Dynamic color-tunable ultra-long room temperature phosphorescence polymers with photo-chromism and water-stimuli response for multilevel anti-counterfeiting
Abstract
Developing dynamic color-tunable ultra-long room temperature phosphorescence (URTP) polymers with afterglow of over 1 s, photo-chromism, and multi-stimuli response for practical anti-counterfeiting and information security applications is attractive but very challenging. Herein, by doping multicolor phosphorescence pyridinium bromide L block or viologen-based photo-chromic V block into polyvinyl alcohol matrixes, the water-stimuli-responsive color-tunable URTP polymer films with afterglow of up to 8 s and the reversible viologen-based photochromic polymer films have been developed. More significantly, a series of dynamic color-tunable URTP polymer films with ultra-long afterglow of over 6 s, photo-chromism, and water-stimuli response have been successfully exploited by integrating L and V blocks into one polymer system. Mechanistic investigations have revealed that their photo-chromism mainly comes from the photo-generated viologen free radicals. Furthermore, their dynamic multilevel anti-counterfeiting applications have been demonstrated. These results pave the way to develop smarter multifunctional URTP materials for anti-counterfeiting and optical sensing.
1 INTRODUCTION
Ultra-long room temperature phosphorescence (URTP) materials with a long lifetime of over 100 ms have attracted extensive attention in recent years, since they could present superior potential applications in anti-counterfeiting, information security, sensing, and optoelectronic devices in comparison to short-lived fluorescent materials.[1] Thus, many different kinds of URTP materials, including inorganic compounds,[2] organic–inorganic hybrid materials,[3] carbon dots,[4] organic crystalline compounds,[5] and polymers,[6] have been widely exploited through various synthetic strategies. Among them, the amorphous URTP polymers have aroused great enthusiasm due to their synthetic scalability as well as their excellent flexibility and processability for practical applications.[7] However, it remains challenging to achieve URTP polymers owing to the fact that organic materials usually possess the weak spin−orbit coupling effect and ultrafast triplet exciton deactivation under ambient conditions. Encouragingly, on the basis of many effective synthetic strategies, such as host–guest doping, supramolecular self-assembly, and polymerization, numerous URTP polymers with a lifetime of several hundred milliseconds or even longer have been created through enhancing the intersystem crossing and suppressing nonradiative transition.[8] For these URTP polymers, dynamic URTP polymers with tunable optical properties would achieve more excellent performance in comparison to static URTP polymers without tunable optical properties, when responding to external stimuli (such as solvent, heat, and light).[9] Accordingly, some novel stimuli-responsive dynamic URTP polymers have been designed by manipulating solvent-stimuli,[10] heat-stimuli,[11] light-stimuli,[12] or two of them.[13] However, most of the reported dynamic URTP polymers are still limited in realizing few adjustable and stimuli-responsive optical properties. In order to further improve their performances for advanced practical applications, dynamic URTP polymers with more switchable properties (simultaneously involving emission color, intensity, phosphorescence lifetime and structural color) and richer external stimuli-responsiveness are highly desirable.
Till now, many efforts have been input on exploring novel dynamic color-tunable URTP polymers with photo-chromism. Unfortunately, most of the reported color-tunable photo-chromic RTP polymers, prepared through the copolymerization method, only display too short afterglow, which is unobservable by naked eyes.[14] Recently, simply doping single photochromic molecules into polymer matrixes has produced some intriguing color-tunable photo-chromic URTP polymers with afterglow captured by naked eyes. For instance, Xu and coworkers have constructed a dithienylbenzothiophene-doped photo-chromic URTP polymer with bright green afterglow (about 0.2 s).[15] In addition, Wang[16] and An[17] have developed naphthalene-diimide-doped color-tunable photo-chromic URTP polymers with red afterglow of about 0.2 s. More recently, doping weak emissive photo-responsive benzoic acid into the PVA matrix could produce photo-chromic URTP polymers with yellow afterglow (less than 0.5 s).[18] Notably, the observed afterglow time of the reported doped photo-chromic URTP polymers is still too short (<1 s). Moreover, most of them are still limited in controlling light stimuli to tune the optical properties. For more advanced applications, it is highly desirable to develop simple methods to obtain dynamic photochromic URTP polymers with the long-lived afterglow (above 1 s) and the good capability of responding to multiple external stimuli.
