GMs (GMH8NV) for localized, MMP9-responsive H8 release to enhance angiogenesis and mitigate oxidative stress and inflammation. The findings indicate that GMH8NV promotes cell migration, angiogenesis, and M2 macrophage polarization, collectively accelerating diabetic wound healing and positioning GMH8NV as a viable targeted treatment strategy.[277] Jiang et al.[216] engineered an innovative hydrogel (ADSC-exo@MMP-PEG) sensitive to the enzyme MMP-2. It is fabricated using four-arm PEG functionalized with maleimide, MMP substrate peptides, a PEG chain with a sulfhydryl group, and ADSC-derived EXO. The creation of this hydrogel involves a Michael addition reaction. The designed hydrogel slowly degrades for 20 days in the presence of MMP-2, steadily releasing ADSC EXO. These EXO have been shown to facilitate cell growth and movement by activating the Akt signaling pathway. When applied in diabetic mouse models, the ADSC-exo@MMP-PEG hydrogel markedly improved the wound healing process. This was evidenced by increased re-epithelialization, collagen synthesis, cellular proliferation, and the formation of new blood vessels. Hydrogel dressings that respond to specific enzymes like MMPs can address the issue of MMP overexpression in diabetic wounds. These dressings are designed to be removed in accordance with the progress of wound healing, enhancing safety. Nonetheless, factors such as the wound's microenvironment and the pH of the dressing are important considerations because they can affect the activity of enzymes. These aspects must be carefully considered during the design, manufacturing, and clinical application of such dressings.
6.2 Exogenous stimulus-responsive hydrogels
6.2.1 Temperature-responsive hydrogels
Enzymatic reactions often vary with temperature, and heat can enhance the rate of wound healing. Clinically, warmth is a traditional sign used to evaluate chronic wounds and can be utilized to activate temperature-sensitive wound dressings.[278] Lin et al. found that elevated temperatures around the wound area facilitated healing in patients with pressure ulcers based on measurements and analyses conducted on 50 individuals.[279] Fierheller et al. observed that in cases of infection, the average temperature of the skin surrounding chronic leg ulcers increased by over 2°F in a study involving 40 patients.[280] Temperature-responsive hydrogels can better adjust to unequal wounds by switching between the glue and solution phases as the temperature changes.
Moreover, temperature-sensitive hydrogels can be made to shrink while releasing loaded medications, allowing for controlled drug delivery. Negative thermo-reversible hydrogels with a lower critical solution temperature (LCST) shrink by heating above the LCST, while polymer solutions with an upper critical solution temperature (UCST) shrink by chilling below UCST in a positive thermo-reversible system.[281] For instance, Zhang et al.[282] created GelMA-PDAASP. This temperature-responsive hydrogel dressing is far more effective than commercial 3 M or gelatin dressings at promoting wound healing in mice and enabling regulated drug release. This hydrogel patch is crafted using a unique approach that combines proteins and polyphenols to form a thermo-responsive network that reacts to body temperature. Its adhesion properties are cleverly designed to activate upon contact with warm skin, while a simple application of an ice bag enables pain-free removal. Hydrogel is engineered to modulate immune responses, reducing the risk of irritation or allergic reactions during extended wear. This makes the patch an ideal, gentle solution for affixing bioelectronics to infants' skin for healthcare purposes without causing harm. Additionally, the patch offers mild adherence to injured skin, creating an optimal environment that accelerates the healing of diabetic wounds by promoting faster recovery.[2]
He et al. have engineered a versatile, photothermally responsive hydrogel (PAG-CuS) designed as an all-in-one solution for wound care. This includes enhancing wound healing, delivering anti-inflammatory treatments, and executing photothermal sterilization. The hydrogel is synthesized through the copolymerization of acrylic acid (AA), methacrylic anhydride-modified gelatin (GelMA), and copper sulfide NPs coated with lipoic acid sodium (CuS@LAS). This composition results in a hydrogel with a porous, three-dimensional structure that encourages cell attachment and retains a significant amount of water but also integrates CuS@LAS to provide photothermal antibacterial capabilities and improve the material's mechanical properties through physical cross-linking. When activated by NIR light, the hydrogel releases CuS@LAS, which then disperses LAS through micelle disassembly, helping to remove intracellular ROS. This action reduces MMP-9 expression, boosts ECM production, and aids healing.
Furthermore, the liberation of Cu2+ ions from the hydrogel fosters CD31 expression in endothelial cells, encouraging the formation of microvessels essential for wound recovery. In diabetic wound studies on GK rats, PAG-CuS significantly decreased ROS levels, increased the number of microvessels, and promoted better epithelialization and wound healing overall. Thus, PAG-CuS hydrogel emerges as a comprehensive, effective solution for managing diabetic wounds through its sterilization capabilities, free radical scavenging, and promotion of angiogenesis, Figure 8A.[283]
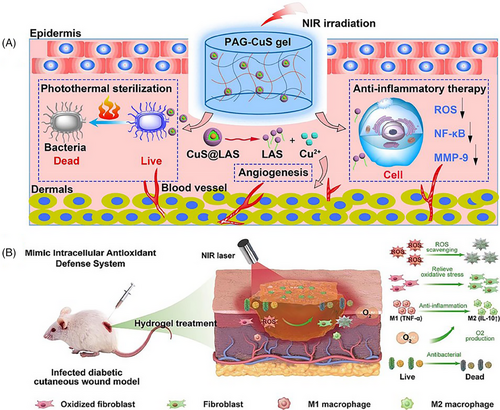
Recently, researchers have engineered an antimicrobial hydrogel dressing, GMH, that combines methacrylic-anhydride-modified gelatin and oxidized HA. This dressing undergoes Schiff base reactions and ultraviolet (UV)-induced double cross-linking and is further enhanced with PDA (polydopamine) NPs, creating GMH/PDA. This innovative composition enables hydrogel to convert photothermal energy and demonstrate effective photothermal antimicrobial activity when exposed to NIR light. This feature significantly reduces inflammation and prevents bacterial infections throughout the healing process. The GMH/PDA hydrogel also boasts remarkable injectability, which means it can be precisely applied to wounds with complex shapes and surfaces, offering a tailored therapeutic approach.
