Full-color-tunable chiral aggregation-induced emission fluorophores with tailored propeller chirality and their circularly polarized luminescence
Abstract
The regulation of emission color, emission efficiency, and asymmetry factor is of great significance for the real applications of circularly polarized luminescent (CPL) materials. Herein, we develop a modular synthetic strategy toward full-color-tunable CPL materials based on chiral macrocyclic aggregation-induced emission (AIE) luminogens via delicate molecular engineering. Modular synthesis of chiral AIEgens with different acceptor moieties has afforded a series of bright solid emitters with tunable emission colors. These chiral cyclic AIEgens have retained high solid-state emission quantum yield and displayed CPL emission from blue to red as nano-aggregates, in liquid crystal matrix and polymer film. The strong acceptor units in the red-emitting chiral AIEgens RBTPE-2CN and SBTPE-2CN have rendered them twisted intramolecular charge transfer properties and solvatochromic luminescence. And polymer matrix with different polarity further facilitates the tuning of CPL emission color from green to red emission. These results have paved a reliable approach toward constructing full-spectra solid-state CPL material.
1 INTRODUCTION
Chiral luminescent materials and related circularly polarized luminescence (CPL) have attracted tremendous attention and achieved flourishing developments in many areas, such as chiral recognition and sensors, photo-electronic devices, and CPL lasers.[1-5] For chiral luminescent molecules and assemblies, the luminescence dissymmetry factor (noted as glum) is the crucial parameter for evaluating the CPL performance, which represents the difference between left-handed and right-handed emission.[6-8] Considering the brightness of CPL materials in a real application, the pursuit of materials possessing both high glum and emission quantum yield (ΦF) represents one of the most important aspects during the exploration of CPL materials.[9] To achieve this goal, modulating the chemical structure and supramolecular assemblies of emissive cores has been extensively investigated in the past decade.[10-12]
Usually, the fabrication of CPL-active organic materials is realized via the delicate synthesis of single-component chiral chromophores or the construction of chiral assemblies containing luminescent components. In these chiral systems, the chiral elements are either structurally endowed into or peripherally substituted onto the central chromophore or non-covalently introduced into the emitting molecular assemblies via supra-molecular co-assembling.[1] To obtain high ΦF in a solid or aggregate state, luminogens with aggregation-induced emission (AIE) characteristics have been proven to be promising candidates for the emissive moiety in CPL materials.[13-15] Via introducing chiral substituents onto AIEgens skeleton or co-assembling the achiral AIEgens with chiral components, a large library of AIE-active CPL materials has been successfully fabricated.[16-18] In these chiral AIE-active systems, the achiral AIEgen would adopt a preferential helical assembly in the assemblies or solid-state (such as the gel[19, 20] and liquid crystal [LC][21, 22]) under the impacts of chiral substituents or chiral co-assembling moieties.[7, 23] To construct bright CPL system with customized emission color, doping chiral assemblies with tailored dye guests,[24, 25] direct structural modulation on chiral dyes,[26, 27] and energy transfer between different fluorophores[28-32] are the prevailing strategies until now. However, donor-acceptor engineering, which has been extensively utilized to synthesize full-color tunable functional dyes with tailored energy levels and emission color,[33-35] is rarely applied in constructing chiral AIE materials. This is mainly attributed to the lack of a precise and efficient synthetic approach toward chiral AIEgens with defined and stable propeller chirality.