Herein, a series of dynamic multi-stimuli responsive color-tunable photo-chromic URTP polymers have been successfully developed by integrating multicolor phosphorescence units and photochromic blocks into one polymer system. Specifically, we first constructed a multicolor luminescent naphthalene-based pyridinium bromide with phenylboric acid (L), which acts as a multicolor phosphorescence block in the construction of multi-color tunable phosphorescence polymer.[19] Next, a viologen-based block V containing a phenylboric acid group was prepared to achieve the interesting photo-chromic functionality inspired by some viologen-based photo-chromic materials.[20] Subsequently, the PVA polymer matrix was selected because of its abundant hydrogen bonds, which could not only provide reactive sites to couple with arylboronic acid to form dynamic B─O covalent bonds, but also effectively block oxygen and restrict rotational motions of luminescent molecules to achieve long lifetime RTP.[10, 21] Importantly, it is demonstrated that, by doping block L or block V into PVA matrixes, the intriguing water-stimuli response color-tunable URTP polymer films (1%-L-PVA and 5%-L-PVA) and the reversible photochromic polymer film 5%-V-PVA have been successfully constructed. Remarkably, the afterglow of the 1%-L-PVA film is up to 8 s. In order to further integrate functionalities of both block L and block V in a polymer system, we have mixed aqueous solution of 1%-L-PVA and 5%-V-PVA at a certain volume ratio. It was found that three films, prepared from both mixed aqueous solutions (1%-L-PVA@5%-V-PVA-1-1, 1%-L-PVA@5%-V-PVA-2-1 and 1%-L-PVA@5%-V-PVA-3-1), exhibited the dynamic color-tunable URTP with ultralong afterglow of over 7 s and color changes in response to light/water stimuli. Moreover, by codoping block L and block V into PVA matrix at a certain mass ratio, three dynamic color-tunable URTP polymer films (PVA@L-V-1, PVA@L-V-2 and PVA@L-V-3) with ultra-long afterglow, photo-chromism and water-stimuli response have also been exploited. Notably, the afterglow of both PVA@L-V-1 and PVA@L-V-2 films are over 6 s. These results pave the way to develop smarter dynamic multifunctional color-tunable URTP materials for anti-counterfeiting and optical sensing.
2 RESULTS AND DISCUSSION
2.1 Color-tunable URTP polymers
In order to develop the color-tunable photochromic URTP polymers, we envisioned that integrating phosphorescence units and photo-chromic blocks into one polymer system would be an effective method. We first prepared a multicolor luminescent naphthalene-based pyridinium bromide with phenylboric acid group (L) through reacting 2,6-di(naphthalen-2-yl)−4,4′-bipyridine (L0) with 4-(bromomethyl)phenylboronic acid (Figure 1A and Supporting Information).[19] The dilute methanol solution (10−3 mol L−1) of L exhibited a weak orange emission (λmax = 615 nm) with the quantum efficiency of 1% (Figure 1B). Then, upon adding water into the solution of L, the resulting solution aggregation state showed an enhanced green emission (λmax = 512 nm) with the quantum efficiency of 6.3% (Figure 1B). Moreover, L in the crystalline state displayed a yellow green emission with the red-shifted maximum emission peak at 535 nm and a quantum efficiency of 14.0%. The differences in these emissions were likely due to the diverse aggregation and packing modes that affect molecular free mobility (Figure S1).[22] As a result, the ionic compound L was capable of displaying multi-color tunable emission similar to the previously reported compounds,[19, 22, 23] which could be readily employed in the construction of multi-color tunable functional polymers.
