Schiff base flexible organic crystals toward multifunctional applications
Abstract
The emergence of flexible organic crystals changed the perception of molecular crystals that were regarded as brittle entities over a long period of time, and sparked a great interest in exploring mechanically compliant organic crystalline materials toward next-generation smart materials during the past decade. Schiff base compounds are considered to be one of the most promising candidates for flexible organic crystals owing to their easy synthesis, high yield, stimuli responsiveness and good mechanical properties. This paper gives an overview of the recent development of Schiff base flexible organic crystals (including elastic organic crystals, plastic organic crystals, and flexible organic crystals integrating elasticity and plasticity) from serendipitous discovery to design strategies and versatile applications such as stimuli responses, optical waveguides, optoelectronic devices, biomimetic soft robots, and organic photonic integrated circuits. Notably, atomic force microscopy-micromanipulation technique has been utilized to bring the multifunctional applications of flexible organic crystals from the macroscopic level to the microscopic world. Since understanding mechanical flexibility at the molecular level through crystal engineering can assist us to trace down the structural origin of mechanical properties, we focus on the packing structures of various Schiff base flexible organic crystals driven by non-covalent intermolecular interactions and their close correlation with mechanical behaviors. We hope that the information given here will help in the design of novel flexible organic crystals combined with other unique properties, and promote further research into the area of mechanically compliant organic crystalline materials toward multifunctional applications.
1 INTRODUCTION
Mechanical flexibility, as one of the most important physical properties, has an impact on the performance of materials, and is a crucial factor in practical applications, traditionally relying upon elastomers, thin films of polymers or liquid crystals.[1] In contrast, organic single crystals were regarded as stiff and brittle entities over a long period of time in virtue of their long-range ordered structure with a regular pattern, dense packing and rigid overall morphology,[2, 3] although they have unique advantages in the field of optoelectronics due to less defects, uniform composition, lightweight, electron transport capacity, high carrier mobility, etc.[4-8] However, in recent years, flexible organic crystals have emerged, which changes the original perception of molecular crystals[9, 10] and sparks a great interest in the exploration of mechanically compliant organic crystalline materials.[11, 12] Flexible organic crystalline materials have a wide range of potential applications in many domains, for instance, organic optoelectronics,[13-17] mechanical actuators,[18-20] artificial mechanosensors,[18] smart biomimetics,[18, 19, 21, 22] and pharmaceutical industry.[23, 24]
Flexible organic crystals show the ability to respond to external stimuli (e.g., light, pressure, temperature, solvent, humidity), accompanied by macroscopic mechanical deformations and/or motions such as bending, jumping, twisting, hopping, curling, swimming, and self-healing.[2, 3, 18, 25] Depending upon whether original shape can be recovered, mechanical deformation is commonly classified as elasticity or plasticity.[3, 11, 12, 26, 27] In general, elastic deformation is attributed to the corrugated and/or interlocked packing structure and the presence of isotropic weak and dispersive intermolecular interactions as “structural buffering”[27-29] while plastic deformation is greatly dependent on active slip planes as well as anisotropic crystal packing through perpendicular strong and weak interactions.[26, 29] Consequently, mechanical flexibilities of organic crystals have a great relation with the underlying crystal packing determined by non-covalent intermolecular interactions as driven forces, for example, halogen bonds, hydrogen bonds, π–π stacking and van der Waals (vdW) forces.[3, 11, 12] Supramolecular chemistry and crystal engineering offer an ideal platform for a deep insight into the correlation between crystal structure and mechanical property. Mechanical flexibilities may be achieved by supramolecular self-assembly of predesigned molecules with the aid of crystal engineering approach.[26, 30, 31] The molecular-level understanding of mechanical flexibility is able to help us in the design and synthesis of novel flexible organic crystals with desired and unique functions/properties.
Over the years, reported flexible organic crystals covered various different kinds of compounds, for instance, Schiff bases,[1] pyridyl derivatives,[32] carbazole derivatives,[33] halogenated π-conjugated derivatives,[34, 35] benzil compounds,[36] indole derivatives,[37] dibenzothiophene derivatives,[38] and anthracene and naphthalenediimide derivatives.[39] Among them, Schiff base compounds are considered to be one of the most promising candidates for flexible organic crystals owing to their easy synthesis, high yield, stimuli responsiveness, and good mechanical properties.[40, 41] Up to now, many flexible organic crystals based on Schiff base have been reported, mainly in several research groups such as Ghosh et al.,[42] Reddy and co-workers,[43] Chandrasekar and co-workers,[44] Zhang and co-workers,[45] and Naumov and co-workers,[46] which could be synthesized by a simple one-step condensation reaction between aldehydes/ketones and amines, and then crystallized by slow evaporation, solution diffusion, etc.