In summary, the GMH/PDA hydrogel stands out for its high antimicrobial efficacy, antioxidant capabilities, and excellent biocompatibility, positioning it as an up-and-coming option for treating infected skin wounds.[284] Wang et al. detail a pioneering method for generating a multifunctional nanocomposite hydrogel by integrating a nanoenzyme (manganese dioxide, MnO2) and non-enzymatic antioxidant elements (PDA) within a dynamic network based on thioctic acid and TA. This hydrogel stands out with its unique adherence and injectability, which help it conform entirely to wounds of modifying shapes and sizes. The presence of PDA@MnO2 NPs provides the hydrogel with an enhanced competence for going through a widespread array of reactive nitrogen and oxygen species, modifying inflammation via altering macrophage polarization. Also, the hydrogel shows a high level of catalytic efficacy in converting hydrogen peroxide (H2O2) into oxygen (O2), facilitating the reduce the hypoxic circumstances of wounds. The nanocomposite hydrogel also possesses a notable photothermal antibacterial consequence in NIR light. These potentials propose that the nanocomposite hydrogel has improved and is expressively probable for clinical therapy in treating diabetic wounds, Figure 8B.[105]
A study created an oxygen-generating matrix, CaO2 NPs, within a temperature-sensitive hydrogel loaded with DHM, resulting in DHM-OTH. This composite demonstrated its ability to maintain a suitable oxygen-rich environment conducive to cell survival around wounds and showcased excellent biocompatibility and a range of biological functions. By developing a T2D wound model, Liu et al. explored the therapeutic potential of DHM-OTH in treating chronic diabetic skin wounds and the underlying healing mechanisms. The application of DHM-OTH decreased inflammatory cell presence and collagen accumulation while enhancing angiogenesis and cellular proliferation, thereby facilitating the healing of diabetic wounds. Both in vitro and in vivo studies indicate that DHM-OTH is an innovative approach for effective oxygen delivery to wound areas, offering a promising avenue for enhancing the healing process of diabetic wounds.[266] The metformin (Met)-infused CuPDA NPs composite hydrogel (Met@CuPDA NPs/HG) was engineered through the dynamic phenyl borate linkage of dopamine-modified gelatin (Gel-DA) and HA that was modified with phenyl boronate acid (HA-PBA), incorporating Cu-loaded PDA NPs (CuPDA NPs). This hydrogel showcases exceptional properties like injectability, self-healing, adhesiveness, and DPPH radical scavenging capability. The controlled release of Met is facilitated through its interaction with CuPDA NPs, boric groups (via B–N coordination), and the physical constraints of the hydrogel network, enabling a slow-release pattern. This release mechanism is intelligently designed to respond to varying pH and glucose levels, tailoring the treatment to different wound environments. Additionally, the CuPDA NPs grant the hydrogel outstanding photothermal responsiveness, allowing for over 95% bacterial eradication within 10 min and a prolonged release of Cu2+ ions for extended protection against infection. The hydrogel also directs cell recruitment and fosters vascularization by releasing Cu2+. Crucially, it reduces inflammation by scavenging ROS and blocking the activation of the NF-κB pathway. Animal studies have shown that Met@CuPDA NPs/HG significantly enhance wound healing in diabetic Sprague Dawley (SD) rats by eliminating bacteria, reducing inflammation, boosting angiogenesis, and speeding up the deposition of ECM and collagen. This positions Met@CuPDA NPs/HG as an up-and-coming candidate for treating diabetic wounds.[285] The development of multifunctional hydrogel presents a significant advancement in diabetic wound healing. By ingeniously combining the properties of thermo-responsive polymers, peptide-functionalized gold nanorods, and a bilayer structure, this hydrogel offers a targeted, sequential release mechanism activated by NIR light. The dual-phase release strategy ensures an immediate antibacterial response followed by a subsequent promotion of angiogenesis, addressing the complex needs of chronic diabetic wounds. The hydrogel's design prioritizes effective infection control through its antimicrobial properties and enhances tissue regeneration by supporting angiogenesis and collagen deposition. The synergy of these features within a single biomaterial platform demonstrates a promising potential for improving outcomes in diabetic wound care. This hydrogel represents a leap in biomaterials research, opening new avenues for developing smart, responsive therapies that adapt to the evolving needs of wound healing processes.[286] Developed a thermo-responsive hydrogel that adjusts to wounds, composed of SA, Antheraeapernyi silk gland protein (ASGP), and poly (N-isopropyl acrylamide) (PNIPAM), creating a self-modifying microenvironment for intelligent drug release and skin regeneration enhancement. PNIPAM's temperature sensitivity allows the hydrogel to contract and release drugs and water molecules when temperatures exceed a certain threshold, providing smart drug delivery. ASGP's inclusion boosts biocompatibility and gives the hydrogel adhesive properties. Our in vitro studies show the hydrogel's excellent biocompatibility, including its promotion of human skin fibroblast cells' adhesion and growth. This research introduces an innovative, thermo-responsive hydrogel method for enhancing wound healing by autonomously adjusting the wound's microenvironment.[287] Created a multifunctional OGF hydrogel, integrating ODex, GA-grafted gelatin (GAG), and Ferric ion, tailored for treating infected wounds in diabetic mice. This hydrogel features double-crosslinking through dynamically formed Schiff-base bonds between Odex's ALD and GAG's amino groups and metal coordination bonds between ferric ions and the polyphenol or carboxyl groups in GAG. These crosslinking mechanisms grant the OGF hydrogel properties such as injectability, self-healing, and adhesiveness. Leveraging the Ferric ion/polyphenol chelate's potent photothermal effect, the hydrogel efficiently eradicates S. aureus and E. coli under NIR light within just 3.5 min. Additionally, hydrogel showcases excellent antioxidant capabilities, promotes hemostasis, and is biocompatible. Notably, it significantly sped up the healing of S. aureus-infected diabetic mouse wounds, achieving complete re-epithelialization in 18 days by eradicating infections, reducing oxidative stress and inflammation, and enhancing angiogenesis. This multifunctional hydrogel thus shows substantial promise for clinical application in diabetic wound management.[288] Poloxham 407 (25 wt%) was used by Heilmann et al.[289] as a morphine carrier for managing serious skin wounds. This hydrogel dressing delivers medicine continuously, preventing frequent dressing changes and relieving pain for up to a day. Of greater significance, temperature-responsive hydrogel dressing can control both the ambient temperature and the skin's temperature. Despite its apparent simplicity, there are severe temperature requirements for the manufacturing, shipping, and storage processes. As a result, it's important to consider both physiological (such as age) and environmental (such as area or season) elements when calculating temperature variations.
6.2.2 Photo-responsive hydrogels
Photoresponsive hydrogels comprise a polysaccharide matrix integrated with a photoreactive component that acts as an effective photochromic chromophore. Upon exposure to light, this photochromic agent undergoes a structural transformation or induces rearrangement in nearby molecules, leading to changes in the hydrogel's physical or chemical characteristics. These light-sensitive hydrogels have significantly facilitated wound healing, particularly in treating DFUs. In their research, Zhao et al. showcased the creation of supramolecular hydrogels from polysaccharides using host-guest interactions to deliver EGF directly to the wound site.[290] EGF enhances collagen accumulation, encourages keratinocyte movement, and promotes re-epithelialization.[291] Traditional hydrogel-based drug delivery systems fail to control EGF's precise release at the injury site. Therefore, developing light-responsive hydrogels could offer a refined approach to administering EGF. In their study, the team introduced a system combining photo-isomerizable azobenzene, capable of existing in cis and trans configurations, with β-cyclodextrin (CD), which forms host-guest interactions. These interactions are modifiable through UV light exposure, disrupting the host-guest affinity via forming the cis isomer, which reverses upon removing the UV light exposure. This innovative mechanism was embedded in an HA-based hydrogel, resulting in a material that transitions from a stiff to a softer gel state under UV light, demonstrating a changeable crosslink density and UV responsiveness, as illustrated by variations in its storage modulus.