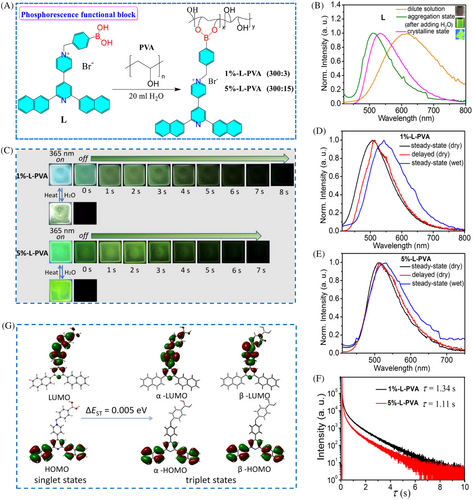
Next, the integration of L into the PVA polymer matrix was performed. First of all, by reacting PVA with L at a mass ratio of 1% in 20 mL water, the homogeneous aqueous solution of polymer 1%-L-PVA (PVA/L = 300:3) was obtained. Then the transparent amorphous film of 1%-L-PVA could be prepared through dripping the corresponding aqueous solution onto a quartz plate and drying at 60°C (Figure 1A and Figure S2). The 1%-L-PVA film exhibited a strong cyan emission when excited by a 365 nm UV lamp (Figure 1C and Movie S1). Impressively, after switching off the UV lamp, the yellow green afterglow could last for about 8 s. Similarly, in the case of polymer 5%-L-PVA (PVA/L = 300:15) with a mass ratio of 5%, its film displayed the bright green emission under 365 nm UV light excitation and yellow green afterglow lasted for about 7 s after switching off the UV lamp (Figure 1C and Movie S2). Furthermore, the steady-state photoluminescence (PL) spectra demonstrated that the maximum emission peaks of 1%-L-PVA and 5%-L-PVA located at 507 and 513 nm upon 365 nm excitation, respectively, and their corresponding photoluminescence quantum yield (Φ) were determined to be 8.7% and 7.4%, respectively (Figure 1D,E, Table 1). In addition, it was found that the delayed PL spectra of two films (λmax = 513 nm, delay 1 ms) were close to both steady-state emission bands (Figure 1D,E). The fluorescence lifetime of 1%-L-PVA (τ = 9.2 ns) and 5%-L-PVA (τ = 9.6 ns) films (Figures S3 and S4; Table 1) and RTP lifetime of 1%-L-PVA (τ = 1.34 s) and 5%-L-PVA (τ = 1.11 s) films (Figure 1F and Table 1) were determined, respectively. Notably, 5%-L-PVA, compared to 1%-L-PVA, displays a relatively shorter phosphorescence lifetime, mainly due to the high concentration of block L, which may easily induce the intermolecular energy transfer, leading to the deactivation of the excited triplet state. These results indicated that doping the phosphorescence block L into the PVA matrix could construct the intriguing URTP polymers, and tuning the doping ratios could regulate the emission properties of the resulting polymers. Especially, the relatively low doping concentration of block L could achieve better performance.
| Polymer films | 𝜆F [nm]a | τF [ns]b | 𝜆P [nm]c | τP [s]d | Φ [%]e | τP [s]f | 𝜆F [nm]g |
|---|---|---|---|---|---|---|---|
| 1%-L-PVA | 507 | 9.2 | 513 | 1.34 | 8.7 | / | 540 |
| 5%-L-PVA | 513 | 9.6 | 513 | 1.11 | 7.4 | / | 531 |
| 1%-L-PVA@5%-V-PVA-1-1 | 490 | 6.8 | 515 | 1.16 | 4.5 | 0.80 | 538 |
| 1%-L-PVA@5%-V-PVA-2-1 | 497 | 8.0 | 513 | 1.17 | 5.0 | / | 537 |
| 1%-L-PVA@5%-V-PVA-3-1 | 497 | 8.3 | 513 | 1.22 | 6.2 | / | 537 |
| PVA@L-V-1 | 511 | 7.7 | 511 | 1.22 | 5.8 | 1.13 | 538 |
| PVA@L-V-2 | 511 | 6.7 | 511 | 1.04 | 5.6 | 0.47 | 538 |
| PVA@L-V-3 | 520 | 5.3 | 511 | 0.86 | 2.8 | 0.15 | 540 |
- a The maximum emission peaks of the steady-state PL spectra in dry states (λex = 365 nm);
- b The corresponding fluorescence lifetime in dry states (λex = 365 nm);
- c The maximum emission peaks of the delayed PL spectra in dry states (λex = 365 nm, delay 1 ms);
- d RTP lifetime in dry states (λex = 365 nm);
- e Photoluminescence quantum yields in dry states (λex = 365 nm);
- f RTP lifetime in dry states after continuous irradiation (λex = 365 nm);
- g The maximum emission peaks of the steady-state PL spectra for various polymer films wetted by water (λex = 365 nm).