In this paper, we summarize the recent advances of Schiff base flexible organic crystals, and focus on their packing structures based on non-covalent intermolecular interactions, the related mechanical properties as well as multifunctional applications, for example, fluorescence, polarization rotators, optical waveguides, optoelectronic devices, biomimetic soft robots, and organic photonic integrated circuits. We hope that the information given here will help to understand the relationship among molecular structure, crystal packing and mechanical property in flexible organic crystals, and thus stimulate further research into the area of flexible organic crystalline materials as future smart materials toward multifunctional applications.
2 SCHIFF BASE ELASTIC ORGANIC CRYSTALS
2.1 Schiff base elastic organic crystals in response to external stimuli
2.1.1 Stress-induced Schiff base elastic organic crystals
The Schiff base elastic organic crystals under mechanical stress were first reported by Ghosh et al. in 2015.[47] Acicular E1–E7 were prepared by slow evaporation of MeOH solution under environmental conditions, containing the corresponding halogen-substituted aniline and dichlorobenzaldehyde in a 1:1 stoichiometric ratio. Single-crystal X-ray diffraction (XRD) analysis revealed that these seven Schiff base crystals showed a similar common packing feature allowing for their high flexibility: (1) the isotropic and uniform distribution of weak/dispersive interactions (including hydrogen bonds and halogen bonds) in three orthogonal directions, which may acted as “structural buffering” during elastic deformation; (2) corrugated packing patterns, namely criss–cross interlocked arrangement of molecules in the same tape or between neighboring tapes in adjacent 2D sheets, restricting long-range molecular movement and sliding of molecular planes. Three-point bending tests indicated that all Schiff base crystals can be bent easily and the maximum elastic strain was about 2% that rivalled and even surpassed most crystalline materials (only about 0.5%), estimated based on the Euler–Bernoulli beam-bending theory and the maximum curvature of bent crystals (Scheme 1).
Subsequently, the other three Schiff base elastic crystals (E8–E10) with similar structural features (corrugated packing with interlocked structures and isotropic weak/dispersive interactions) were obtained by the synthetic method mentioned above.[48] To quantitatively capture their elastic responses, nanomechanical characterizations (nanoindentation and three-point bending) were performed. Among them, E9 displayed a lower elastic modulus (E) and moderate hardness (H) (average values of E and H: 12.76 ± 0.82 GPa and 226 ± 12 MPa for E8, 8.43 ± 0.31 GPa and 291 ± 30 MPa for E9, 12.54 ± 0.33 GPa and 367 ± 25 MPa for E10, separately), indicating a greater flexibility, which could be rationalized by its greater number of variable weak interactions as “elastic strain buffer”, for example, hydrogen bonds (C–H···O, C–H···N, C–H···F), halogen bonds (Cl···F), and π···π stacking.
In order to extend their research on the exploration of relationships among molecular structures, intermolecular interactions and mechanical properties, a series of new halogenated Schiff base crystals were synthesized via a similar method, and yet only needle-shaped E11 and E12 were elastically bendable, crystallizing in the orthorhombic P212121 and monoclinic Pc space group, respectively.[49] The elastic flexibility was probably due to their isotropic criss–cross packing with the existence of multiple weak/dispersive interactions. The former was significantly stiffer and harder in nature than the latter, indicated by their elastic recovery (∼80% for E11 and ∼70% for E12, separately), which was ascribed to different kinds of weak interactions (more supple Cl···Cl and C─H···Cl in E11; relatively stronger C─H···N, C─H···O, and C─H···F in E12). It has been observed that slight variations in molecular structures, non-covalent intermolecular interactions and packing features all play a decisive role in tuning mechanical properties of molecular crystals.