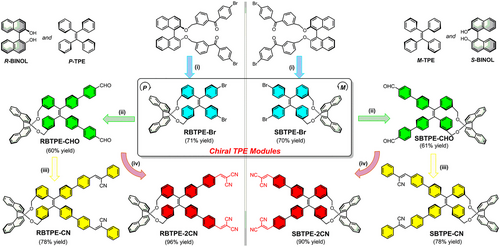
Classical AIE-active luminogens, represented by tetraphenylethenes (TPEs), are usually featured with a propeller-like structure and can adopt a helical conformation with blade-like phenyl rings twisted in one direction.[32, 36-38] However, the P- and M- isomers of AIEgens, in which the phenyl rings are arranged respectively with clockwise and anti-clockwise torsion, usually undergo rapid inter-conversion via ring flipping. Eventually, racemization equilibrium for AIEgens is reached owing to the intramolecular motions, and hence most AIEgens reported are composed of equal amounts of P- and M- isomers which display no chiral optical properties. To obtain optical-pure AIEgens with defined and stable chirality, the propeller-shaped configuration is firstly required to be fixed via delicate molecular design, and then tedious chiral resolutions via chiral chromatography are needed to separate and purify the P- and M- isomers.[39-41] Recently, the commercially available BINOL (1,1′-binaphth-2,2′-diol) moiety with defined axis chirality has been leveraged to tether the helicity of TPE cores via chirality transfer in the macrocyclic skeleton.[42-44] Based on the intramolecular McMurry coupling,[45] our group has readily synthesized a pair of chiral macrocyclic TPE derivatives (denoted as RBTPE and SBTPE) with high yield and stereo-selectivity.[42] The helicity of propeller-like TPE moiety within the macrocyclic backbones is feasibly pre-determined by the chiral BINOL moiety and unambiguously confirmed via single crystal analysis.
Herein, we developed a modular synthesis approach toward full-color-tunable chiral AIEgens based on the chiral macrocyclic building blocks RBTPE and SBTPE with well-defined chirality (Scheme 1). Relative to most reported AIE-active macrocycles, these chiral macrocyclic AIEgens had combined tailored helicity via choosing the appropriate BINOL unit and feasible emission color modulation by delicate donor-acceptor engineering on the TPE unit. Embracing this modular strategy, a series of chiral macrocyclic AIEgens with chiral TPE as the universal electron donor and different electron acceptors had been feasibly synthesized. Identical to most TPE-based derivatives reported,[37, 46] these chiral cyclic AIEgens all retained the AIE property with high emission efficiency in solid and aggregates state and displayed twisted intramolecular charge transfer (TICT) process when decorated with strong electron-withdrawing groups.[47] Ultimately, four pairs of chiral AIEgens exhibited emission color ranging across the visible light spectrum and displayed mirror-imaged circular dichroism (CD) and CPL spectra as nano-aggregates in tetrahydrofuran (THF)-H2O mixture as well as in diverse matrixes. The intermolecular packing and aggregate structures contributed to the signal inversion of CD and CPL spectra for these chiral AIEgens. Among these chiral emitters, the CPL emission of RBTPE-2CN and SBTPE-2CN with malononitrile as an acceptor unit could be further modulated via tuning the structure of the polymer matrix, whereas their CPL color had red-shifted from green to yellow, and ultimately to red via introducing more polar polymers.
2 RESULTS AND DISCUSSION
2.1 Synthesis and structural analysis
First, the chiral TPE synthons, namely the bromide-substituted chiral AIEgens (RBTPE-Br and SBTPE-Br), were readily obtained with a high yield of 70% based on the intramolecular McMurry cyclization of benzophenone substituted chiral BINOL precursor (Scheme 1 and Schemes S1–S3). Based on this pair of chiral building blocks, Suzuki coupling was leveraged to introduce reactive and electron-withdrawing aldehyde groups onto this chiral TPE core in the yield of approximately 60% to afford RBTPE-CHO and SBTPE-CHO with extended π-conjugation. Subsequent Knoevenagel condensation reaction of RBTPE-CHO or SBTPE-CHO with phenylacetonitrile or malononitrile had directly yielded chiral TPEs with stronger electron-withdrawing groups, denoted as R/SBTPE-CN and R/SBTPE-2CN (Schemes S2 and S3).
The chemical identities of these chiral AIEgens were evidently confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectra and mass spectrometry (Figure 1 and Figures S1–S10), whereas all proton signals had been unambiguously assigned with the assistance of 1H−1H 2D correlation spectroscopy and nuclear Overhauser effect spectroscopy. Notably, the proton signals originating from BINOL moiety were independent of the donor-acceptor engineering on the TPE core (Figure 1A). By contrast, after the introduction of acceptor units, the signal originating from the central TPE unit (especially Hf and Hg in Figure 1A) had obviously shifted to a low field relative to RBTPE-Br and SBTPE-Br. This de-shielding effect further confirmed the decreased electron density on the TPE core after the introduction of acceptor moiety.