In order to gain deeper insight into photophysical characteristics of polymer 1%-L-PVA, time-dependent density functional theory (TDDFT) calculations for the optimized geometry of L have been carried out at the B3LYP/6-31G(d) level, and the calculated energy levels and electronic transition characters of singlet states (Sn) and triplet state (Tn) of L are given in supporting information (Figure 1G; Tables S1 and S2). As shown in Figure 1G, the highest occupied molecular orbitals (HOMOs) of singlet states and triplet states are predominantly distributed on two naphthalene rings, and a small amount was distributed on the adjacent pyridine ring, and the lowest unoccupied molecular orbitals (LUMOs) of singlet states and triplet states were mainly distributed on pyridinium ring and the adjacent methylene benzene. These results clearly suggested the extended intramolecular electron transfer characteristics. Moreover, excited states 17, 19, 24, 38, 39, 51 of the calculated singlet states in monomer state were 3.0567 eV (405.61 nm), 3.2026 eV (387.14 nm), 3.5081 eV (353.42 nm), 3.9984 eV (310.08 nm), 4.0153 eV (308.78 nm), 4.3945 eV (282.13 nm), which are in good line with the experimental absorption band (Figure S5). Excited states 14, 15, 17, 30, 32, 40 of the calculated triplet states in monomer state were 2.1783 eV (569.18 nm), 2.2458 eV (552.06 nm), 2.3456 eV (528.59 nm), 2.7585 eV (449.46 nm), 2.8779 eV (430.82 nm), 3.1720 eV (390.87 nm), which are in accordance with the experimental phosphorescence emission spectra (Figure 1D,E). Notably, it was found that L exhibited small singlet-triplet energy gaps (∆EST = 0.005 eV), beneficial for the intersystem crossing process according to the El-Sayed's rule, then endowing the higher likelihood in achieving long lifetime phosphorescence.[24]
We then switched our attention to water-stimuli response of these polymer films. By wetting 1%-L-PVA film and 5%-L-PVA film with water, we observed that the wet 1%-L-PVA film had the emission at 540 nm while the wet 5%-L-PVA film emitted light at 531 nm when excited at 365 nm (Figure 1D,E and Table 1), suggesting water-stimuli response in term of the apparent color differences of wet and dry films (Figure 1C). Such water-stimuli responsive color-tunable emission can be ascribed to that intramolecular/intermolecular multiple hydrogen bonds of polymer films are destructed by water invasion, allowing free rotation of the luminescent block L.[10, 17] Therefore, the afterglow of both 1%-L-PVA and 5%-L-PVA films in wet states disappear (Figure 1C). After drying at 60°C, their corresponding emission color could be restored to the origin states, and the phosphorescence could be observed again. All these results indicated that both 1%-L-PVA and 5%-L-PVA films could not only achieve reversible URTP, but also could exhibit the reversible water-stimuli responsive color-tunable emission. These results allowed us to believe that stimuli-responsive multiple-color URTP polymers would be successfully developed by combining the multiple-color phosphorescence functional block L into polymer matrix through tuning mass ratios.
2.2 Photochromic polymer
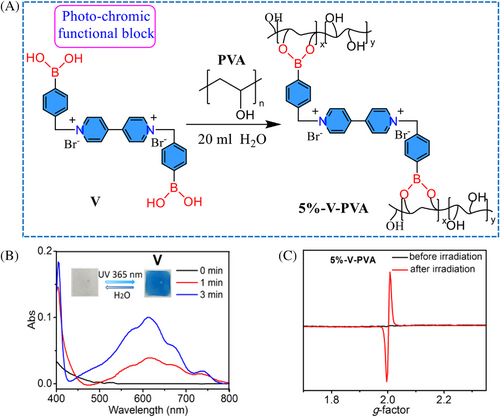
To achieve photochromic polymers, a viologen-based derivative containing arylboronic-acid group (V) block was selected because this block has been widely used in photochromic materials for anti-counterfeiting (Figure 2A and Supporting Information).[20] By reacting V with PVA in aqueous solution, a viologen-based polymer (5%-V-PVA) solution was obtained. Subsequently, the colorless 5%-V-PVA dry film was prepared by dripping the corresponding aqueous solution into a quartz plate and drying at 60°C. As expected, this non-emissive 5%-V-PVA dry film could realize the interesting photo-chromism, that is, the film quickly turned blue after 365 nm UV light irradiation (Figure 2A). The UV–vis absorption spectrum indicated that a new absorption at 613 nm appeared under 365 nm UV light irradiation for 1 min, and the new absorbance band gradually increased with continuous irradiation (Figure 2B). We believe that the appearance of the new absorbance band is likely due to the photo-generated free radical.[20] Moreover, the in situ EPR measurements indicated that no obvious free radical signal could be detectable before 365 nm UV irradiation, but the strong free radical signal (g = 2.0) could be observed after light irradiation (Figure 2C), further verifying that the photo-generated free radicals can be the cause of the photo-chromism. Noteworthy, the produced free radical could be stable for several days in dry air, which is mainly due to that PVA could effectively prevent the oxygen penetration.[17] In contrast, after wetting the colored film by water or water vapor, the blue viologen-based polymer film rapidly discolored because the permeated oxygen in wet state could quench free radicals.[20] In addition, the viologen-based polymer (10%-V-PVA, PVA/V = 100:10) has been prepared by tuning the doping ratio of block V. It is found that the 10%-V-PVA dry film also could realize the photochromism after 365 nm UV light irradiation, and then it could recover the initial state by adding water (Figure S6). In short, the viologen-based polymer film could achieve the reversible photo-chromism by light-irradiation and water stimuli.
