Unveiling the nanoscale architectures and dynamics of protein assembly with in situ atomic force microscopy
Zhaoyi Zhai and Sakshi Yadav Schmid contributed equally to this work.
Abstract
Proteins play a vital role in different biological processes by forming complexes through precise folding with exclusive inter- and intra-molecular interactions. Understanding the structural and regulatory mechanisms underlying protein complex formation provides insights into biophysical processes. Furthermore, the principle of protein assembly gives guidelines for new biomimetic materials with potential applications in medicine, energy, and nanotechnology. Atomic force microscopy (AFM) is a powerful tool for investigating protein assembly and interactions across spatial scales (single molecules to cells) and temporal scales (milliseconds to days). It has significantly contributed to understanding nanoscale architectures, inter- and intra-molecular interactions, and regulatory elements that determine protein structures, assemblies, and functions. This review describes recent advancements in elucidating protein assemblies with in situ AFM. We discuss the structures, diffusions, interactions, and assembly dynamics of proteins captured by conventional and high-speed AFM in near-native environments and recent AFM developments in the multimodal high-resolution imaging, bimodal imaging, live cell imaging, and machine-learning-enhanced data analysis. These approaches show the significance of broadening the horizons of AFM and enable unprecedented explorations of protein assembly for biomaterial design and biomedical research.
1 INTRODUCTION
Proteins can assemble into multi-component hierarchical structures at solid‒liquid interfaces through a delicate balance of covalent, hydrophobic, ionic, hydrogen bond, entropic, etc., interactions between their sequences and folded structures.[1-4] This complex orchestration leads to the formation of intricate architectures with orders across scales. These hierarchies offer unique potential to assemble biomaterials that have versatile structures, biocompatibility, and enhanced but diversified properties at interfaces. Second, their biodegradability and potential for drug delivery, tissue engineering, and functional surfaces make proteins valuable candidates for medical and biotechnological applications.[5-11] Third, their roles in controlling biomineralization also improve mechanical properties and biocompatibility of biomaterials.[12, 13] However, organizing protein complexes with precise geometries and orders and tunable intermolecular interactions are profoundly complex features of biomaterial engineering.[14, 15] Hence, understanding the structure and dynamics of protein assembly is essential for developing biomaterials with pre-defined properties and functions.[16] Additionally, emulating the sophisticated structures of protein complexes grants access to a new class of intelligent biomaterials with potential applications in medicine, energy, and nanotechnology.[17, 18]
In addition, proteins perform intricate and highly regulated functions in all living beings.[19-27] For example, protein supramolecular complexes, such as membrane receptors, cytoskeletal filaments, molecular transporters, and ribosomes, control the critical cellular activities of signaling, growth, division, motility, and homeostasis.[24-36] These highly dynamic protein complexes can reversibly assemble into different polymorphs in response to cellular demands and environmental triggers. Their organization requires a sophisticated regulation that, when perturbed, can lead to medical problems.[22, 37, 38] Hence, elucidating the structural and regulatory mechanisms that govern the molecular folding and assembly dynamics of protein complexes can provide insights into the biological processes underpinning health and disease.[1]
Technological and methodological advances have enabled researchers to develop profound understandings of protein assembly: X-ray crystallography, nuclear magnetic resonance spectroscopy, electron microscopy, and super-resolution light microscopy can reveal the atomic and structural details of protein assembly (Table 1);[1, 4, 39-49] cross-linking mass spectrometry can provide insights into molecular interactions in protein complexes;[50, 51] and computational modeling can yield the simulated views of assembly principles at the time scales, dimensional scales, and assembly conditions, which are hard to validate by experiments alone.[52-54]
| Spatial resolution | Temporal resolution | Physiological condition | Temperature | Label | Imaging (I)/spectrum (S) | |
|---|---|---|---|---|---|---|
| AFM |
X, Y: <10 Å Z: <1 Å[55] |
∼0.5‒10 min | Yes | RT | No | I |
| HS-AFM |
X, Y: <10 Å Z: <1 Å[56] |
∼50 ms[57] | Yes | RT | No | I |
| X-ray | <1 Å[46] | N.A. | No | RT | No | S |
| NMR | ∼2 Å[47] | ∼ms[49] | Yes | RT | Yes | S |
| Cryo-EM | ∼1.25 Å[48] | N.A. | Yes (cryo-preserved) | Cryogenic | No | I |
| Confocal microscopy | ∼250 nm[45] | ∼ms[45] | Yes | RT | Yes | I |
- Abbreviations: Cryo-EM, cryo-electron microscopy; HS-AFM, high-speed AFM; NMR, nuclear magnetic resonance; RT, room temperature.
Atomic force microscopy (AFM) is a highly potent imaging method to capture and describe protein assembly in physiological conditions with sub-molecular resolution. Since its invention in 1986, AFM has been extensively used in biology, biomaterials, and biomedical sciences.[58] It has a unique capability for in situ visualization of the structures and dynamics of biomolecular assemblies illuminating nanoscale architectures, inter- and intra-molecular interactions, geometric constraints, kinetic responses, and environment-regulating elements.[57, 59-63] Specifically, high-resolution AFM (HR-AFM) can capture the nuances of protein assembly in physiological environments with environmental stimuli, such as cations, pH, and temperature.[55, 64, 65] For example, HR-AFM allows visualization of single amino acids and DNA double strands.[66, 67] In addition to sub-nanometer spatial resolution, high-speed AFM (HS-AFM) makes in situ/in vivo filming of dynamic processes of active biomolecules possible at an image acquisition speed of up to 50 frames per second (fps)—the time scale close to protein dynamics in native conditions.[68] For example, high spatiotemporal imaging of proteins in native and artificial membranes offers the potential to study proteins on a single molecular level and obtain their real-time oligomerization, arrangement, association, and dissociation dynamics.[69-71] Hence, AFM provides unique advantages in understanding the nuances of protein assembly across spatial and temporal scales in physiological conditions.
In this review, we first discuss the technical developments of AFM and HS-AFM. Second, we select recent understandings in protein assembly and dynamics at solid‒liquid interfaces using in situ HR-AFM. Third, we summarize the capability of HS-AFM to track the intermediate states and kinetic pathways involved in protein assembly. Finally, we summarize the current advancements to push the boundaries of AFM capabilities in studying protein assembly and potentially overcome the current limitations of AFM for more profound comprehensions of protein assembly dynamics, structures, and functions.