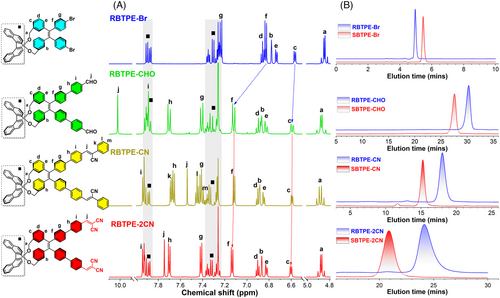
Then, all these chiral compounds were analyzed on chiral high-performance liquid chromatography (HPLC) to evaluate their optical purity and enantiomeric excess (ee) value (Figure 1B). The elementary chiral modules RBTPE-Br and SBTPE-Br showed distinctive peaks with different retention times of 4.96 and 5.46 min in n-hexane/isopropyl alcohol/diethylamine (90/10/0.1, v/v/v) as eluent. The ee values for this pair of chiral compounds were both as high as 99.9%. Additionally, other chiral TPE derivatives also retained high ee values above 90% after stepwise structural modification on the TPE core. The successful synthesis and high ee values of chiral TPEs directly validated the efficiency of this synthetic strategy toward chiral TPEs, and the high tolerance of this strategy to transitional metal or alkaline-catalyzed reactions. Thus, RBTPE-Br and SBTPE-Br were proven to be reliable building blocks for constructing diverse chiral TPE derivatives via molecular engineering.
To elucidate the chiral conformation of these chiral macrocyclic TPE fluorophores, a single crystal analysis was applied to determine their precise structures. Unfortunately, we failed to grow suitable crystals for these four pairs of chiral cyclic AIEgens. Therefore, the precise molecular geometry and inherent chirality of these chiral AIEgens were defined based on the crystal structures of previously reported building blocks RBTPE and SBTPE.[42] As previously reported, the introduction of R-BINOL moiety would induce the P-helicity for TPE fluorophores within the cyclic skeleton, whereas M-helicity was thermodynamically preferred for the TPE core when conjugated with S-BINOL moiety. Owing to the identical chiral cyclic skeleton with RBTPE, P-helicity was more favored and dominant for the TPE moiety in RBTPE-Br, RBTPE-CHO, RBTPE-CN, and RBTPE-2CN, whereas the TPE unit in their counterparts preferred M-helicity. These results were also supported by the theoretical modeling of these chiral AIEgens (Figures S11–S14), which indicated that the P- and M- helicity were thermodynamically more stable and preferential in RBTPE and SBTPE skeleton, respectively.
2.2 Full-color-tunable AIE
Ultraviolet (UV)-visible absorption spectra of these chiral AIEgens were first measured in THF to investigate the influences of donor-acceptor engineering on their photo-physical properties (Figure 2A and Figures S15–S18). The results obviously illustrated a gradually red-shifted absorption band parallel with the raising electron-withdrawing strength, which was also consistent with the UV spectra based on time-dependent (TD) density functional theory (DFT) calculation (Figure S19). And their optical band gap had decreased from 3.38 eV for SBTPE-Br to 3.02 eV for SBTPE-CHO, 2.90 eV for SBTPE-CN, and ultimately to 2.69 eV for SBTPE-2CN. This trend also agreed with the decreased band gap from theoretical calculation, whereas the theoretical band gap had gradually decreased from 3.71 eV for SBTPE-Br to 2.43 eV for SBTPE-2CN (Figures S20–S21). For this series of chiral macrocyclic AIEgens, the highest occupied molecular orbital level located on the BINOL units remained almost unchanged, and the least unoccupied molecular orbital level distributed on the TPE moiety was gradually lowered owing to the introduction of acceptor units.