2.3 Photo-chromic URTP polymers from polymer blending method
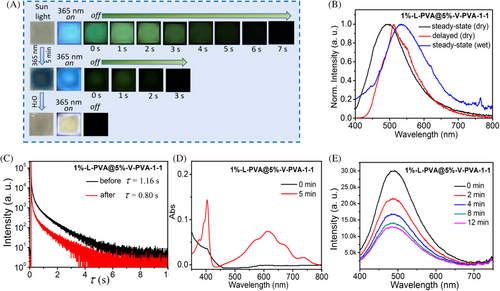
With the fundamental photo and chemical properties of L-PVA and V-PVA in hand, the following key challenge is how to integrate their merits into one polymer system to construct the more intriguing photo-chromic URTP polymer. Since the polymer blending method can allow researchers to realize the combination of the different functionalities of various functional polymers into polymer composites, we adopted this method to prepare the homogeneous aqueous solution of the polymer blend 1%-L-PVA@5%-V-PVA-1-1 by directly mixing the aqueous solution of 1%-L-PVA and 5%-V-PVA at a volume ratio of 1:1. Subsequently, the amorphous films of the corresponding polymer blends were fabricated by dripping the corresponding aqueous solutions onto quartz plates and drying at 60°C (Figure S7). We found that 1%-L-PVA@5%-V-PVA-1-1 film emitted the cyan emission upon 365 nm UV light excitation and a green afterglow of 7 s after switching off the UV lamp (Figure 3A and Movie S3). As shown in Figure 3B, the maximum emission peaks of the steady-state emission and the delayed URTP are located at 490 and 515 nm, respectively (Table 1). Moreover, the fluorescence lifetime of 1%-L-PVA@5%-V-PVA-1-1 film was found to be 6.8 ns (Figure S8) and the RTP lifetime at 515 nm was up to 1.16 s, which was slightly shorter than that of the 1%-L-PVA film (Figure 3C and Table 1). The photoluminescence quantum yield of 1%-L-PVA@5%-V-PVA-1-1 film was determined to be 4.5%. After 5 min irradiation, the color of 1%-L-PVA@5%-V-PVA-1-1 film clearly changed from colorless to dark blue (Figure 3A), and the colored film absorbed at 613 nm (similar to that of 5%-V-PVA), due to the photo-generated viologen free radical (Figure 3D). By continuously illuminating with a 365 nm UV lamp, the emission intensity of the film gradually decreased (Figure 3A,E and Movie S4). Moreover, the observable green afterglow of the blue film weakened and the related afterglow lasted for about 3 s (Figure 3A and Movie S4). The corresponding RTP lifetime decreased to 0.80 s (Figure 3C and Table 1). Noteworthy, the emission attenuation phenomenon can be ascribed to the energy transfer from the exited state of the emission center L to the increased photo-generated viologen free radical.[20] This may be supported by the fact that the broad steady-state and delayed emission spectrum of 1%-L-PVA@5%-V-PVA-1-1 partly overlapped with the absorption spectrum of the colored film containing the viologen free radicals (Figure S9). Subsequently, after dropping water on the blue film, the blue color of the film rapidly faded away (Figure 3A). Meanwhile, yellowish green emission at 538 nm but without afterglow (Figure 3A,B) was investigated, which is likely due to the fact that water could destruct intramolecular/intermolecular multiple hydrogen bonds of the polymer film.[10] More interestingly, the polymer film of 1%-L-PVA@5%-V-PVA-1-1 prepared by blend strategy could simultaneously realize multiple properties of URTP, photo-chromism, and water-stimuli responsive color-tunable emission.