2 ATOMIC FORCE MICROSCOPY
2.1 Working principle of conventional AFM
AFM was initially invented in 1986 to obtain topographical information on low-conductive and nonconductive materials.[58, 72] It can also image samples in various environments, such as vacuum, solutions, and air, with sub-nanometer spatial resolution for sensitive biological samples.[73, 74] In the last three decades, AFM has been exhibited as a powerful imaging technique for biological samples, from molecules to living cells to better understand their assembly dynamics, mechanics, and functionality.[65, 75, 76]
In brief, AFM is mainly comprised of four components: (1) a mechanical cantilever with a nanoscale sharp probe, (2) a detection system tracking the cantilever deflection, (3) X, Y, Z piezo scanners, and (4) a feedback loop (Figure 1A). When the AFM probe raster scans sample surfaces, the probe‒sample interactions deflect the cantilever. The feedback loop keeps the cantilever deflection (referred to as probe‒sample interaction force and measured by the detection system) constant (Figure 1A). Specifically, the piezoelectric element on the Z-axis either extends or retracts at each scanning point (X, Y) to keep the probe‒sample interaction force (the deflection of the cantilever) as close to the setpoint as possible. The moving distance of the piezoelectric element (ΔZ) on each scanning point is recorded as the relative height. In the end, the recorded matrix (X, Y, ΔZ) generates morphology images. Until now, optical beam deflection, developed by Meyer and Amer, has been the most successful and widely applied detection technique to monitor cantilever deflection.[77] A laser beam is reflected from the back side of the cantilever onto a photodiode with four quadrants (Figure 1A). Slight movements of the reflected laser beam caused by probe‒sample interactions introduce potential differences in the four quadrants of the photodiode, and the vertical and torsional deflections of the cantilever are monitored and calculated based on the potential differences.
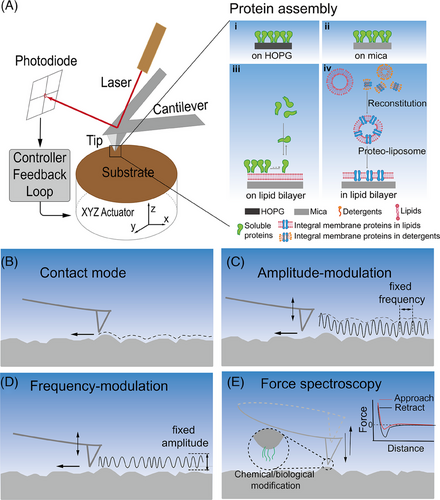
2.2 AFM operation modes
In general, AFM has four widely used operation modes: contact mode (CM), amplitude modulation mode (AM), frequency modulation mode (FM), and PeakForce tapping mode (PeakForce, detailed in Section 2.3).
CM-AFM is the original operation mode, in which the probe is in constant contact with the sample while raster scanning across it (Figure 1B). CM-AFM usually operates in the repulsive regime of the Lennard‒Jones inter-atomic interaction potential. When scanning native membrane proteins, CM-AFM can apply minimal interaction forces ranging from 50 to 100 pN.[62, 73] These gentle forces allow CM-AFM to successfully characterize purple membranes in aqueous solution, such as the water channel aquaporin Z of Escherichia coli bacteria, and the Rhodopseudomonas viridis photosynthetic core complex in native membranes.[63, 78-80] However, the AFM tip may exert a large shearing force when using CM-AFM to scan over isolated biomolecules on a substrate due to the feedback lag. Therefore, soft biomolecules are liable to be damaged and weakly adsorbed objects may be distorted or pushed away by the AFM tip.
AM-AFM mechanically excites the AFM cantilever at a frequency close to its first flexural resonance frequency (Figure 1C).[81] When the AFM tip approaches sample surface, the cantilever's oscillation amplitude is dampened due to the increasing repulsive interaction force between the sample and the AFM probe. Hence, in AM-AFM, the cantilever's oscillation amplitude, instead of the cantilever deflection, is monitored by optical beam deflection (Figure 1C).[77] The amplitude setpoint is usually close to 80%‒90% of the free oscillation amplitude to gain a strong signal-to-noise ratio, but keep the tip‒sample interaction minimal. During scanning, the motion (ΔZ) of the Z piezo is considered equal to the relative height at every scanning point. In addition, the phase lag of the cantilever oscillation to the drive signal can be recorded. It qualitatively indicates the mechanical heterogeneity across sample surfaces.[82] AM-AFM has exhibited great potential for studying various biomolecular assemblies and complex bio-architectures.[83-85] Möller et al. first demonstrated that native protein surfaces can be imaged with AM-AFM at molecular scale.[86] Chung et al. demonstrated that AM-AFM can provide unique insights into protein crystal growth.[87] In addition to imaging surface morphologies with high spatial resolution, AM-AFM can directly measure the mechanical properties of supported lipid bilayers.[88]
FM-AFM excites the cantilever close to its resonance frequency but monitors shifts in resonance frequency due to the interaction force between the probe and sample after locking in the oscillation phase (Figure 1D).[89, 90] The feedback mechanism compensates for constant change in the resonant frequency (). FM-AFM in air and liquid media requires precise control of piezo movement and high-speed feedback; thus, it is widely utilized in ultra-high vacuum AFM. With optimized hardware and operation, FM-AFM has been used efficiently in ambient and liquid media to measure molecular structures at solid‒liquid interfaces with sub-nanometer spatial resolution.[90-93]
2.3 AFM-based force measurements
As its name states, AFM tries to measure and track the atom/nanoscale interactions between samples and AFM probes. In addition to imaging surface morphologies, AFM can also collect force‒distance curves as force spectroscopy (FS) and generate force‒volume maps.[94] For example, AFM-FS measures the interaction when an AFM probe approaches a sample surface, “presses into” the sample, and then withdraws from the sample (Figure 1E). This probe movement is controlled via programmed approach-withdraw velocities and dwell time. The cantilever displacement versus probe‒sample distance is recorded and converted to measured interaction force with the Hooke's law. For instance, when an adhesion force occurs between the probe and sample surface, a downward force increases upon cantilever retraction until the adhesion bond breaks and the force versus distance curves are recorded. FS can quantify nonspecific and specific inter-/intra-molecular interactions by modifying the AFM probe and substrate surface with target chemicals/biomolecules.[94]
AFM-FS has been used to elucidate various phenomena in biological systems, ranging from biomolecules to cells, such as the mechanical characteristics of molecular machines, protein affinity, and folding mechanism. For example, the mechanical properties of proteins, such as I band titin and titin-mimicking artificial elastomeric proteins, were determined by single-molecule AFM-FS.[95, 96] In addition, the receptors on living cell surfaces were successfully mapped by probing individual molecular interactions.[97] During the COVID-19 pandemic, binding between SARS-CoV-2 and cellular angiotensin-coverting enzyme 2 (ACE2) receptors was evaluated by AFM-FS, providing valuable information for assessing the preclinical potential of a new drug.[98, 99]