Moreover, the EGF-loaded light-responsive supramolecular hydrogel (EGF@PR-S gel) released significantly more EGF upon UV light exposure compared to a non-responsive control gel (EGF@S gel), displaying superior biocompatibility, negligible cytotoxicity towards L-929 fibroblast cells, and minimal hemolysis against red blood cells. When applied to full-thickness wounds in rats, the gel enhanced EGF delivery through light activation and notably improved healing rates, evidenced by faster wound closure and elevated transforming growth factor beta-1 levels at the wound site. These findings underscore the potential of such polysaccharide-based photo-responsive hydrogels in advancing wound care.[290] Creating an innovative in situ injectable hydrogel that integrates photo-crosslinking capabilities and is embedded with Ag@ZnO NPs forms a cutting-edge approach to wound care. This hydrogel is remarkable for its quick gelation upon exposure to 395 nm UV light, taking just five seconds, allowing it to conform perfectly to the contours of any wound surface. Once applied, the hydrogel releases silver and zinc ions, initiating the healing process. A key feature of this hydrogel is the heterojunction between silver and zinc oxide NPs, which facilitates the absorption of oxygen and water, thereby catalyzing the production of a potent burst of ROS. This ROS generation is crucial for its antibacterial action, disrupting bacterial cell walls and causing the release of their contents.
Additionally, it fosters wound healing by activating key factors like VEGF and CD31, which are essential for angiogenesis. The sustained release of silver and zinc ions and ROS synergizes to combat bacteria, reduce inflammation, encourage cell growth, and enhance collagen formation and new blood vessel development.[292-294] These combined actions significantly quicken the healing process of chronic diabetic wounds, offering a promising new direction in treating such conditions.[295] For example, Mao et al. combined Ag/Ag@AgCl and ZnO within a hydrogel to make a gel that reacts to light acquires antibacterial characteristics, and accelerates wound healing. In this framework, the hydrogel's design enhances the exploitation of Ag/Ag@AgCl for the improved production of hydroxyl radicals upon light exposure. Also, this system takes gain of the natural pH variations in bacterial microenvironments to offer a combined antimicrobial result while increasing immune responses. This is accomplished through the adjusted and prolonged dispersal of Ag+ and Zn2+ ions over 21 days, Figure 9A.[296]
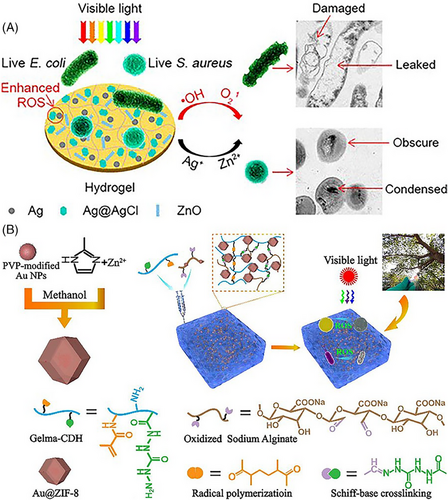
The PAG-CuS hydrogel is a state-of-the-art, photothermally responsive material created for comprehensive wound care, promoting healing, providing anti-inflammatory benefits, and facilitating photothermal disinfection. It's crafted through the copolymerization process combining AA, GelMA, and lipoic acid sodium-coated copper sulfide NPs. This results in a porous, adaptable structure supporting cell attachment and effectively retaining moisture. The embedded CuS@LAS lends the hydrogel its photothermal antibacterial properties and is a physical cross-linker, enhancing its mechanical strength. When irradiated with NIR light, the hydrogel exhibits a photothermal reaction, triggering the release of CuS@LAS, dispersing lipoic acid sodium as it disassembles, targeting and neutralizing ROS within cells. This significantly decreases the expression of MMP-9 and promotes the synthesis of the ECM, contributing to improved wound healing dynamics.
Furthermore, the liberation of Cu2+ ions from the hydrogel fosters CD31 expression in endothelial cells, stimulating the formation of microvessels essential for wound healing. Tested on diabetic GK rats, the PAG-CuS hydrogel demonstrated its ability to lower ROS levels, increase microvessel density, improve epithelial regeneration, and accelerate the wound healing process. Thus, this innovative photothermal hydrogel presents a versatile, all-encompassing approach to addressing the complex challenges associated with diabetic wound care.[283] In wound care, particularly for chronic wounds necessitating long-term and recurrent dressing changes, there's a substantial demand for a dressing solution that can be managed easily without direct contact. A groundbreaking advancement in this area is the development of an all-light-operated hydrogel dressing designed to facilitate rapid and remotely controllable dressing changes. This innovative dressing can transition to a gel state within 30 seconds and dissolve within 4 min upon exposure to light, streamlining the process of chronic wound management. Utilized in a diabetic murine model, this dressing has significantly expedited wound healing within 2–3 weeks, primarily by reducing secondary damage typically caused by frequent dressing replacements.