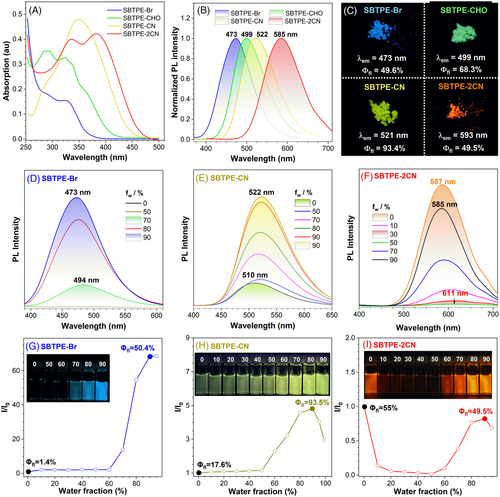
In addition, these chiral AIEgens all exhibited bright and tunable emission from blue to red, as nano-aggregates in the THF-H2O mixture (water faction fw = 90 %, Figure 2B) and as powders (Figure 2C). For instance, SBTPE-Br, SBTPE-CHO, SBTPE-CN, and SBTPE-2CN emitted at 473, 499, 521, and 593 nm in a solid state, respectively, whereas their absolute emission quantum yields (ΦF) in the solid state were as high as 49.6%, 68.3%, 93.4% and 49.5% (Figure 2C). In order to elucidate their photoluminescence (PL) behavior, the PL spectra of these chiral AIEgens in the mixture of THF and water were further investigated (Figure 2 and Figures S22–S26). The results showed that SBTPE-Br, SBTPE-CHO, and their enantiomeric counterparts with opposite chirality were all classical AIEgens (Figure 2D,G and Figures S22–S24). When the water fraction is below 60%, these two pairs of chiral AIEgens exhibited almost no emission with low ΦF in the solution. Parallel with increasing water content, the emission intensity increased significantly, and the emission color had slightly blue-shifted owing to the formation of the aggregates (Figure 2D,G). The diameter for nano-aggregates of SBTPE-Br and SBTPE-CHO in the THF-H2O mixture (fw = 90 %) was determined to be 91 and 79 nm, respectively (Figure S27). In addition, the nano-aggregates for these chiral AIEgens mostly existed as spheres or irregular particles with an amorphous nature, which was indicated by their fluorescence microscope and scanning electron microscopy (SEM) results (Figure S28). The ΦF of SBTPE-Br and SBTPE-CHO had risen from 1.4% and 3.3% in pure THF to 50.4% and 81.3% as nano-aggregates in THF-H2O mixture (fw = 90 %), whereas their emission lifetime had also increased from 0.02 ns and 0.16 ns in THF to 3.69 ns and 2.90 ns as nano-aggregates, respectively (Figures S29 and S30). Owing to the π-conjugation extension and electron-withdrawing effects from the aldehyde group, the emission color of SBTPE-Br had obviously shifted from blue (473 nm) to green (499 nm) for SBTPE-CHO.
For SBTPE-CN and RBTPE-CN, π-extension with cyano-stilbene moiety on chiral TPE skeleton had rendered this pair of chiral fluorophores medium emission in pure THF (Figure 2E and Figure S25). Parallel with the formation of the aggregates, their emission was further enhanced and slight red-shifted from 510 nm in THF to 522 nm in THF-H2O mixture (fw = 90 %), whereas the diameter for the nano-aggregates was approximately 106 nm (Figure S27C). The ΦF of SBTPE-CN was enhanced from 17.6% in THF to 93.5% as nano-aggregates (Figure 2H) with increasing emission lifetime from 0.59 ns to 2.25 ns (Figure S29). The intermolecular π-π stacking between cyano-stilbene moieties contributed to the red-shifted emission after aggregate formation. Consequently, SBTPE-CN and RBTPE-CN exactly displayed aggregation-enhanced emission properties.
By contrast, the donor–π–acceptor (D–π–A) structure with TPE as donor moiety and malononitrile groups as acceptors had converted SBTPE-2CN and RBTPE-2CN into fluorogens combining both the TICT process and AIE property (Figure 2F,I and Figure S26). As shown in Figure 2F, the PL intensity of SBTPE-2CN gradually decreased with red-shifted emission from 587 to 611 nm when the fw increased from 0% to 50%, which was mainly owing to the enhanced ICT effect with increasing content of more polar water in the solvent mixture. When fw increased from 50% to 90%, a significant emission enhancement with blue-shifted emissions from 611 to 585 nm was further recorded due to the nano-aggregates formation with a diameter of 68 nm (Figure S27d). This indicated that AIE behavior was overwhelming over the ICT effect under high water content. Due to the high ΦF of 55% in THF and 49.5% in THF-H2O mixture (fw = 90 %), SBTPE-2CN and RBTPE-2CN were both dual-phase emitters with intense emission in solution and solid. And CIE color coordinates for RBTPE-Br, RBTPE-CHO, RBTPE-CN, and RBTPE-2CN in THF-H2O mixture (fw = 90 %) clearly exhibited the color modulation of these chiral AIEgens via donor-acceptor engineering (Figure S31).