Another widely used AFM-based force measurement approach is PeakForce tapping mode. It was developed to obtain force‒distance curves at kilo-Hz speed by modulating the Z-scanner movement with a sinusoidal wave.[100] Within a modulation cycle, the maximum repulsive force, known as the peak force, is reached when the AFM probe approaches the sample surface. The sample morphology was measured by controlling Z-scanner movement with the peak force as the set point. After calibration, this process enables to extract samples’ mechanical information, such as Young's modulus, deformation, adhesion force, and energy dissipation, from the force‒distance curves recorded at each scanning pixel. It is efficient to obtain quantitative nanomechanical maps and correlate them with protein structures.[101, 102]
2.4 High-speed atomic force microscopy
Although conventional AFM can capture high-resolution images of biomolecular hierarchical structures and phase transitions, tracking the dynamics of most biological events that occur at (milli) second time scale is challenging. To break through the temporal resolution limit, HS-AFM was invented around 2008 with the development of various hardware components and control approaches, such as ultra-short cantilevers, optical beam deflection detectors optimized for small cantilevers, fast scanners, fast deflection-to-amplitude converters, active vibration damping techniques, and dynamic feedback controllers.[103-111] Reviews by Ando et al. have discussed the working principle and design of HS-AFM in detail.[103, 112, 113]
In addition, HS-AFM can integrate multiple bio-functional modules and characterization methods. For example, bio-enhanced HS-AFM was developed by Miyagi et al. with a buffer exchange perfusion system and a pulse UV laser system.[68] This allows for the exchange of buffer and uncage-caged compounds to mimic various environmental conditions and stimuli, including homeostasis shift, sudden cation flux into the cytoplasm in a membrane lesion, and upregulation of photon influx that stimulates photosynthetic proteins. Combining HS-AFM with temperature control offers opportunities to study the temperature-dependent biological processes, for example, membrane ripple transition.[114] Furthermore, the successful development of probe-scan HS-AFM has opened new opportunities to combine HS-AFM with various optical techniques, such as optical tweezers and super-resolution fluorescence microscopy techniques.[115-118] An integrated system of tip-scan HS-AFM and total internal reflection fluorescence microscopy can simultaneously collect the topographic AFM images and single-molecule fluorescence images at 5 fps.[119]
Three imaging modes are commonly used in HS-AFM: AM mode, FM mode, and off-resonance tapping (ORT).[74, 103, 120] AM/FM-HS-AFM is based on tapping mode, where the AFM tip oscillates vertically and periodically probes a sample surface during scanning.[121] The basic configuration and principle of the AM-HS-AFM instrument are similar to those of conventional AFM setups (Figure 2A).
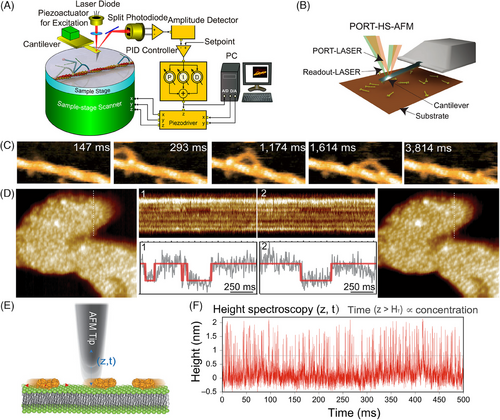
ORT HS-AFM based on force‒distance curve imaging modes with cycle-to-cycle feedback was recently established by Nievergelt et al.[120, 122] They presented an advanced ORT imaging mode using photothermal actuation called high-speed photothermal ORT (HS-PORT). In HS-PORT (Figure 2B), the top and bottom layers of the AFM cantilever are generally coated with different materials that have different thermal expansion coefficients.[123] When exposed to a modulated laser beam, the coating surfaces (top and bottom surfaces) produce differential thermal expansion, which causes the cantilever to bend, referred to as the bimorph effect, enabling the actuation of the AFM probe for imaging or manipulation purposes. In ORT, the achievable imaging speed is restricted by the cantilever oscillation rate in a controlled manner. The oscillation rate of the cantilever moving up and down is generally smaller than its resonance frequency. In conventional ORT instrumentation, the motion is manipulated by a piezo that moves either the sample or the cantilever chip. In HS-PORT, only the cantilever and probe need to drive when utilizing photothermal actuation; hence, the mass is much smaller. This allows force-curve speeds at several hundred kilohertz, limited only by the HS-AFM cantilever resonance.
HS-AFM can capture images with high spatiotemporal resolution at ∼1 nm in lateral resolution, ∼0.1 nm in vertical resolution, and ∼100 ms in temporal resolution. It has emerged as a valuable tool to understand the behaviors and functions of proteins at single-molecule level, where bioprocesses occur on a time scale of hundreds of milliseconds to minutes (Figure 2C).[68, 114, 124, 125] However, numerous molecular processes are still too fast to be resolved by HS-AFM. To investigate molecular dynamics at the millisecond or even microsecond scale, enhanced HS-AFM techniques such as high-speed AFM line scanning (HS-AFM-LS) (Figure 2D) and high-speed AFM height spectroscopy (HS-AFM-HS) (Figure 2E) have proven highly potent.[126-130] By fixing one scan axis as zero (X- or Y-direction) and repeatedly scanning over a single line [(X, Z, t) or (Y, Z, t)], HS-AFM-LS supplies sub-millisecond to millisecond temporal resolution (Figure 2D). The generated line scan images show the sample height profiles (Y-axis) versus time (X-axis). Thus, line scan kymographs can reveal the biomolecular structural transition dynamics with angstrom precision.[131-140]
Inspired by fluorescence spectroscopy, Heath and Scheuring developed HS-AFM-HS by holding the AFM probe at a fixed X‒Y position and monitoring the tip height fluctuation in Z-direction with Angstrom accuracy (Z, t) and ∼10 µs of temporal resolution (Figure 2E,F).[127] The probe sonicating resonance frequency and feedback loop time delay mainly determine the temporal resolution. HS-AFM-HS allows to directly detect the unlabeled molecules motion underneath the AFM probe and simultaneously measures surface diffusion rates, concentrations, and oligomer sizes.[127]
In addition to image modes, HS-AFM-FS was developed by Rico et al. by using a short and soft cantilever (0.035 pN µm−1 s−1) and a miniature piezoelectric actuator.[142] This provides a wide range of pulling velocities from 0.0097 to 3870 µm s−1 (six orders of magnitude), and microsecond time resolution.[142] The pulling speed is ∼2.5 orders of magnitude faster than that of conventional AFM-FS and reaches the limit of molecular dynamics simulations. Thus, HS-AFM-FS bridges the gap between simulation and conventional low-velocity FS experiments; for example, it allows to directly compare experimental and simulated DNA and protein unfolding forces.[142, 143] In addition, improving protein unfolding experiments to the microsecond time range is vital for studies of biomechanical processes such as receptor‒ligand unbinding, lipid membrane dynamics, and mechanical protein folding and unfolding.[144-147] HS-AFM-FS has revealed the multistep unfolding mechanisms of titin I91 protein: the protein fluctuates between the native and intermediate states under moderate forces. Still, it remains in the intermediate state under increasing forces.[142] The ability to directly compare experimental observations with simulations is essential for studying biomechanical processes.