Additionally, it has demonstrated a remarkable capacity to enhance the wound healing process, including improved epithelialization, increased collagen deposition, heightened cell proliferation, and effective inflammatory regulation. These findings highlight the hydrogel dressing's synergistic approach to improving therapeutic outcomes for chronic wounds, showcasing its potential as a highly efficient and innovative solution in wound management.[298] The valuable metal gold was combined into zinc-based metal-organic frameworks to establish an Au@ZIF composite, which reformed the ZIF's light fervor from ultraviolet to the visible spectrum. To increase its effectiveness, Au@ZIF was merged with sodium oxide alginate and carbohydrazide-modified gelatin methacrylate, leading to the development of the Au@ZIF@GCOA. This ground-breaking mix, engaging both Au-induced surface plasmon resonance and Schottky junctions at the edge of the metal-MOFs within the Au@ZIF@GCOA hydrogels, led to amplified ROS generation upon contact with visible light (wavelengths over 400 nm). Accordingly, these developments significantly supported the hydrogels' abilities for antibacterial activity, decreasing inflammation, and endorsing wound healing, Figure 9B.[297]
6.2.3 Electro/conductive responsive hydrogel
Electro-responsive hydrogels, composed of conductive materials that deform when subjected to electrical stimuli and combined with polysaccharides, are emerging as significant materials within skin tissue engineering and the creation of artificial skin. This area of research is becoming increasingly important as it explores the potential of these hydrogels in therapeutic applications and medical advancements.[299, 300] Moreover, the therapeutic potential of these hydrogels in treating diabetic wounds has been observed, given their capability to boost cellular growth and movement, which are pivotal to the healing process.[301, 302] Liu et al. achieved a considerable breakthrough by developing a conductive hydrogel wound dressing that combines polymerized ionic liquid with konjac glucomannan. This innovation addresses the challenges associated with conventional ES in wound treatment.[303] This ES technique boosts fibroblast proliferation and migration alongside increased ECM secretion but often fails to reach the entire wound area, thus limiting healing effectiveness. To tackle the limitations of traditional ES in wound care, the study introduced an electro-responsive hydrogel made of konjac glucomannan, which was enhanced with polymerized ionic liquids. A specific ionic liquid, [1-vinyl-3-(3-aminopropyl)-imidazolium tetrafluoroborate], was copolymerized with acrylamide to form a stable polymer network. This polymer was subsequently aminated and linked to the carboxyl group of AA grafted onto KGM. By adjusting the molar ratios of the ionic liquid to KGM, they created hydrogels that were not only highly conductive and capable of significant swelling but also exhibited substantial mechanical strength. These hydrogels demonstrated conductivity between 0.2 and 0.8 mS/cm and biocompatibility with minimal cell death and hemolysis. In vivo, tests on full-thickness wounds in Kunming mice revealed that these electro-responsive hydrogels significantly expedited healing, underscoring their potential in wound healing applications.[303] A groundbreaking flexible conductive hydrogel dressing endowed with inherent ROS-scavenging capabilities and electroactivity has been engineered to treat and monitor wounds. This innovative antioxidant hydrogel, when used in conjunction with ES, has been shown to enhance the healing of chronic diabetic wounds significantly. It achieves this by effectively managing oxidative stress, reducing inflammation, and encouraging re-epithelialization, angiogenesis, and collagen synthesis. Remarkably, in addition to its therapeutic benefits, this hydrogel exhibits excellent mechanical properties and conductivity, enabling it to also serve as a tool for monitoring potential stresses at the wound site. This dual functionality positions the hydrogel as an “all-in-one” bioelectronic solution, combining treatment and monitoring capabilities, which holds great promise in advancing the care and recovery process for chronic wounds (Figure 10A).[304]

A sophisticated multifunctional electronic skin, E-skin, has been developed using a unique hydrogel called PPMAg. This hydrogel integrates a specially engineered MXene nanocomposite, PMAg, into a poly(acrylamide-co-sulfobetaine methacrylate) matrix. The PMAg nanocomposite stands out for its unique heterostructure, with AgNPs anchored onto PDA-coated MXene nanosheets. The PPMAg-based E-skin possesses wide-ranging functionality. It offers a broad spectrum of operation, high sensitivity, excellent reproducibility, quick response times, and outstanding durability. These properties allow the E-skin to precisely monitor diverse human motions, facial expressions, and real-time heartbeat signals in vivo.
Furthermore, this E-skin has the added functionality of facilitating diabetic wound healing when applied with ES. Such multifaceted utility underscores the potential of this newly developed E-skin system to act as a foundational element for future generations of flexible health management solutions, marking a significant advancement in the field of wearable healthcare technologies.[306] When shown to a magnetic field, magnetic NPs can perform as micro-nanorobots that automatically constrain. Sun et al. constructed a new magnetic hydrogel micromachine via PNIPAm for the gel matrix with a combination of Fe3O4 NPs and NdFeB microparticles. This proposed hydrogel works in three main antibacterial ways. First, it can transition between two kinds of movement, flat spinning, and oscillation, which can be keenly controlled through an exterior magnetic field to split bacterial biofilms mechanically. Secondly, the MHM hydrogel forces the magnetocaloric prompt from the magnetic field to create located heating, permitting heat-responsive PNIPAm to execute the H2O2 that is loaded with antibacterial mediators. Finally, Fe3O4 NPs within the hydrogel perform as nanocatalysts for the Fenton reaction, producing hydroxyl radicals (·OH) recognized for their strong antibacterial properties. Together, this hydrogel responsive to magnetic fields proposes an innovative and deliberate means for addressing infections linked with biofilms, particularly in confined environments, Figure 10B.[305]
A novel self-powered wound dressing has been developed and equipped with a unique electric field (EF) “Lock-ON/OFF” mechanism for controlled, on-demand release of hydrophilic antibiotics, significantly enhancing infected wound repair. This dressing intelligently releases drugs under mechanical stress, with an 88.57% cumulative release rate, dramatically higher than the 0% release in its absence. It utilizes a piezoelectric effect to regulate drug release and reshape the wound's EF, thus speeding up healing. Combined with ES therapy, this approach yields a 1.26-fold faster healing rate, heralding a breakthrough in automated wound care and precision medicine.[307] Composite hydrogels made from gelatin and CMC, enhanced with aloe vera juice for anti-inflammatory effects and crosslinked with glutaraldehyde, were developed and studied for their potential in wound care. These hydrogels demonstrated excellent elasticity, self-healing capabilities, and a shear-thinning property for skin application. The addition of aloe vera improved their structure, mechanical strength, and swelling behavior, closely mimicking human skin's compressive strength.
Furthermore, they showed high biocompatibility with HFF-1 cells and effective antibacterial action against E. coli and S. aureus. Drug release studies using lomefloxacin indicated a fast initial release, continuing effectively over 12 h with a release rate above 20%. This suggests these gelatin-based hydrogels could be highly effective as wound dressings.[308]
6.2.4 Bacteria-responsive hydrogel
Microbial colonization, a common issue in the progression of DFUs, leads to extended inflammation, infection spread, increased wound size, and delayed healing, necessitating antibiotics or anti-infective agents. However, due to the challenges associated with systemic antibiotic therapy, localized delivery methods are preferred for effectively treating DFUs. Researchers have thus explored drug delivery systems that activate in response to bacterial presence. Cheng et al. developed a bacterial infection-responsive hydrogel that is breathable, moisture-permeable, and capable of absorbing wound exudates. This hydrogel, combining quaternized CS, photothermal antibacterial NPs, and a specific PEG formulation, demonstrates rapid gelation, is sprayable, has photothermal properties, and has significant antibacterial activity, particularly when exposed to a bacteria-induced acidic microenvironment. Its photothermal effect under xenon light further disrupts bacterial membranes, enhancing healing in vivo, Figure 11A.[309]
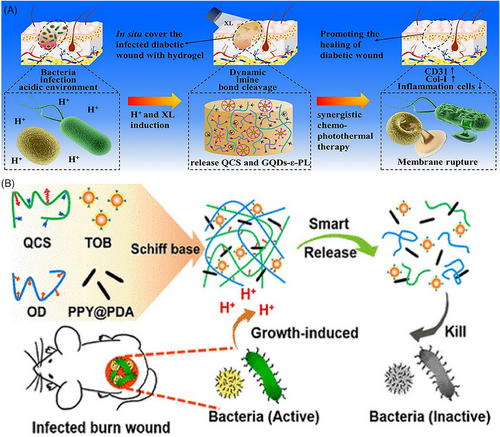
Similarly, Huang et al. created a hydrogel that releases antibacterial agents in response to the acidic conditions produced by bacterial growth. This hydrogel, made with ODex, quaternized CS, PDA-coated polypyrrole nanowires, and tobramycin, offers a targeted bactericidal effect that accelerates wound healing Figure 11B.[217] These advances suggest promising directions for treating DFUs and controlling microbial infections through responsive hydrogel systems.