In addition, chiral SBTPE-2CN and RBTPE-2CN also showed obvious fluorescence solvatochromism with tunable emission color in diverse solvents owing to the dependence of the ICT process on solvent polarity. Their PL spectra in different solvents were measured accordingly (Figure S32). Parallel with increasing solvent polarity, the maximal emission wavelengths for SBTPE-2CN and RBTPE-2CN had gradually red-shifted from 529 nm in toluene, 535 nm in hexane, 579 nm in chloroform, 585 nm in THF, and ultimately to 636 nm in acetonitrile. The dependence of the Stokes shifts on solvent polarity parameter (∆f) suggested the TICT process for R/SBTPE-2CN (Figure S32), in which the band gap between ground and excited states decreased owing to the increased solvent polarity resulting in the red-shifted emission spectrum. This significant solvatochromic luminescence behavior suggested the possibility of tuning the chiral optical properties for these chiral AIEgens via modulating the solvent or matrix polarity.
2.3 Full-color-tunable CPL via donor-acceptor engineering
As propeller-like molecules, TPE units usually showed silent chiral optical signals in solution due to the rapid exchange between their P- and M- isomers. Owing to the introduction of a chiral tether and stabilization effects from the cyclic skeleton, TPE moiety within these cyclic AIEgens would adopt one preferential helical configuration. Therefore, the optical rotation and CD spectra for these chiral macrocyclic AIEgens were first recorded in the THF solution (Table S1, Figure 3A, and Figures S33–S36). Remarkably, these four pairs of chiral AIEgens all exhibited opposite optical rotation in THF, for instance, the specific optical rotation of RBTPE-Br and SBTPE-Br was determined to be approximate −5.32 × 104 and 3.97 × 104 deg cm2 mol−1, respectively. In addition, strong and fully mirror-symmetric CD spectra were also observed for these compounds in solution, which were also consistent with their corresponding UV-vis absorption spectra. Identical to the red-shifted absorption curve, their CD signals beyond 350 nm also displayed gradual elevation from RBTPE-Br to RBTPE-2CN parallel enhancing the electron-withdrawing capability of the acceptors. These CD bands also agreed well with the simulated CD spectra based on TD-DFT calculation (Figure S37).
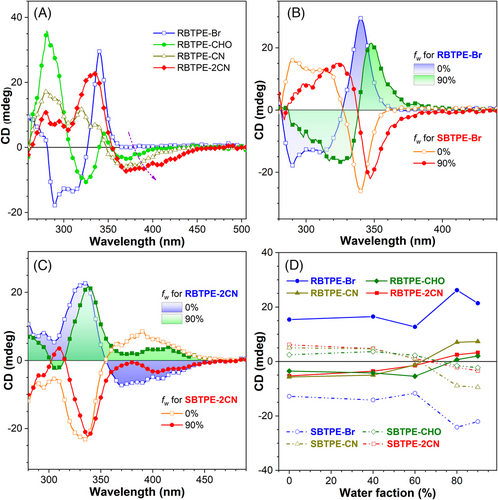
Then, the influence of aggregate formation on CD spectra for these chiral AIEgens was also studied. Along with increasing water content in the solution, the CD signals of SBTPE-Br and RBTPE-Br were slightly attenuated and red-shifted after the formation of the aggregates (Figure 3B and Figure S33). By contrast, for the other three pairs of chiral AIEgens with extended π-conjugation and acceptor units, their CD signal above 350 nm experienced remarkable signal inversion after aggregates formation (Figure 3C and Figures S34–S36). As shown in Figure 3D, the CD signal remained almost unchanged when fw was below 40%, whereas the CD inversion started to occur with the water content above 60%. Similarly, when R/SBTPE-Br and R/SBTPE-CN were dispersed in poly(methyl methacrylate) (PMMA), concentration-dependent CD inversion was also observed for R/SBTPE-CN whereas the CD band of R/SBTPE-Br in PMMA was independent of the concentration (Figure S38). In detail, when the concentration of R/SBTPE-CN in PMMA was increased beyond 5% (w/w), the CD band was inverted owing to the aggregation of AIEgens under high concentration. By contrast, the CD spectra of all these AIEgens in 4-pentyl-4-biphenylcarbonitrile nematic LC (5CB) exhibited no signal inversion after raising the content from 1% to 5% (Figures S39 and S40), this result suggested that the chiral macrocyclic AIEgens was homogeneously dissolved in 5CB without aggregation.