3 SELECTED STUDIES OF PROTEIN ASSEMBLY BY AFM
AFM has been used to investigate protein assembly on diverse substrates, including inorganic surfaces, such as mica and HOPG, and support lipid membranes under physiologically relevant conditions (Table 2). It enables high-resolution imaging and analysis of nanoscale protein interactions, morphology, and assembly dynamics in situ. The selected studies in this section provide valuable insights into the mechanisms and processes governing protein assembly and its interplay with different substrates by shedding light on fundamental biological phenomena, paving the way for applications in various scientific disciplines.[70, 73, 93, 148]
| Substrate | Protein name | Protein solubility | Research focus | Variable | References |
|---|---|---|---|---|---|
| Mica | C98RhuA | Soluble | Surface-templated assembly polymorphs | K+ concentration | Zhang et al.[149] |
| Septins | Soluble | Surface-templated hierarchical assembly | Electrolyte concentration | Jiao et al.[150] | |
| S-layer protein | Soluble | Multiple-stage assembly | Temperature | Shin et al.[148] | |
| HOPG | Cytochrome b562 | Soluble | Multidimensional assembly | Heme analogs | Kitagishi et al.[151] |
| Lipid bilayer | S-layer protein | Soluble | Multistage protein crystallization | Protein concentration | Chung et al.[87] |
| Perforin-2 | Soluble | Conformational change | pH | Jiao et al.[152] | |
| Aquaporin Z | Lipophilic | Diffusion, oligomerization | Lipid acyl chain length | Jiang et al.[71] |
3.1 Investigating protein assembly on inorganic surfaces
Protein assembly on inorganic surfaces is highly relevant in design of biodevices and protein‒mineral composites. In addition, the protein's patchiness and tunable intermolecular interactions can be harnessed to engineer assembly dynamic and multiple phases with emergent capabilities akin to those of living systems.[153, 154] Insights from protein assembly can guide the design of biomimetic materials with organizational sophistication and responsiveness for emergent applications. AFM enables the observation of protein assembly and disassembly processes at high spatiotemporal resolution, provides insights into the protein dynamics under varying environmental conditions, and sheds light on the intricate mechanisms governing their assembly into hierarchical structures across scales.[155-162]
3.1.1 L-rhamnulose-1-phosphate aldolase on mica
Recently, Zhang et al. reported a modified functional protein as a highly patchy building block that assembles into variable 2D lattices via tunable interactions (Figure 3).[149] The engineered protein, L-rhamnulose-1-phosphate aldolase with Cys residues at its corners (C98RhuA, Figure 3A), was modulated into four different patterned 2D crystals (Figure 3C–F). AFM imaging and simulation show that physiochemically distinct growth pathways are controlled by the modulated intermolecular interactions (Figure 3G). C98RhuA protein self-assembles into 2D p4212 crystals in solution (pathway 1) via the formation of intermolecular disulfide bonds. In addition, because the N-terminus and C-terminus of the C98RhuA molecule have opposite charges (Figure 3A), the neighboring C98RhuA molecules need to adopt an out-of-plane antiparallel (up‒down) arrangement to avoid the out-of-plane dipole moment accumulation (Figure 3C,G).
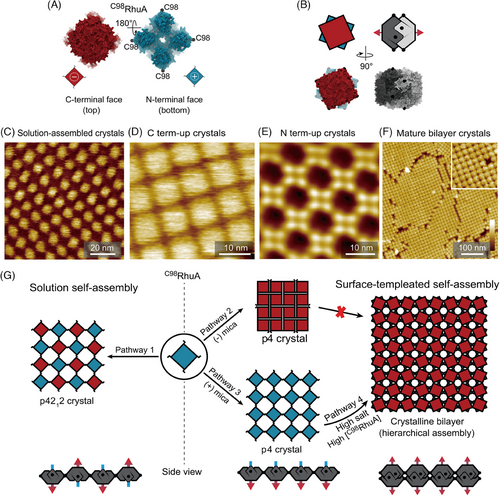
On the contrary, C98RhuA assembly on negatively charged mica could compensate for the accumulated dipole moment by modulating electrostatic interactions between the anisotropically charged C98RhuA and mica at different concentrations of K+. C98RhuA forms close-packed 2D crystals in p4 symmetry with the positively charged N-terminus facing mica in 5 mM K+ solution because the negatively charged mica can compensate for the accumulated dipole moment (Figure 3D). When incubated in solution with >100 mM K+, porous C98RhuA crystals with the C-terminus down grow on K+ occupied mica surface (Figure 3E). Upon further increase in the K+ and protein concentrations, a C98RhuA bilayer crystalline forms exclusively (Figure 3F). The bilayer height is slightly smaller than two times of the monolayer thickness, indicating that tail-to-tail packing dimers form via interpenetration of the corrugated N-term faces (Figure 3B,G). The bilayer structure is formed by self-templating growth from the underlying p4-symmetric monolayer. The underlying layer subunits undergo a 22.5° rotation to bring the second-layer monomers close enough for disulfide bonding (Figure 3G).
The results show the mechanism of the patchy protein assembly into alternate 2D crystalline structures by modulating protein‒protein and protein‒substrate interactions. The interactions, including electrostatic, entropic, dipole‒dipole, etc., can be manipulated. If properly exploited, the building blocks with unique sensitivity and angular dependence, such as C98RhuA, could allow external fields to direct multi-phase assembly in solution, potentially realizing the template-free self-assembly of tunable biomaterials.