6.2.5 Ultrasound-responsive hydrogels
Ultrasound, known for its simplicity and convenience as a physical stimulus, can be utilized in low-frequency (20–100 kHz) and high-frequency (20.7 MHz) ranges to trigger controlled drug release. Its appeal lies in its non-invasive approach, ability to penetrate tissues deeply, and precise control over time and space, making ultrasound-responsive wound dressings a growing interest.[310] Applying ultrasonic energy can disrupt the molecular arrangement by breaking intramolecular hydrogen bonds or π-π interactions, leading to a reorganized intermolecular structure. This capability allows for manipulating hydrogel materials with ultrasound, facilitating rapid gelation and promoting the formation of a uniform, entangled, three-dimensional fiber network.
A novel approach introduces the development of a flexible ultrasonic patch to treat chronic wounds effectively. This patch incorporates piezoelectric ceramic elements, organized linearly, and mounted on a flexible circuit board. A dual-purpose thin hydrogel layer encodes the device and facilitates ultrasound transmission, preventing wound infection while ensuring deep tissue penetration of the ultrasonic waves. The design of the patch allows it to be soft, lightweight, and fully adaptable to the contours of the wound area. An innovative feature is the patch's ability to focus ultrasound beams on the center of its bending radius, enabling precise targeting of the treatment zone. Experimental application of this ultrasonic treatment on type-II diabetic rats demonstrated its efficacy; immunohistochemical analysis revealed that ultrasound expedited wound closure by activating Rac1, a signaling molecule in skin tissue. Comparatively, wounds treated with the ultrasound patch healed significantly faster, with recovery times reduced by approximately 40%, underscoring the potential of this technology in advancing chronic wound care.[311] A pioneering antibacterial strategy utilizing stanene nanosheets has been created. These SnNSs possess a characteristic nanosheet architecture and are remarkable for their capacity to produce ROS upon ultrasound exposure. By integrating these nanosheets with a thermosensitive polymer, specifically poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide), a new composite material named Sn@hydrogel has been formulated. This Sn@hydrogel exhibits potent sonodynamic antibacterial properties, significantly enhancing wound healing. In vivo, tests on a full-thickness wound model infected with MRSA demonstrated that Sn@hydrogel effectively combats infection and promotes wound recovery. Utilizing stanene nanosheets within a hydrogel opens a new pathway for effectively minimizing bacterial infections and facilitating wound healing through sonodynamic action.[312] An innovative multifunctional hydrogel has been crafted to treat bacterial wound infections using a novel ultrasonically induced piezocatalytic method. This hydrogel contains dispersed barium titanate (BaTiO3, BT) NPs, which, when ultrasonic energy is applied, generate ROS through a powerful intrinsic electrical field, giving the hydrogel exceptional antibacterial qualities. This innovative treatment surpasses traditional photodynamic therapy by offering deeper tissue reach and bypassing potential skin phototoxicity that can occur with the systemic introduction of photosensitizers. The hydrogel's blend consists of N-[tris(hydroxymethyl)methyl] acrylamide, N-(3-aminopropyl) methacrylamide hydrochloride, and oxidized HA. These ingredients confer notable self-healing and bioadhesive functionality, essential for recuperating full-thickness skin wounds. The adhesive ability of this hydrogel is more effective than mussel-inspired adhesives, attributable to its robustness against oxidation and its capacity for repeated use, supported by the numerous hydrogen bonds created by its tri-hydroxyl structure. The hybrid hydrogel places BT NPs directly at the wound site, focusing on targeted delivery. Ultrasound activation triggers precision piezoelectric catalysis to combat and remove bacterial contaminants. This technique underscores the significance of therapeutic safety and marks a considerable leap forward in the noninvasive management of wounds plagued by bacterial infections, Figure 12A.[313] An innovative nanozyme hydrogel spray has been engineered to tackle multiple challenges of diabetic foot ulcer treatment, including reducing inflammation, alleviating hypoxia, controlling blood glucose, and promoting angiogenesis. This multienzyme-like hydrogel, activated by ultrasound and the ulcer's microenvironment, has demonstrated in-vitro its efficacy in accelerating wound healing, presenting a promising all-in-one therapy for DFUs, Figure 12B.[314]
7 DUAL-STIMULI RESPONSIVE HYDROGEL DRESSING
Diabetic wounds, due to their complex nature, often require more than single-responsive treatments for effective healing. This has led to the development of multi-responsive hydrogel dressings, which cater to various stimuli simultaneously, enhancing the precision and effectiveness of drug delivery. Such dressings synergistically combine different therapeutic actions, offering superior results compared to single-modality treatments. For instance, Guo et al. pioneered a hydrogel responsive to both pH and ROS, designed to counteract the adverse microenvironment of stubborn wounds by incorporating Schiff base bonds and ROS-responsive micelles.[218] This hydrogel degrades more rapidly in environments rich in H2O2 and acidity than in neutral phosphate-buffered saline solutions, facilitating the phased release of therapeutic agents for effective wound care.