In most reports, the changing dihedral angle of the binaphthyl rings in BINOL moiety had contributed to the aggregation-induced CD annihilation phenomenon for chiral small molecules and polymers based on BINOL moieties,[48] and the signal inversion in CD and CPL spectra.[49-52] According to the simulated geometry, the dihedral angles within the BINOL unit for these four pairs of chiral AIEgen were calculated to be around 107.5°, whereas the dihedral angle for BINOL moiety in the crystal structure of pristine RBTPE and SBTPE was 106.1°.[42] This small difference suggested that the aggregation of chiral AIEgens in the solid state probably had neglectable impacts on the dihedral angle of BINOL moiety owing to the constraint from the cyclic skeleton. Thus, the signal attenuation and signal inversion in CD spectra were probably related to specific aggregate structure instead of simply aggregate formation, and the specific intermolecular packing between chiral AIEgens in aggregates was responsible for the CD inversion.[53, 54] For SBTPE-Br and RBTPE-Br, the highly twisted structure diminished the π-π stacking between luminogens, which was also supported by the reported crystal structure of SBTPE and RBTPE. On the contrary, the extended conjugation (such as the phenyl rings, or cyano-stilbene units) within the other three pairs of chiral AIEgens provided planar conjugated segments, which allowed for efficient intermolecular π-π stacking. The intermolecular π-π stacking of R/SBTPE-CHO, R/SBTPE-CN, and R/SBTPE-2CN in aggregates probably contributed to the CD signal inversion observed in the THF-H2O mixture and PMMA matrix.[55, 56]
Then, the CPL spectra of these chiral AIEgens were further investigated in solution. Owing to the low emission efficiency of R/SBTPE-Br and R/SBTPE-CHO, their CPL spectra failed to be recorded precisely. Furthermore, the TPE moiety or cyano-stilbene unit within these chiral compounds probably underwent cis-trans isomerization in THF under UV-light irradiation, which was indicated by their 1H NMR spectra after irradiation (Figures S41–S43). This led to the failure of CPL measurement for these chiral AIEgens in the THF solution. In addition, the 1H NMR spectra of R/SBTPE-CN and R/SBTPE-2CN samples, which had experienced UV irradiation in the solid state for 60 s, validated the photo-stability of these compounds as aggregates (Figures S44–S47). Therefore, the CPL spectra for these chiral AIEgens were recorded as nano-aggregates in the THF-H2O mixture (fw = 90 %). As shown in Figure 4, four pairs of chiral AIEgens all displayed mirror-imaged CPL spectra whereas their emission wavelength ranged from 486 nm for R/SBTPE-Br, to 603 nm for R/SBTPE-2CN. These results strongly supported the potential of these chiral macrocyclic AIEgens as full-spectra tunable solid-state CPL emitters combining high solid-state emission efficiency and tunable emission colors.
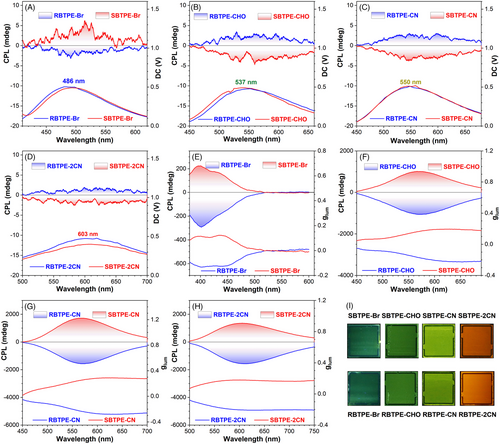
In detail, the chiral module RBTPE-Br displayed right-handed CPL emission at 486 nm with a negative signal (Figure 4A). By contrast, RBTPE-CHO, RBTPE-CN, and RBTPE-2CN with identical chiral cyclic skeleton and extended π-conjugation, exhibited gradually red-shifted positive CPL signals which corresponded to left-handed CPL emission (Figure 4B–D). The glum value for RBTPE-Br, RBTPE-CHO, RBTPE-CN and RBTPE-2CN was determined to be about −4.35 × 10−4, 4.7 × 10−4, 4.06 × 10−4, and 3.85 × 10−4, respectively. On the other hand, SBTPE-Br, SBTPE-CHO, SBTPE-CN, and SBTPE-2CN all displayed mirror-imaged CPL spectra relative to their counterparts, and CPL inversion phenomenon was also observed from SBTPE-Br to its derivatives. The glum values for these compounds were comparable with that of previously reported chiral macrocycles consisting of TPE and BINOL units (Table S2).