3.1.2 Septin assembly on mica
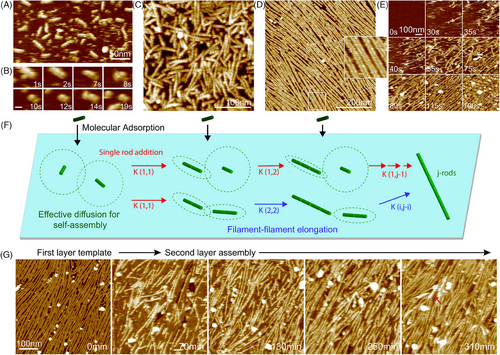
Self-templating protein assembly is observed not only in C98RhuA assembly on mica but also in septin assembly, an essential family of guanosine triphosphate (GTP)-binding proteins. Septins are highly conserved among eukaryotic cells, functioning as a scaffold recruiting various proteins in cell polarity, spore formation, and cell division.[163-166] They are P-loop-NTPase proteins, in which a shared globular core is opposite in the polybasic domain—a highly conserved GTP-binding domain flanked by the C-terminus and N-terminus.[163, 167] The septin rod in Saccharomyces cerevisiae is composed of four septins by forming a linear palindromic hetero-octamer: Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11.[168] The interactions between adjacent septins dominate with alternate GTP interface and the N-terminal and C-terminal helices interfaces. The octamer rod is a basic unit for further assembly into filaments, filament pairs, and other higher order structures.[169, 170] In vivo, septin filaments and further assembled bundles are present at sites of cell division.[171, 172] They act as scaffolds to recruit and interact with the actomyosin network that drives membrane ingression.[173, 174] The fundamental polymerization mechanism of septins is essential for biophysical properties and functions, yet their assembly mechanism is still unclear.[175] Jiao et al. used HS-AFM and kinetic modeling to explore septin filament assembly mechanisms and formations of other higher order structures.[150]
In agreement with previous studies, only the subunits and rod fragments (<32 nm) of septins could be observed in the absence of monovalent ions (Figure 4A,B).[169, 176, 177] In contrast, objects smaller than septin rods were rarely found in a salt solution (Figure 4C). After incubating septin on mica surface in high salt concentration, septin rods quickly assemble into long filaments with lengths up to several micrometers, and the filaments are readily paired (Figure 4D). Septin filament assembly kinetics were investigated with HS-AFM. Rapid surface binding, filament elongation, and pairing of septins were observed (Figure 4E). Once the septin filaments started to assemble, additional filaments grew in their proximity, regulated their orientation, and formed pairs. The formation of long, well-aligned filaments was fast and could be completed within ∼3 min. The observed assembly kinetics data were consistent with the molecular diffusion-driven annealing model. Filament diffusive volumes strongly influenced the septin polymerization (Figure 4F). Once a single layer of well-aligned and well-paired septin filaments formed on the surface, the septin filaments of the second layer started to assemble (Figure 4G). The growth of second-layer septin filaments is due to a template-initiating assembly process with the first-layer filaments as a template. The templating correlation of the second- and first-layer filaments was quantitatively assessed with ∼80% on average. After the second layer was formed, the filament assembly of the third layer was initiated with the underlying filaments as a template (Figure 4G, red arrowhead). These experiments indicate that septin multilayered hierarchical structures are assembled with a complex process synergistically affected by septin vertical and lateral interactions. In short, the assembly of septins is a diffusion-driven process, while the higher order structure formation is a complex process related to self-templating.
3.1.3 S-layers lattice on mica
In addition to the classical nucleation process shown in the study of septin filament, multistep assembly involving intermediate states,[178-181] is also common in protein assembly. In the folding and assembly of native proteins at solid‒liquid interfaces, it is highly possible that one portion of protein molecules reaches the lowest-energy state, while the rest of the molecules get kinetically trapped in a metastable state along the energy landscape. For example, in situ AFM results showed that S-layer protein goes through a multistage assembly pathway at the mica‒liquid interface[148] (Figure 5): (1) the main portion of S-layer molecules goes through the classical nucleation and formed domains in a stable tall phase (T phase, Figure 5C,D); (2) a small portion of S-layer molecules fold to form domains in a metastable short phase (S phase, ΔE = 1.6 kJ/mol, Figure 5D); (3) the S-phase domains transform to the T-phase domains (Figure 5A,B) in an exponential dependence; and (4) the ratio between S and T phases depends on the incubation temperature. Both states emerge from monomer clusters but follow two pathways—one leads to the kinetically trapped state, and the other directly reaches the final low-energy state. However, the trapped state transforms into a stable state by overcoming an energy barrier, Es (Figure 5D). The transition initiated at the free edge of the short domain and gradually propagated from one side to the other rather than coinciding across the whole domain (Figure 5B).
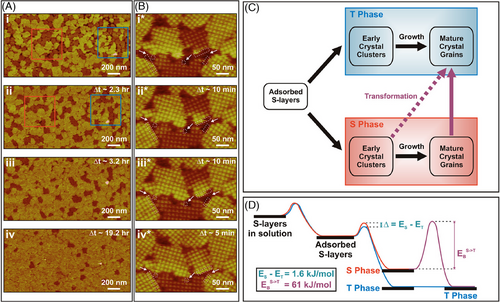
These results demonstrate that kinetic traps, which might be triggered by the properties of substrate‒solution interfaces, play an essential role in determining the pathways and phases of protein assembly (Figure 5C,D). Extended protein arrays require conformational changes from a monomeric structure to a long-range order during protein assembly. They could fold into a metastable state (a local energy minimum) and slowly relax into a thermodynamic equilibrium state. The described picture of protein multistage assembly, which is based on in situ AFM observations, could be generally applicable to the phenomenon of native protein folding and assembly.
3.1.4 Cytochrome b562 (cyt b562-(H63C)) assembly on HOPG
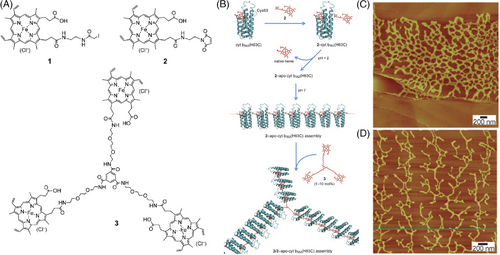
In addition to hydrophilic surfaces, protein assemblies on hydrophobic surfaces have also been investigated by in situ AFM. For example, Kitagishi et al. studies the assembly of the hemoprotein system on HOPG.[151] Cytochrome b562 is a subclass of cytochromes that play a vital role in energy transfer by undergoing reduction and oxidation.[182] The heme group bonded to the protein can also undergo these reversible reactions. Externally introduced heme analogs, such as heme 1, can bind to the surface of a H63C single mutant of cytochrome b562 (cyt b562-(H63C)) through a thiol-reactive group after knocking out the native heme of the modified protein. Variable morphologies of synthetic heme analogs on HOPG substrate were characterized by AFM, which have regioisomers regarding the substitution position in the two heme propionate side chains of protoheme IX (Figure 6A).[151] Compared to analog 1, heme analog 2 has a higher reactivity to the thiol group of cyt b562-(H63C), enabling the formation of long linear protein fibers through interprotein heme‒heme pocket interactions on HOPG, 2-apo-cyt b362-(H63C) (Figure 6B). In the presence of heme triad 3 having a threefold center, cyt b562-(H63C) can form dispersed protein particles. However, the coexistence of heme 2 and 3 at appropriate molar ratios results in the assembly of cyt b562-(H63C) into a two-dimensional (2D) protein monolayer network, 3/2-apo-cyt b362-(H63C), on HOPG (Figure 6B–D). The heme triad 3 incorporates into the linear fibers as a branching point for the trident hemoprotein assembly. The assembly continues to expand and diverge, eventually forming unique 2D hemoprotein networks on HOPG. This design strategy for controlling 1D and 2D supermolecular architectures on HOPG holds promise in developing protein-based materials.