Next, investigations on the ratios between aqueous 1%-L-PVA and 5%-V-PVA were conducted. Two amorphous polymer blends (1%-L-PVA@5%-V-PVA-2-1 (2:1), 1%-L-PVA@5%-V-PVA-3-1 (3:1)) were prepared (Figure S7). It was found that both 1%-L-PVA@5%-V-PVA-2-1 and 1%-L-PVA@5%-V-PVA-3-1 films exhibited similar cyan emission under 365 nm UV light excitation (Figures S10 and S16, and Movie S5 for 1%-L-PVA@5%-V-PVA-2-1 film and Movie S7 for 1%-L-PVA@5%-V-PVA-3-1 film). The related steady-state spectrum further revealed that the maximum emission peaks of both 1%-L-PVA@5%-V-PVA-2-1 and 1%-L-PVA@5%-V-PVA-3-1 films are located at 497 nm (Table 1; Figures S11 and S17). Moreover, two films exhibited the close fluorescence lifetime (about 8.0 ns) (Table 1; Figures S12 and S18), and photoluminescence quantum yields of 1%-L-PVA@5%-V-PVA-2-1 and 1%-L-PVA@5%-V-PVA-3-1 films were determined to be 5.0% and 6.2%, respectively. After removing the light resource, two films could also emit green afterglow lasting for about 7 s (Figures S10 and S16). The emission decay curves indicated that the RTP lifetime of 1%-L-PVA@5%-V-PVA-2-1 film and 1%-L-PVA@5%-V-PVA-3-1 film could reach up to 1.17 s and 1.22 s, respectively (Table 1; Figures S13 and S19). Relatively, the RTP lifetime of 1%-L-PVA@5%-V-PVA-3-1 film became longer, indicating that properly increasing the ratio of the phosphorescence functional block of the polymer blend could prolong the phosphorescence lifetime. With continuously illuminating with a 365 nm UV lamp, we could observe the gradually decreased emission of two films (Figures S10, S14, S16 and S20). After continuous irradiation for 5 min, two nearly colorless films became blue with a new absorption at 613 nm owing to the photo-generated viologen free radical (Figures S10, S15, S16 and S21). It should be noted that the 1%-L-PVA@5%-V-PVA-3-1 film only showed a light blue color because of the relatively low doping amount of 5%-V-PVA in the PVA polymer, suggesting that colored characteristics of the polymer blend are mainly dependent on the amount of the viologen block. Meanwhile, the afterglow decreased to about 5 s (Figures S10 and S16, Movie S6 for 1%-L-PVA@5%-V-PVA-2-1 film with 5 min irradiation and Movie S8 for 1%-L-PVA@5%-V-PVA-3-1 film with 5 min irradiation). After treating two films with water, the blue color of both films faded away, and the wet films exhibited the yellow-green emission, and no RTP was observed. The corresponding steady-state spectrum further indicated that two wetted films showed the similar red-shifted broad emission band with a maximum emission peak at about 537 nm (Table 1; Figures S11 and S17). After drying at 60°C, two films could recover the initial states. In brief, both 1%-L-PVA@5%-V-PVA-2-1 and 1%-L-PVA@5%-V-PVA-3-1 films exhibited the properties of URTP, photo-chromism and water stimuli-response color-tunable emission, confirming the effectiveness of the polymer blending strategy in developing novel multifunctional photo-chromic URTP polymers.
2.4 Photo-chromic URTP polymers from co-doping method
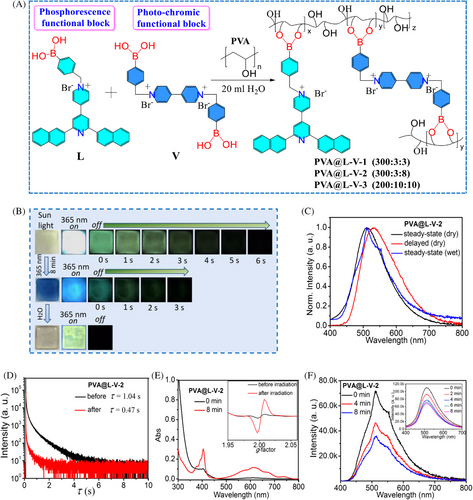
Inspired by these results, we would like to verify the effectiveness of the co-doping method in integrating the merits of both phosphorescence L block and photo-chromic V block in one polymer matrix. At first, aqueous solutions of three different polymers (PVA@L-V-1 (300:3:3), PVA@L-V-2 (300:3:8), PVA@L-V-3 (200:10:10)) were obtained by modifying mass ratios of PVA/L/V in 20 mL water (Figure 4A), and then their amorphous films were prepared by dropping their corresponding aqueous solution into the quartz plates and then drying at 60°C (Figure S22). Under a 365 nm UV lamp, both PVA@L-V-1 and PVA@L-V-2 films exhibited similar cyan emission at 511 nm, and PVA@L-V-3 film showed red-shifted green emission at 520 nm (Figure 4B, Figures S23, S24, S31 and S32; Table 1). The fluorescence lifetime of three films (PVA@L-V-1 and PVA@L-V-2 and PVA@L-V-3) were determined to be 7.7 ns, 6.7 ns and 5.3 ns, respectively (Table 1; Figures S25, S30 and S33). Photoluminescence quantum yields of three films PVA@L-V-1, PVA@L-V-2 and PVA@L-V-3 were determined to be 5.8%, 5.6% and 2.8%, respectively (Table 1). These results suggested that emission properties of the co-doping polymers could be modified