Identical to the CD signal inversion discussed above, the intermolecular π-π stacking originated from the extended π-conjugated segments on R/SBTPE-CHO, R/SBTPE-CN, and R/SBTPE-2CN was probably responsible for their inversed CPL spectra when compared with R/SBTPE-Br.[57-59] To confirm this hypothesis, their CPL spectra in LC with a low concentration of 1% (w/w) were measured. As indicated by their CD spectra in 5CB (Figures S39 and S40), these chiral AIEgens were homogeneously dissolved in 5CB and thus the CPL spectra in 5CB originated from the single molecule instead of their aggregates. Surprisingly, the CPL spectra of RBTPE-Br and SBTPE-Br in 5CB had remarkably blue-shifted to 400 nm, which was probably attributed to the photo-cyclization reaction of TPE moiety (Figure 4E).[60] For their derivatives, RBTPE-CHO, RBTPE-CN, and RBTPE-2CN all displayed right-handed CPL emission with negative signal whereas SBTPE-CHO, SBTPE-CN, and SBTPE-2CN all displayed left-handed CPL emission with positive signal (Figure 4F–H). These results indicated that their CPL in the LC matrix was opposite to that recorded as nano-aggregates. Consequently, the π-π packing and aggregate structure of chiral AIEgens in nano-aggregates contributed to the CD and CPL signal inversions observed above.[61] In addition, the glum for these chiral AIEgens were all significantly amplified to the order of 10−1 promoted by the self-assembly in the well-organized LC system, for instance, the glum for RBTPE-2CN and SBTPE-2CN had reached −0.204 and 0.179. And color-tunable CPL behavior was also well retained indicated by fluorescent images of LC cells doped with chiral AIEgens (Figures 4I and S48). The combination of chiral macrocyclic AIEgens and LC suggested the feasibility of constructing color-tunable CPL emitters with high glum and intense solid-state emission.[62]
2.4 Full-color-tunable CPL via polymer matrix engineering
Considering the real application of these CPL molecules as thin films, the CPL spectra of these chiral AIEgens in a polymer matrix were then studied. To precisely record the CPL signal, the concentration of chiral AIEgens was raised to 10% (w/w). Their CPL spectra in PMMA clearly indicated mirror-imaged emission (Figure 5A–D), which was also identical to that of nano-aggregates. Notably, the emission color of R/SBTPE-Br, R/SBTPE-CHO, R/SBTPE-CN, and R/SBTPE-2CN in PMMA gradually changed from blue (459 nm) for R/SBTPE-Br to greenish yellow (535 nm) for R/SBTPE-2CN (Figure 5), whereas the CIE color coordinates shifted from (0.15, 0.12) to (0.31, 0.52) (Figure S49). Relative to their nano-aggregates in the THF-H2O mixture, the emission wavelength of these chiral AIEgens had all obviously blue-shifted, which was probably owing to their congested conformation in the polymer matrix and the low polarity of the PMMA main chains (Figure S50). Meanwhile, these chiral emitters also displayed similar glum when compared with nano-aggregates, for example, glum were recorded to be +2.0 × 10−4 for RBTPE-2CN and −2.0 × 10−4 for SBTPE-2CN in PMMA (Table S3).