3.2 Imaging of protein dynamics on lipid membranes
One key aspect of protein pivotal roles in modulating the biological membrane functionality is their ability to oligomerize and self-assemble on lipid membrane surfaces. These protein complexes and supramolecular structures contribute to the membrane organization, stability, and function.[19, 22, 23, 28, 32] Protein‒protein/protein‒lipid interactions and the self-assembly processes are tightly regulated and essential for various cellular processes. Conversely, certain proteins can perturb a membrane integrity and disrupt its structural organization. For instance, specific toxin proteins can interact with membranes, leading to membrane disruption, pore formation, or alterations in membrane permeability.[183] The interplay between proteins and membranes is a complex and dynamic, underlying fundamental biological processes. AFM provides opportunities to in situ study the dynamics of these proteins on lipid membranes in a near-neutral environment.
3.2.1 Soluble protein on supported lipid membrane
S-layers lattice on lipid membrane
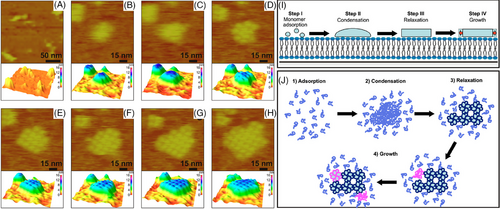
Similar to multistep assembly on mica, in situ AFM can be used to observe the assembly of S-layer proteins on supported lipid bilayers through a multistep process.[87, 148] Unlike the assembly on mica controlled by mica‒liquid interfaces and showing the phase transition between a metastable crystal phase and a thermodynamically stable phase (Figure 5); S-layer nucleation on supported lipid bilayers is mainly controlled by the folding kinetics between condensed liquid-like amorphous clusters and a crystalline structure (Figure 7). Monomers initially from a mobile adsorbed phase on the lipid membrane and transform into amorphous/liquid-like clusters (Figure 7A–C). These clusters then further undergo a phase transition into crystalline arrays composed of compact tetramers through simultaneous folding, and rearranged tetramers occur in the clusters (Figure 7C,D). Following growth occurs through new tetramer formation at the array edges—a template-guiding process with the existing tetramer matrix as a template (Figure 7E–H).[87] Growth kinetics reveal that tetramer formation of the protein folding is the growth-limiting step (Figure 7I,J). The barrier of the new tetramer formation is significantly reduced by the pre-folded tetramers at the edges of the crystal clusters.
Perforin-2 (PFN2, MPEG1)
Membrane attack complex/perforin-like (MACPF) proteins are a huge family of pore-forming proteins.[184] The primary role of MACPF proteins is to form transmembrane pores in membranes with various downstream biological effects, including targeted cell destruction by transport of granzymes and solute homeostasis devastation.[185-190] These proteins play a critical function in immune defense and characteristically undergo a multistep conformational transition from water-soluble monomers into large β-barrel pores.[137, 186, 189-206] The multistep pore formation mechanism commonly includes the following steps: (i) soluble MACPF monomers are recognized and recruited by membrane receptors or lipids onto membrane surface and (ii) MACPFs activate and oligomerize into ring- and/or arc-shaped pre-pore and pore structures upon membrane binding.
As a member of the MACPF family, PFN2 is a crucial effector in killing engulfed bacteria in the phagolysosome by forming pores in the cell membranes.[207] PFN2 is a critical component of the innate immunity conserved throughout the animal kingdom and is close to the MAC and perforin-1 in evolution.[208-211] Despite extensive exploration of the MACPF pore formation mechanism and recent structure advances of both pre-pore and pore states of PFN2 and MAC using cryo-electron microscopy (cryo-EM), the pre-pore-to-pore transition mechanisms and related dynamics of MACPF remain primarily unknown.[189, 190, 198, 200, 202, 203, 212]
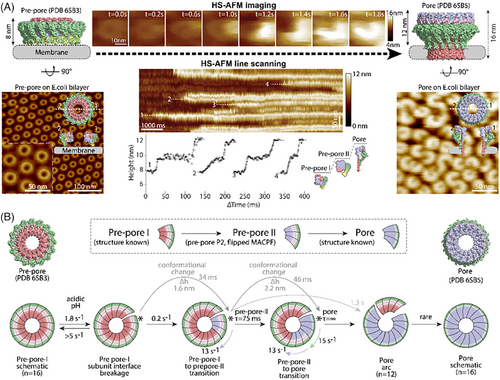
With the help of HS-AFM and HS-AFM-LS, Jiao et al. investigated the pre-pore-to-pore transition mechanism of PFN2.[137] Using HS-AFM with sub-second temporal resolution (200 ms) (Figure 8A, top center), PFN2 oligomers were revealed to proceed in a multistep, clockwise conformational transition. In this transition: (i) pre-pore rings broke open upon triggering by acidification, (ii) the subunit located clockwise at the breakage site proceeded a conformational transition from the original pre-pore state (referred to as pre-pore-I) to an intermediate state (referred to as pre-pore-II) with ∼1.6 nm increase in height, (iii) the conformational transition further propagated to adjacent subunit in clockwise direction, (iv) the subunit in initiating site proceeded further conformational transition from the intermediate pre-pore-II state to the pore state by ∼2.2 nm increase in height, and (v) was also propagated clockwise to the neighboring subunit. The pre-pore-to-pore transition occurred within a few seconds. In addition, HS-AFM-LS experiments were performed to explore the transition dynamics of PFN2 (Figure 8A, bottom center). Due to the increased temporal resolution (2 ms) of HS-AFM-LS, state transition dynamics could be quantified with discernable height plateaus. The height increases were in accordance with the HS-AFM data and showed a height increase of 1.6 ± 0.4 nm and 2.2 ± 0.2 nm of pre-pore-I to intermediate pre-pore-II and the pre-pore-II to pore, respectively. These HS-AFM-LS data revealed the conformational change speed by fitting the height profiles (Figure 8A (bottom center), B). Together, the HS-AFM imaging and HS-AFM-LS experiments determine the progressive clockwise transition mechanism and dynamics of the PFN2 pore formation (Figure 8B).
3.2.2 Integral membrane proteins reconstituted in lipid membranes
Aquaporin Z water channels in lipid membranes
Biological membranes are not passive and actively organize their proteins.[213, 214] Long-range membrane-mediated interactions, modulated by membrane properties, drive initial protein assembly.[215] Rather than direct protein‒protein contacts alone, these interactions yield nonrandom organization and domains.[216] Fundamental studies of how membranes physically orchestrate protein assembly promise to enhance our understanding of membrane organization. However, directly studying membrane‒protein interactions at single-molecule spatiotemporal resolution is still challenging.