by the doping ratio of the phosphorescence block L, which is in line with the foregoing observation. After withdrawing light irradiation, all three polymer films still displayed long-life yellow-green afterglow (Figure 4B, Figures S23 and S31, Movie S9 for PVA@L-V-1 film, Movie S11 for PVA@L-V-2 film and Movie S13 for PVA@L-V-3 film). The maximum emission peak of their RTP was at 511 nm, very close to that of the 1%-L-PVA film (Figure 4C and Figures S24 and S32). The afterglow captured by naked eyes of PVA@L-V-1 film and PVA@L-V-2 film lasted for about 8 s and 6 s, respectively, but PVA@L-V-3 film only showed the relatively short afterglow for about 4 s (Figure 4B and Figures S23 and S31). The recorded emission decay curves revealed that the RTP lifetimes of PVA@L-V-1 film, PVA@L-V-2 film and PVA@L-V-3 film were 1.22 s, 1.04 s and 0.86 s, respectively (Figure 4D and Figures S26 and S34), indicating that increasing the doping ratio of the viologen block could result in the decrease of the phosphorescence lifetime. Furthermore, the absorption spectra of three films before and after light irradiation (Figure 4E and Figures S29 and S37) suggested the formation of a new enhanced absorption at 615 nm, which is close to that of the colored 5%-V-PVA film and is likely induced by photo-generated viologen free radicals. To verify the radical formation, the in situ EPR measurements of PVA@L-V-2 film before and after light irradiation were implemented, suggesting that the colored PVA@L-V-2 film has a strong free radical signal (g = 2.0) (Figure 4E). Additionally, the steady-state and delayed emission intensity of three colored films became weak after light irradiation (Figures S27, S28, S35 and S36). Moreover, the afterglow captured by naked eye of PVA@L-V-1 film and PVA@L-V-2 film descended to about 6 s and 3 s, respectively, and the weak afterglow of PVA@L-V-3 film could be barely observed (Figure 4B, Figures S23, S31, and Movie S10 for PVA@L-V-1 film with 8 min irradiation, Movie S12 for PVA@L-V-2 film with 8 min irradiation and Movie S14 for PVA@L-V-3 film with 8 min irradiation). The detectable RTP lifetimes of the colored three films (PVA@L-V-1 film, PVA@L-V-2 film and PVA@L-V-3 film) decreased to 1.13 s, 0.47 s and 0.15 s, respectively (Figure 4D, Table 1; Figures S26 and S34). Consequently, the high ratio of the viologen block could shorten the phosphorescence lifetime of the colored polymers to a certain extent, which may be due to the fact that the higher the concentration of free radicals, the easier the energy transfer between the excited state of the emission center L and the violet radical occurs.[20] With respect to water stimuli response, three wetted films showed no phosphorescence, but displayed different emission colors in comparison to their corresponding dry states (Figure 4C, Figures S24 and S32).
2.5 Multilevel anti-counterfeiting applications
In view of the multiple dynamic characteristics of those smart color-tunable URTP polymers, investigations on their multilevel anti-counterfeiting applications were carried out (Figure 5A). As a proof-of-concept of the dynamic multi-level anti-counterfeiting mode, we first prepared the film of the connected five half-loops by dripping the aqueous solution of PVA@L-V-2 on a groove model and then drying at 60°C (Figure 5B). Under sun light, five half-loops were transparent with light yellow color. By irradiating five half-loops with a 365 nm UV lamp, five half-loops emitted cyan light (regarded as the first Level anti-counterfeiting). After turning off the lamp, five half-loops still emitted bright green phosphorescence lasting for about 6 s in the dark (second Level). After steadily irradiating five half-loops with a 365 nm UV lamp for 8 min, five half-loops turned blue (third Level). Then, the five half-loops only exhibited very weak emission under a 365 nm UV lamp (fourth Level). Next, after turning off the lamp again, five half-loops could only emit weak phosphorescence lasting for about 3 s in the dark (fifth level). Subsequently, the colored five half-loops could discolor in response to water vapor and then recover the initial state after drying (sixth level). Hence, the reversibly dynamic six-level anti-counterfeiting mode could be realized based on PVA@L-V-2 film.