Addressing diabetic wounds complicated by multidrug-resistant bacterial infections remains a formidable challenge, exacerbated by the infections' resilience, high recurrence, and significant mortality rates. Wang et al. introduced an innovative FeMI hydrogel through a Schiff group reaction, offering a pH/redox dual-responsive system for insulin release, markedly enhancing healing by reducing inflammation and encouraging tissue regeneration.[75] Furthermore, using stimuli such as alternating magnetic fields and NIR radiation presents unique advantages, including deep tissue penetration and the capacity for high-sensitivity remote activation.[315-317] A notable advancement in this area is the work of Yang et al., who developed a hydrogel system incorporating MXene-wrapped magnetic colloids within a temperature-sensitive polymer network. This system uniquely controls the release of therapeutic agents, like AgNPs, through temperature-induced contractions in response to external magnetic or infrared stimuli, demonstrating significant potential for chronic wound treatment.[248] Paeoniflorin (Pf)-coated micelles and antibacterial zinc oxide NPs (nZnO) were added to pH/ROS-responsive hydrogels.[218] Gelatin modified with ethylenediamine and ODex forms a dynamic Schiff base that enables the pH responsiveness of hydrogels. Strong hemostatic properties are exhibited by gelatin alone, but Pf is an angiogenic substance found in micelles formed of the amphiphilic polymer DSPE-TK-PEG2k-NH2. Thioketal (TK) has a mercaptan group, which gives the micelles their ROS sensitivity. Because of this, the hydrogel dressing not only improves hemostasis but also makes it possible for antibacterial and angiogenic drugs to be released one after the other, greatly hastening the diabetic wound healing process in diabetic rats. The antibacterial polypeptide ε-polylysine comprises catechol groups that form boron ester connections and amino groups that form Schiff base bonds. Schifbase and boron ester linkages are two dynamic covalent bonds forming hydrogels using oxyglucan grafted with PBA and ε-polylysine (CE) grafted with cafeic acid.[49] The anti-inflammatory medication diclofenac sodium and the angiogenic medication mangiferin (MF)-containing pH-responsive micelles were put into the hydrogels. MF is administered in a slow and sustained manner to enhance angiogenesis. In contrast, DS and CE are delivered rapidly to foster antibacterial environments and minimize inflammation at the diabetic wound site, characterized by acidic pH and elevated ROS levels. In diabetic rats, such precisely timed and localized drug delivery could expedite wound healing.[7] Zhao et al.[247] developed a hydrogel dressing that responds to pH and glucose levels for the treatment of diabetic wounds, featuring benzoic-imine linkages and phenylboronate ester groups to deliver insulin along with fibroblasts. Fibroblasts aid in angiogenesis through the production of ECM components and the release of growth factors. In the acidic and glucose-rich environment of diabetic wounds, insulin is continuously released, which promotes healing by activating insulin signaling pathways. The in vivo studies demonstrated that insulin/L929 hydrogel dressings improved wound healing, neovascularization, and collagen formation in diabetic rats with streptozotocin-induced diabetes. Additionally, L-arginine is suggested to assist in the healing of diabetic wounds by encouraging insulin release. In treating type II diabetes, Met is a first-line medication that can improve local cell sensitivity to insulin; however, its oral bioavailability is restricted, and its half-life is brief.[247] A multipurpose hydrogel dressing called PC/GO/Met was created and has conductive, adhesive, antimicrobial, and antioxidant qualities. For instance, the hydrogel can achieve both targeted drug release and good removal ability due to the Schiff-base and phenyl borate bonds produced between PEGS-PBA-BA/CS-DA-LAG (PC) and their capacity to respond to pH and glucose at the wound site. The quality of regenerated skin in type II diabetic SD rats treated with PC hydrogel was superior to that of the control group. The regenerated skin displayed increased angiogenesis, abundant granulation tissue, increased collagen deposition, and better hair follicle regeneration.[48] To combat bacterial infection at the wound site, Haidari et al. developed a hydrogel that reacts to changes in pH and temperature. This hydrogel was crafted by cross-linking N-PNIPAM with AA and incorporated ultrafine AgNPs. The copolymerization of N-PNIPAM with AAc resulted in a lower critical solution temperature of 36.5°C, allowing the hydrogel to transition at a temperature close to the human body's natural warmth. AAc, being a weak acid, enables the hydrogel to expand as pH levels rise, which allows for the gradual release of AgNPs. In vivo studies demonstrated that this PNIPAM-PAA-AgNPs hydrogel could discharge Ag ions as required, achieving a substantial antibacterial effect for treating rat wounds infected with S. aureus. This advancement holds promise for the management of chronic wounds and therapeutic innovation.[318] The block copolymers Pluronic F127, composed of PEG-PPG-PEG structures, also possess a distinctive reverse thermal gelation property. Their aqueous solutions, at concentrations of 20–30% w/w, remain liquid at temperatures as low as −4°C but transform into a gel at room temperature.[46] To create injectable FHE hydrogels (F127/OHA-EPL) that deliver adipose-derived mesenchymal stem cell-exo in response to weakly acidic environments, synthesized oxide HA, which forms Schiff-base bonds with poly-ε-L-lysine (EPL) and added thermally responsive F127. Mice wound healing was sped up, and granulation tissue development was promoted by this multifunctional hydrogel with regulated EXO release.[178] Liu et al. created a system where CUR NPs, generated through the self-assembly of CUR, were encapsulated within gelatin, referred to as CNPs@GMs.[182] In response to injury, gelatin reacts to elevated levels of MMP-9 by heightening the concentration of CUR. Integrating CNPs@GMs with the thermoresponsive hydrogel F127 creates a dual-responsive hydrogel dressing, enabling time- and temperature-regulated drug delivery. This hydrogel patch is designed to selectively release CUR at the wound site, optimizing the therapeutic efficacy of the drug while minimizing cellular damage. Consequently, this promotes restoring the skin structure and function in diabetic mice wounds. An MXene-based hydrogel was selected to encapsulate Fe3O4@SiO2 magnetic NPs (MNPs@MXene), which were then included in a PNIPAM-alginate dual network hydrogel. AgNPs were added to bolster the hydrogel's antimicrobial capabilities. The MNPs can be periodically activated by an external alternating magnetic field or by exposure to NIR light, generating heat that causes the hydrogel to warm, shrink, and rapidly release drugs. Experimental studies on the release of AgNPs indicate that this MXene-based hydrogel is capable of precise and controlled drug release when stimulated externally. The NIR-stimulated intelligent drug release hydrogel effectively treats deep-seated wound infections.[248] In a pioneering development, PVA CS/SA CUR hydrogels, denoted as PCSA hydrogels were formulated, demonstrating outstanding performance via the Michael addition reaction between CS and CUR. These PCSA hydrogels possess dynamic bonds that afford them superior mechanical strength, showcased through a tensile stress of approximately 0.980 MPa, a toughness of around 258.45 kJ/m3, and a compressive strength of about 7.38 MPa under 80% strain. These mechanical features contribute to an optimal cellular microenvironment that supports cell movement and division and prompts early blood vessel formation. Significantly, the PCSA hydrogels have demonstrated an ability to convert M1 macrophages, which are pro-inflammatory, to M2 macrophages, which exhibit anti-inflammatory properties in vitro, without the need for other agents. When applied to full-thickness diabetic wounds in rats, the hydrogels significantly improved healing by scavenging ROS, diminishing IL-1β levels, and augmenting CD31 expression. This facilitates angiogenesis and collagen production. This advancement not only proposes a simple and secure CUR-infused hydrogel for diabetic wound management but also highlights the potential of incorporating ingredients from traditional Chinese medicine into innovative biomaterials designed for diabetic wound treatment.