Using HS-AFM, Jiang et al. successfully visualized and quantified the membrane-mediated interactions of E. coli water channel Aquaporin Z (AqpZ) at high spatial and temporal resolution.[71] Initially, the membrane was reconstituted with AqpZ at a low lipid‒protein ratio and covered only a tiny portion of the surface. A continuous fluid lipid membrane was then formed by adding lipids with defined hydrophobic thicknesses for the membrane proteins diffusion and interaction. Membrane protein association/dissociation (Figure 9A) and unbounding diffusion (Figure 9B) were measured in C14, C16, C18, and C20 phosphatidylcholine lipid membranes by HS-AFM and HS-AFM-LS. Based on these measurements and a mechanical model of the lipid membrane, the researchers revealed that the interaction energies are proportional to the hydrophobic mismatch between the protein and lipid membranes. The oligomerization energetics of AqpZ-W14A were estimated based on statistical observations of non-tetrameric complexes (Figure 9C). The protomer interaction is weakest (∼ −2kBT) in C20 lipids, which matches the protomer interface hydrophobic thickness. AqpZ diffusion speed is inversely proportional to the hydrophobic mismatch of the lipids (thicker and thinner). The most favorable membrane‒protein interaction was estimated (∼ −6.5kBT) in lipids with a good match in the membrane thickness.
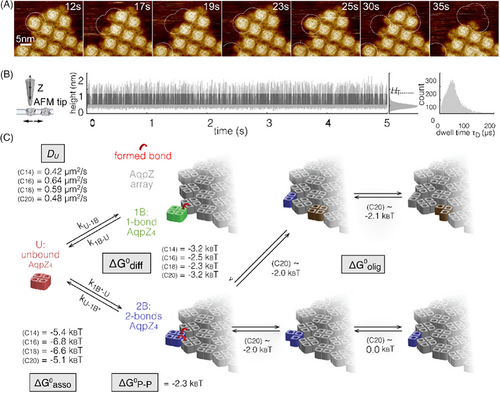
Elucidating the principles of membrane assembling and regulating protein organization is crucial to understanding cellular processes. These results demonstrate how membrane properties directly control AqpZ interactions and dynamics at the single-molecule level. By revealing how lipid membranes assemble and regulate model membrane proteins, this work establishes a framework for understanding the active role of membranes in orchestrating protein function. The methodology can be broadly applied to determine membrane contributions to the structure and function of other proteins.
4 SUMMARY AND PERSPECTIVE
AFM is an essential tool for studying protein assembly, which allows researchers to unravel the intricate mechanisms governing the hierarchical organizations and structural dynamics of protein complexes across spatial and temporal scales.[64, 65, 94, 130, 217] It provides opportunities to directly observe the intermediate states and pathways involved in the assembly process. By capturing conformational evolutions, AFM provides unique knowledge of how molecular folding and structural rearrangements affect protein complexes and their functions.[124, 126, 129, 139, 218] Furthermore, AFM can elucidate the inter- and intra-molecular interactions involved in protein assembly, providing valuable information on the structures, dynamics, properties, and functions of native protein complexes to further inspire artificial protein engineering.[81, 125, 140, 150]
Specifically highlighted in this review are investigations of the structure-dependent biological functions of membrane proteins in physiological environments with in situ AFM. AFM can reveal the spatial distribution and organization of protein domains within lipid bilayers. In situ AFM provides insights into protein clustering, lateral mobility, and interactions to describe the mechanisms of signaling pathways, membrane-bound enzymes, and artificial biomaterials.[70, 73, 93, 148] The knowledge of the protein assembly associated with diseases could help shed light on the development and progression of neurodegenerative disorders, as well as facilitate the formulation of targeted therapies.[70] In addition, researchers can uncover the fundamental principles governing the formation, stability, and function of engineered and de novo-designed proteins.[57, 68, 75, 130, 141, 219] These achievements have significant applications in various fields, including biophysics, biomaterials engineering, and medicine.
However, AFM still encounters challenges in many aspects. Initially, the high spatial resolution of AFM is limited by the probe sizes and shapes, requiring suitable probes and data processing to optimize resolution across sample types. Second, AFM is predominantly suited for surface analysis and faces challenges while dealing with sub-surface structures or sample thickness. Third, although tapping mode and PeakForce tapping mode can reduce probe‒sample interaction, AFM tips may still cause potential damage or distortions to soft and fragile bio-samples, leading to imaging artifacts on sample morphologies and alterations to sample properties. Consequently, professional practice is crucial to minimize the potential damage and preserve sample integrity. Furthermore, AFM is unable to directly measure chemical information. In the case of protein assembly, although several methods have been developed to indirectly correlate protein's chemistry fingerprints, such as the secondary structures and the functional groups, with the morphologies of protein complexes, AFM is not a chemistry-sensitive approach. Last, the more immense data generated by AFM necessitates more sophisticated analysis.
- 1)
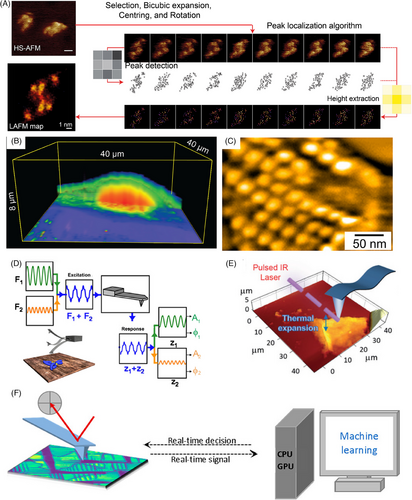
Inspired by super-resolution fluorescence microscopy, localization-based AFM (LAFM) achieves super-resolution imaging of biomolecules under physiological conditions by locating features in multiple HS-AFM images and reconstructing a single high-resolution image (Figure 10A).[66] It could visualize near-native biomolecular structures.
- 2)
Nano-endoscopy AFM enables 3D visualization of native proteins and organelles inside living cells, allowing to systematically study cellular machinery across spatiotemporal scales—from molecules to complexes to functional domains (Figure 10B).[220] By probing living systems at the nanoscale, nano-endoscopy AFM can reveal principles of biological complexity that emerge from coordinated molecular interactions. Integrating nano-endoscopy AFM with HS-AFM may correlate dynamics across multiple levels of cellular organization to understand how cells function as integrated systems. Illuminating life's elemental building blocks in their native states, nano-endoscopy AFM promises foundational discoveries in cell biology and beyond.