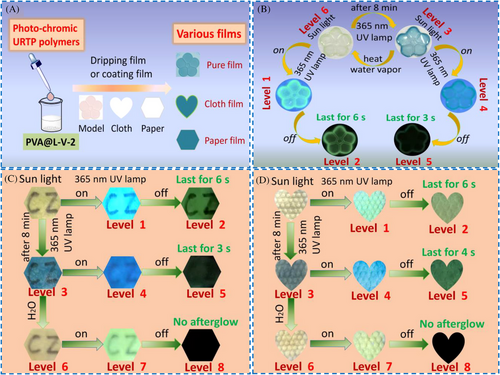
Furthermore, we explored the anti-counterfeiting application of PVA@L-V-2 in a label paper. At first, the label paper was prepared by dropping the aqueous solution of PVA@L-V-2 onto a sheet of non-luminous paper with two letters ‘CZ’ and then dried at 60°C (Figure 5C). Under sunlight, the labeled paper was yellow (Figure 5C), which displayed cyan light under a 365 nm UV lamp (first Level). After switching off the lamp, the labeled paper still could emit green phosphorescence lasting for about 6 s in the dark, and two letters ‘CZ’ could be seen clearly (second Level). After continuously illuminating the label paper with a 365 nm UV lamp for 8 min, the labeled paper became blue (third Level). Moreover, the labeled paper showed weak emission, and two letters became obscure under a 365 nm UV lamp (fourth level). After turning off the lamp again, the labeled paper only emitted weak phosphorescence lasting for about 3 s in the dark, and two letters were not clearly identified (fifth level). Next, the blue label paper discolored at a wet state (sixth level). Moreover, it was found that the labeled paper wetted by water exhibited yellow-green emission under a 365 nm UV lamp (seventh level) and showed no phosphorescence after turning off the lamp (eighth level). Finally, the labeled paper recovered the initial state after drying. Therefore, as a proof-of-concept, PVA@L-V-2 has been demonstrated to show excellent performance in the reversible dynamic eight-level anti-counterfeiting using the support of paper. Additionally, we also investigated the anti-counterfeiting application of PVA@L-V-2 using the support of cloth (Figure 5D). Similarly, the reversible dynamic eight-level anti-counterfeiting mode was verified. More importantly, by applying dynamic color-tunable photo-chromic URTP polymer as an anti-counterfeiting material, a dynamic multilevel anti-counterfeiting mode could be achieved on the basis of a solution-processable method.
3 CONCLUSION
In summary, by doping the multicolor tunable pyridinium-based phosphorescence block L and the viologen-based photo-chromic block V into PVA matrixes, respectively, the intriguing water-stimuli responsive color-tunable URTP polymer films (1%-L-PVA and 5%-L-PVA) and the reversible photochromic polymer film 5%-V-PVA have been constructed. Remarkably, phosphorescence lifetime of the 1%-L-PVA film is up to 1.22 s and the corresponding observable afterglow lasts for 8 s. More importantly, we have successfully exploited a series of dynamic color-tunable URTP polymers with ultra-long afterglow of over 6 s, photo-chromism and water-stimuli response by mixing the aqueous solution of block L and V polymers and by co-doping block L and V into PVA matrix, respectively. We confirmed that the photo-chromism is mainly resulted from the photo-generated viologen free radicals. Owing to that the dynamic photo-chromic URTP polymers could synergistically achieve unique multiple switchable optical features including emission lifetime, color, and intensity in response to light stimuli and water stimuli, these materials could present the dynamic multilevel anti-counterfeiting capability. As a proof-of-concept, the eight-level dynamic anti-counterfeiting modes have been demonstrated in different conditions on the basis of a solution-processable method. Clearly, these results pave the way to develop smarter multifunctional color-tunable URTP materials for anti-counterfeiting and optical sensing.
ACKNOWLEDGMENTS
The authors are thankful for financial support from the Priority Academic Program Development of Jiangsu Higher Education Institutions. This work was supported by the Applied Basic Research Programs of Science and Technology Commission Foundation of Jiangsu Province (No. BK20231340) and Changzhou Introduction Program of Innovative Leading Talents (No. CQ20220111), the Natural Science Foundation of Jiangsu Province (BK20170290), the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (17KJB150002), the Opening Project of Zhejiang Engineering Research Center of Fat-soluble Vitamin (202107), and the National Natural Science Foundation of China (51803143 and 22175181). The authors are thankful for measurement service from Engineering Center of School of Petrochemical Engineering in Changzhou University. Qichun Zhang thanks the support from City University of Hong Kong (9380117, 7005620, 7020040, and 7020089).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding authors upon request.