[319] Designing stimuli-responsive smart hydrogel dressings for bacteria-infected diabetic wounds remains a significant challenge, particularly in developing materials that can effectively respond to external stimuli or the wound microenvironment throughout all stages of healing. Recent studies have highlighted the roles of NO and oxygen (O₂) in enhancing diabetic wound healing by promoting angiogenesis and alleviating hypoxia. To address these challenges, innovative NIR/glucose stimuli-responsive multifunctional smart hydrogels with dual NO/O₂ gas-releasing capabilities have been developed. These hydrogels are composed of N-carboxyethyl CS-grafted-PBA (CECP), ODex, ε-polylysine (EPL)-coated MnO2 (EMn), and CD-reduced graphene oxide/N,N′-di-sec-butyl-N,N′-dinitroso-1,4-phenylenediamine (rGB) through dual dynamic crosslinking.The incorporation of rGB and EMn as photothermal agents enables the hydrogels to effectively eliminate bacteria via PTT. Furthermore, rGB facilitates NO release under NIR stimulation, which promotes angiogenesis, while the nanozyme EMn, exhibiting catalase (CAT) and superoxide dismutase (SOD)-like activities, scavenges ROS (ROS) and releases O₂ to counteract hypoxia and oxidative stress. The PBA in CECP forms a phenylboronate ester structure that allows glucose-controlled release of doxycycline, enhancing targeted therapy. In full-thickness skin wound models of bacteria-infected diabetic wounds, these hydrogels demonstrated significant benefits, including reduced inflammation, enhanced wound contraction, improved collagen deposition, stimulated angiogenesis, and alleviation of hypoxia. These multifunctional hydrogels offer a promising and innovative approach to the treatment of complex bacteria-infected diabetic wounds, highlighting their potential as advanced therapeutic strategies in wound care, Figure 13A.[320]
Dynamic multifunctional hydrogels were crafted by uniting gelatin with 5-carboxy 3-nitrophenyl boronic acid and Epigallocatechin gallate to create a boronic acid-linked network. These hydrogels are engineered to solidify at a pH level of 7.4 quickly. The design of this hydrogel system enables the electrostatic interaction between EGCG and positively charged antimicrobial peptides, allowing for their effective embedding within the hydrogel matrix. These injectable hydrogels can adhere to skin defects, offering a protective barrier and immediate hemostatic effects for diabetic patients. Particularly effective in high glucose conditions, they release AMPs swiftly to combat bacterial presence. In contrast, EGCG is released responsively to neutralize ROS and encourage the transition of macrophages towards an anti-inflammatory M2 phenotype. The hydrogel displays outstanding biocompatibility and degradability, completely disintegrating within three days after being injected subcutaneously, and it shows no signs of toxicity. This is confirmed through H&E staining of crucial organs and serum liver function assessments conducted in mice. As a multifunctional injectable hydrogel, it marks a considerable breakthrough in treating diabetic skin wounds, offering a promising option for meticulously treating such wounds.[323] The presented study details a versatile hydrogel consisting of catechol-modified CS and ODex, referred to as CDP-PB. This hydrogel demonstrates antibacterial, antioxidant, and pro-angiogenic properties, which are beneficial for improving the healing of diabetic wounds. It achieves an impressive antibacterial efficiency of 99.9% by utilizing the synergistic effects of PBA-modified polyethyleneimine and the photothermal properties of PDA NPs that contain the NO donor BNN6 (PDA@BNN6). The hydrogel excels against MRSA, inactivating around 3.6 log10 CFU/mL when exposed to an 808 nm NIR laser light. Chi-Ca's role in the hydrogel combats oxidative stress by undergoing Schiff-base reactions with Dex-CHO, showcasing its competence in neutralizing a wide array of radicals (>85%). Cell-based experiments indicate that CDP-PB hydrogel promotes cell migration and proliferation, particularly under stress conditions. Meanwhile, animal model research validates the hydrogel's efficacy in eradicating infections, encouraging the formation of blood vessels, stimulating tissue regeneration, and increasing collagen deposition in wounds. These promising results underscore the CDP-PB hydrogel's substantial promise for diabetic wound management in clinical environments, Figure 13B.[321] A groundbreaking double-layered hydrogel, combining TA, CMC, ZIF-90@i-PPOPs, and phenol red, has been developed for its unique stimuli-responsive and antibacterial qualities. The inner layer offers impressive injectability, biodegradability, and antibacterial action by strategically incorporating ZIF-90@i-PPOPs with TA and CMC. The outer layer, made of polyacrylamide and phenol red, is designed for excellent tissue adhesion and real-time pH monitoring of the wound environment. This innovative hydrogel system efficiently delivers antibacterial agents directly to the wound site, enabling effective bacterial elimination and providing a novel means to monitor healing progress with minimal impact on healthy tissue. Its dual functionality positions it as a promising theragnostic tool for treating bacterial infections.[324] Haidari et al. define the formation of a hydrogel that reacts to multiple stimuli, created through crosslinking N-isopropyl acrylamide with AA and integrating enormously small AgNPs, which enables the controlled release of Ag+ ions in response to changes in the wound's microenvironment. The hydrogel shows a high sensitivity to the pH and temperature differences representative of a wound, showcasing a conventional Ag+ ion release at acidic pH levels (less than 5.5) and an improved release at alkaline pH levels (more than 7.4), with over 90% of ions released. The release mechanism, dependent on pH values, illustrates insignificant antibacterial action at pH 4 or 5.5; however, it becomes highly active at pH 7.4 and 10, decreasing over 95% of infection-causing agents. The antibacterial efficacy and safety in live models effectively eliminate S. aureus infections in wounds, remarkably improving the rate of wound closure. This versatile hydrogel exploits a smart delivery system that responds to bacterial presence, aiming to minimize adverse effects and amplify antibacterial activity adapted to the physiological conditions of the wound. It connects significant promise to progress wound infection cures with direct clinical application, fulfilling a strong solution for complete wound care management, Figure 13C.[318]
A groundbreaking approach has been developed for the treatment of diabetic wounds, particularly those complicated by bacterial infections, through the creation of glucose-responsive hydrogels. These hydrogels, formed using TA-modified ceria nanocomposites (CNPs) and a zinc metal-organic framework (ZIF-8) as nodes, are synthesized to enhance mechanical properties and allow for the targeted delivery and glucose/pH-responsive release of medications. The TA@CN gel variant is designed to combat ROS and foster oxygen generation, promoting neovascularization and wound repair. On the other hand, the TA@ZMG gel, infused with GOx, intelligently responds to glucose levels to regulate pH and facilitate the programmed release of Met, contributing to antibacterial action, hair regeneration, and blood glucose control. This innovative nanocomposite hydrogel technology signifies a significant advancement in treating complex diabetic wounds, showcasing the potential for broader biomedical applications.[325] The advanced hydrogel P-LP-PMX-CA-L@E is ingeniously formulated by integrating PDA-grafted MXene, chlorogenic acid, L-ascorbate-2-phosphate trisodium salt (L), and adipose-derived stem cell-derived EXO (E) with PBA modified flaxseed gum. This composition is chemically cross-linked with PVA, forming a hydrogel with dynamic phenyl boronate ester bonds, suitable for precise therapeutic uses. The hydrogel is designed with a ROS/glucose-responsive system that facilitates the release of EXO, effectively lowering ROS levels and inflammatory responses by helping to mend the electron transfer chain. This contributes to skin repair in a mouse model with type I diabetes. The P-LP-PMX-CA-L@E hydrogel's multifaceted construction and its stimulus-triggered exosome release mechanism not only expedite the healing of chronic diabetic wounds but also introduce an innovative, dual-responsive exosome delivery strategy for the treatment of type I diabetes-related wound conditions.[326] An innovatively designed fluorinated peptide hydrogel wound dressing has been developed to regulate the pH and oxygen levels at the wound site, thus fostering a more conducive healing environment. This hydrogel comprises fluorinated peptides with a specific sequence that allows it to assemble into a nanofiber network under slightly acidic conditions (pH = 6.0), like the skin's natural pH. The F5–Pep hydrogel maintains the wound pH within the range of 6.0–7.3 for up to a day and provides a steady oxygen supply for approximately four h. Coagulation tests have indicated that the F5–Pep hydrogel significantly accelerates blood clotting in vitro and effectively stops bleeding in a mouse liver hemorrhage model.