- 3)
Due to the dynamic movement of membrane proteins and the flexibility of the plasma membrane, directly imaging the membrane proteins in live cell cytoplasmic membranes remains challenging. The low invasiveness of HS-AFM has improved the feasibility of AFM characterization on the surface of live cells, as proven by the evidence of AQP0 arrays on lens fiber cells, the actin cortex bound to the cytoplasmic face of the plasma membrane, the mosaic outer membrane of live negative bacteria, structure movements in living Mycoplasma (Figure 10C), etc.[60, 61, 221-223]
- 4)
The advent of bimodal AFM enables the measurement of molecular mechanics while contouring topography at high imaging speeds (Figure 10D).[59, 224, 227] Functionalizing AFM probe also enables probing bio-specific interactions and biochemical imaging, offering a singular platform to quantify, visualize, and manipulate non-covalent interactions and processes.[94, 228] Despite these advancements, further progress in refining the imaging mode and AFM probe design is still required to address the ongoing need for comprehensive characterization of sample properties while fulfilling the demand for high-speed morphology imaging.
- 5)
Combining AFM with infrared spectroscopy (AFM-IR) has proven highly efficient in simultaneously obtaining morphological and chemical analyses of biomacromolecule assemblies (Figure 10E).[225, 229] Upon light adsorption, biomolecules exhibit thermal expansion, which an oscillating AFM probe can detect. The modulated amplitude of the AFM cantilever scales with the IR adsorption coefficient at each wavenumber.[225] AFM-IR has been widely used to investigate the secondary structures and intermolecular interactions of peptides and/or proteins at sub-10-nm resolution.[225] Furthermore, recent advances have made this technique applicable to study in situ/in vitro biomolecular self-assembly.[230, 231]
- 6)
The emergence of HS-AFM and LAFM applicable in multiparametric experiments has dramatically increased data size and dimensionality.[232] To cope with the massive, multidimensional datasets generated by the advancing scanning probe microscopy techniques, artificial intelligence (AI) frameworks for exploring structural and material properties are rapidly emerging.[233-236] With constantly expanding datasets and improved workflows, deep learning is essential to fully exploit the potential of AFM for obtaining theoretical mechanisms and guiding rational design across nanoscopic to macroscopic scales.[237] Combining AFM experimentation, simulation, and AI could systematically unravel the dynamic complexity of protein assembly at multiple scales.[15, 16, 238] Furthermore, the development of automated AFM empowered by the recent breakthrough in machine learning/AI is underway, such as the human-in-the-loop automated AFM (Figure 10F).[226, 239] The interactive AI-driven AFM presents a promising avenue for optimizing imaging parameters, reducing the invasiveness of the samples, and promoting the investigation of new principles behind the structure‒function relationship.
In summary, AFM is a powerful tool for investigating protein assembly, offering unique capabilities to image and characterize the hierarchical organization, structural dynamics, and functional properties of biomolecular assemblies. AFM contributes to our understanding of fundamental biological phenomena and biomaterial structures and properties through its ability to probe the intricacies of protein assembly processes. AFM has the potential for continued transformation into a multifaceted platform integrating multimodal and multidimensional analysis through technological, methodological, and computational progress. By further coalescing tools for correlating structure with function across otherwise inaccessible spatiotemporal scales, AFM can systematically decode the complexity of hierarchical assembly in living systems and reconstruct it in silico, in service of science and technology alike. Broader horizons await as AFM's ever-expanding capabilities converge at the nexus of the physical and life sciences.
ACKNOWLEDGMENTS
Z.Z. and F.J. acknowledge support from the National Natural Science Foundation of China (NSFC 32371525, T2221001, 92353304, and T2350011) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB37020105). S.S. and S.Z. acknowledge support from the U.S. Department of Energy (DOE), Office of Science (SC), Office of Basic Energy Sciences (BES), Division of Materials Science and Engineering, Biomolecular Materials Program under award no. FWP 65357 at Pacific Northwest National Laboratory (PNNL). S.Z. and Z.L. acknowledge support from the U.S. DOE-SC-BES as part of the Energy Frontier Research Centers (EFRC) program: Center for the Science of Synthesis Across Scales (CSSAS) at the University of Washington under award no. DE-SC0019288. S.Z. acknowledges support from the U.S. DOE-SC-BES as part of EFRC program: CSSAS (award no. DE-SC0019288) through FWP-72448 at PNNL. PNNL is a multiprogram national laboratory operated for DOE by Battelle under contract no. DE-AC05-76RL01830.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Biographies

Zhaoyi Zhai earned his master's degree from the National Center for Nanoscience and Technology of China in 2021. He joined Professor Fang Jiao's research group as a research assistant in the same year and is pursuing a joint PhD program. His research interests focus on the assembly and dynamics of pore-forming proteins.

Sakshi Yadav Schmid is an NCCR WINS Fellow (Center of Competence in Research for Bio-Inspired Material, Women in Science) and a scientific collaborator in the SunMIL Laboratory at École Polytechnique Fédérale de Lausanne, Switzerland. Previously, she was a Postdoctoral Research Associate at Pacific Northwest National Laboratory. She obtained her PhD in Chemical Engineering from Lamar University, USA. Her research focuses on biomineralization, biomolecule self-assembly, and organization at nanoscale. She is currently investigating the dynamics of biomolecular building block assembly into hierarchical structures and exploiting these building blocks' designed interfaces and anisotropic nature. To study the emergence of order and the thermodynamic and kinetic parameters that control the assembly at nanoscale, atomic force microscopy and X-ray scattering at high spatiotemporal resolution are used.

Shuai Zhang is a Materials Scientist at Pacific Northwest National Laboratory (PNNL) and an affiliate faculty in the Department of Materials Science and Engineering (MSE), University of Washington (UW). He obtained his PhD at the Interdisciplinary Nanoscience Center, Aarhus University, Denmark, in 2014. He began to work at PNNL as a postdoctoral research associate in 2015 and became a research assistant professor at UW MSE in 2019. He rejoined PNNL as a materials scientist in 2023. Dr. Zhang's research delves into the design of biomolecular materials through assembly mechanisms, focusing on elucidating the principles governing assembly dynamics, structures, and hierarchies, employing cutting-edge in situ/operando scanning probe microscopies. Additionally, he spearheads investigations in mineralization, two-dimensional materials, and surface sciences with applications in clean energy technologies.

Fang Jiao is an Associate Professor at the Institute of Physics, Chinese Academy of Sciences, Beijing, China. He obtained his PhD from East China Normal University, Shanghai, China, in 2016. After the doctorate, he continued as a postdoctoral researcher at Weill Cornell Medicine, New York, NY, USA, and the Swiss Federal Institute of Technology Lausanne (École Polytechnique Fédérale de Lausanne), Lausanne, Switzerland. His research interests include developing and using in situ and high-speed atomic force microscopy to understand biomolecules (pore-forming proteins, membrane proteins, cytoskeletal proteins, etc.), self-assembly, dynamics, and working mechanisms in physiological environments.