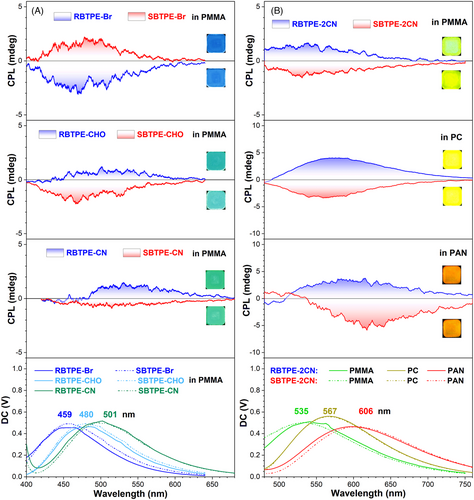
On the other hand, the dependence of CPL spectra on their concentration was also studied to verify the impacts of aggregation in the polymer matrix. As shown in Figure S51, the CPL spectra of R/SBTPE-Br remained almost unchanged parallel with increasing concentration from 1% to 10% (w/w). By contrast, the PMMA film of R/SBTPE-CN experienced CPL inversion when the concentration was increased to 5%. These results were well consistent with the CD spectra in PMMA, and efficient intermolecular π-π stacking of R/SBTPE-CN and analogs had contributed to the inverted CPL signal under high concentration.
Owing to the solvatochromic luminescence of RBTPE-2CN and SBTPE-2CN, their emission colors were highly dependent on the polarity of the surrounding environment. Thus, the chemical nature of the polymer matrix was further leveraged to tune the CPL color of RBTPE-2CN and SBTPE-2CN in polymer films, whereas polymers with different polarity were chosen, including PMMA, medium polar poly(bisphenol A carbonate) (PC), and strongly polar PAN. Obviously, the emission color of RBTPE-2CN and SBTPE-2CN had remarkably changed from greenish yellow in PMMA, to yellow in PC, and ultimately to red in PAN (Figure 5D–F), whereas the emission color coordinates correspondingly shifted from (0.31, 0.52) in PMMA, to (0.38, 0.58) in PC, and finally to (0.50, 0.49) in PAN (Figure S49). The CPL emission wavelength had gradually red-shifted from 535 nm in PMMA to 567 nm in PC, and finally to 606 nm in PAN. The glum of SBTPE-2CN in these polymer films had slightly increased from 2.0 × 10−4 in PMMA, to 4.1 × 10−4 in PC, and to 6.0 × 10−4 in PAN. Therefore, the solid-state CPL color of the chiral macrocyclic AIEgens can be feasibly tuned across the visible light range via both molecular engineering and materials processing, as well as their combinations.
3 CONCLUSION
In summary, four pairs of chiral macrocyclic AIEgens with different acceptors and tailored propeller chirality were readily synthesized via a modular synthetic approach. Their chemical identities, chiral geometry, and optical purity were further confirmed via structural characterization, theoretical calculation, and chiral HPLC analysis. Owing to the incorporation of TPE moiety and diverse electron withdrawing units, these chiral cyclic AIEgens all displayed high solid-state emission efficiency across the visible light range. Distinctive from their analogs featured with classical AIE phenomena in THF-water mixture, RBTPE-2CN and SBTPE-2CN with strong malononitrile acceptors exhibited TICT progress before aggregate formation, and AIE phenomenon under high water content. Mirror-imaged CD spectra for these four pairs of chiral AIEgens further confirmed their enantiotopic relationships, whereas these chiral fluorophores also displayed mirror-imaged CPL signal as nano-aggregates and amplified CPL signal in LCs. The aggregate structure and intermolecular π-π stacking between chiral fluorophores had reverted the CD and CPL signal of chiral TPE derivatives when compared with their modular precursor RBTPE-Br and SBTPE-Br. Based on the donor-acceptor engineering on the chiral TPE skeleton and polarity modulation of the polymer matrix, full-color-tunable CPL could be realized based on this series of chiral AIEgens. These results have paved a highly promising strategy toward developing chiral AIEgens with tailored propeller chirality and customized emission color. This modular approach will also further promote the structural exploration of chiral AIE materials and facilitate the development of new chiral organic emitters. Embracing this donor-acceptor engineering and chiral modules, the construction of near-infrared chiral AIEgens is currently under progress in our lab.
ACKNOWLEDGMENTS
This work was financially supported by the Guangdong Basic and Applied Basic Research Foundation (2023A1515030228). We thank the Instrumental Analysis Centre of Shenzhen University (Xili Campus) for NMR measurement.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