Additionally, cell migration studies in the lab and in vivo experiments have shown that the F5–Pep hydrogel promotes cell movement and greatly enhances the healing of wounds. This novel approach is a step forward in wound care, highlighting the potential for advanced materials in wound healing applications (Figure 13D).[322] A novel all-light-operated hydrogel dressing has been developed, specifically designed for non-contact, easily refreshable applications. It is ideal for managing chronic wounds that require frequent and long-term dressing changes. This innovative dressing can rapidly transition from liquid to gel in 30 seconds and dissolve within 4 min under light exposure, enabling fast and remote-controlled changes. Tested in a diabetic murine model, the dressing significantly enhances wound healing within 2–3 weeks by minimizing secondary damage typically caused by regular dressing replacements. It also effectively promotes key healing processes such as epithelialization, collagen buildup, cell growth, and inflammation control, showcasing the photo-responsive hydrogel dressing's synergistic benefits for therapeutic effectiveness.[298] These advancements underscore the promise of multi-responsive hydrogels in revolutionizing diabetic wound care through targeted, efficient, and multifaceted therapeutic approaches.
8 CONCLUSION AND PERSPECTIVE
Diabetic wounds pose a significant challenge to healthcare providers due to the intricate nature of the wound's internal environment. This complexity arises from issues such as high blood sugar levels, acidic conditions, increased amounts of ROS, an excess of enzymes, and poorly regulated levels of growth and inflammatory factors. These conditions can lead to persistent delays in the wound's inflammation stage and elevate the risk of infection. Among the therapeutic strategies available for diabetic wounds, wound dressing is a key component in the management and healing process. Nevertheless, the static effects of the current hydrogel wound dressings in medicine may lead to maceration in wounds that heal slowly. It is also debatable whether to utilize them as an anti-infection agent. This may prevent infection in diabetic wounds when paired with antibiotics and other medications. Still, the rate of drug release and breakdown cannot be precisely controlled in response to changes in the surrounding environment. In the past few years, advances have been made in creating multifunctional hydrogel dressings for wounds that are sensitive to stimuli. These dressings are engineered to deliver medications in a controlled manner, adjusting to fluctuations in temperature, pH, glucose, ROS, enzymes, or other factors present in the wound area. Such responsive hydrogel dressings do more than dispense medication; they also facilitate the severe conditions of diabetic wounds, aiding overall wound management. Thus, these dressings have the potential to significantly improve clinical diabetes wound treatment outcomes by hastening the healing process.
Additionally, when considering the length of treatment, how often dressings need to be changed, rates of wound recurrence, and similar considerations, the actual cost of employing responsive hydrogel wound dressings could be lower than that of traditional or other currently available medical gel dressings. However, there may still be some pressing issues that need to be resolved before responsive hydrogel wound dressings may be considered clinically used. First, the intelligent drug-release capacity of responsive hydrogels, which serve as a drug delivery system, can be influenced by the pH, glucose levels, or enzyme activity within the wound environment. Given the many reactions smart hydrogels can undergo, evaluating how pH, glucose, ROS, temperature, or enzyme activity changes could alter the hydrogel's drug-release behavior is crucial. Furthermore, stimuli-responsive hydrogel wound dressing must be stable before being applied.
However, other environmental conditions during production, storage, and transportation, including oxidizing agents, enzymes, and temperature fluctuations, may also impact how hydrogel wound dressings behave regarding drug loading, drug release, and gel formation. Additionally, this raises the bar for wound dressing manufacturing, shipping, and storage. Second, before advancing responsive hydrogels to clinical trial phases, it's essential to consider the animal models employed in preclinical studies. Common animal models such as mice, rats, and pigs are utilized to simulate diabetic wounds, and each comes with a set of advantages and limitations that need to be carefully examined in the context of wound healing research. Mice diabetic wound models, for instance, are inexpensive, simple to use, and require little upkeep. Rats may test numerous reagents per animal, although they are larger than mice.
Regarding architecture and physiology, porcine skin is more like human skin but more costly and challenging. An animal model that more closely resembles the human model will have a higher success rate in clinical transformation, even if no animal model can replicate the diabetes wound healing process and alterations. Clinical trials may encounter notable discrepancies in the gel formation time, drug release behavior, and degradation time of responsive wound dressings due to substantial variations in glucose levels, pH values, enzyme expression levels, and other factors in the chronic wound environment of diabetic patients compared to those in experimental animal diabetic wound models. These differences can lead to errors and should be considered when translating preclinical findings into human clinical settings. This may lead to unsatisfactory treatment outcomes.
Furthermore, the timeframe for animal experiments in preclinical research, approximately 2–3 weeks, is shorter than required for wound healing in individuals with diabetes, such as the more than 4-week DFU clinical healing cycle, and is more prone to recurrence. Thus, more research is required to determine how smart hydrogel affects diabetic wound remodeling over the long run. In conclusion, it is best to choose animal models closely resembling human skin structure in preclinical research. Additionally, extending the treatment and observation duration can boost the confidence of clinical trials. Lastly, because the process of wound healing is dynamic, fluctuations in the pH, ROS, glucose level, and other variables near the wound site may impact the healing process. For instance, modest concentrations of ROS exhibit angiogenic and antibacterial properties, whereas excessive concentrations of ROS can damage cells and impede the healing of wounds. The therapeutic application of responsive hydrogel dressings in diabetes patients necessitates a prompt and precise evaluation of the alterations in the wound environment.
Additionally, diabetes patients may have unique wound environments, but they may also experience variations in their wound environments depending on the stage of development. To direct clinical applications, developing trustworthy clinical monitoring indicators is vital. Patients' wound states must also be considered when choosing responsive wound dressings. Clinical tools such as infrared thermometers, pH strips, and glucose monitors can be used to measure temperature, pH, and glucose levels at the site of a wound. The wound dressing is unnecessary for these sensors, and they cannot monitor real-time environmental changes like pH and glucose levels. Numerous research studies have recently developed wound dressings that combine diagnosis and treatment to monitor pH, glucose levels, or wound infections while promoting healing. This is thought to be the future course of development for smart hydrogels. But no single product works flawlessly on every diabetic wound. Thus, to enable more customized wound care for diabetic patients, the development of an external control system and a systematic monitoring system is required. While preparing, storing, and using smart, responsive hydrogels might seem challenging, these advanced materials often yield better outcomes for complex chronic diabetic wounds, such as DFU. Given the potential reduction in wound recurrence, infection risks, and the costs associated with frequent dressing changes, responsive hydrogels could ultimately lessen the overall economic impact of managing chronic diabetic wounds, especially as the population ages and diabetes prevalence increases. The market is steadily growing for intelligent, quality-driven, responsive hydrogels, with a heightened focus on innovative wound care solutions. Therefore, it's anticipated that with the progressive resolution of current challenges, responsive hydrogel wound dressings will increasingly transition into clinical use and emerge as the preferred choice for wound care in diabetic patients and healthcare professionals.
ACKNOWLEDGMENTS
This work is supported by Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars (LR23C100001).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