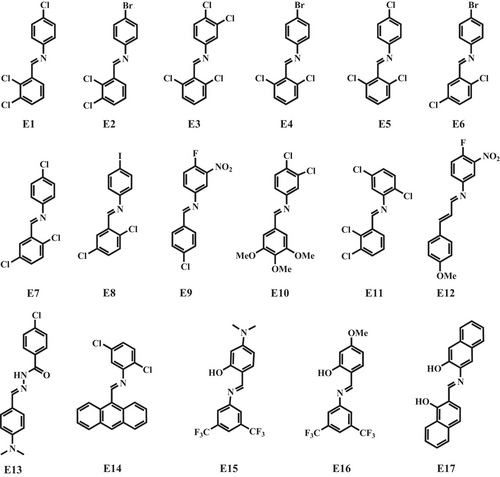
Furthermore, solvent molecules play an important part in regulating the mechanical and photophysical properties of crystals, as in the case of hydrazide-based E13.[50] Solvent-free E13a, growing during solution diffusion (dichloromethane/hexane), exhibited a weak luminescence with the maximum at 449 nm (quantum yield ΦF = 0.03) while its polymorph E13b, produced by vacuum deposition at 200°C, displayed a strong green emission with the maximum at 518 nm (ΦF = 0.19), which was attributed to the more planar conformations in E13b with smaller dihedral angles of 23.68° and 63.99° compared with a more twisted conformation in E13a with the dihedral angle of 72.69° (Figure 1A). The former presented good elasticity whereas the latter could not be bent, presumably as a result of their different packing modes where molecular chains in E13b, constituted by neighboring molecules through hydrogen bonds C═O···H─N along the c axis, possessed different orientations unlike the same molecular orientations in E13a, leading to an unhomogeneous stacking that prevented it from elastic bending.
With a view to combining the advantages of such two solvent-free polymorphs, three solvated crystals (E13c–E13e) with good elasticity and bright emission were obtained by solution diffusion methods, using dichloromethane and different alcohols (methanol, ethanol, and propanol) as solvents.[50] Solvent molecules interacted with hydrazide-based E13 through intensive hydrogen bonds that could serve as a buffer and disperse energy during the bending process, forming infinite molecular chains and presenting similar packing structures (Figure 1B). As compared with E13a, all the three crystals showed bright emissions peaking at 473 nm for E13c, 465 nm for E13d, and 461 nm for E13e with greatly enhanced quantum yields (ΦF = 0.48, 0.55, and 0.59, respectively). Intensive hydrogen bonds owing to the insertion of solvent molecules would make their conformations tend to be planar, and indirectly enhance fluorescence intensities of crystals, thus benefitting the improvement of emission behaviors. Prominently, these solvated crystals were potential candidates for flexible optical waveguides with the calculated optical loss coefficients of 0.149, 0.147, and 0.089 dB mm−1 for the straight crystals, and 0.167, 0.166, and 0.093 dB mm−1 for the bent crystals, respectively. Accordingly, molecular conformations and packing modes are both crucial factors to regulate mechanical properties and photophysical properties of crystals.
Since polymorphs possess different molecular conformations, non-covalent interactions or/and underlying crystal packings, polymorphic strategy is often utilized to tune physicochemical properties of molecular crystals, for example, solubility, granularity, permeability, tabletability, optical, and mechanical properties. Concomitant polymorphs (E14a and E14b) of anthracene Schiff base grew from solvent hexane.[51] Long needle-shaped E14a was elastically bendable whereas block-shaped E14b was brittle in nature, which were closely correlated with their underlying crystal packings, namely isotropic packing with crisscross arrangement of neighboring tapes and interpenetrating ladder-type network structure, respectively.
In a similar manner, two salicylaldehyde aniline derivatives (E15a and E16a), together with their polymorphs (E15b and E16b), were successfully prepared by adjusting crystallization conditions,[40] which exhibited different photophysical properties and mechanical behaviors. E15a showed orange emission at 590 nm while E15b presented yellow emission at 579 nm. The stronger π⋯π interactions in E15a were responsible for the red shift relative to E15b. For E16a, emission spectrum illustrated a bright yellow–green fluorescence at 538 nm. And yet, that of E16b could not be got owing to the existence of photochromic phenomena caused by its distorted molecular conformation. On the part of mechanical behaviors, E15a displayed better elasticity than E15b, mainly resulting from the reversible variation of stronger π⋯π interactions and relatively isotropic packing with the interlocked arrangement; the presence of separated layers in E16a determined its plasticity while the highly distorted conformation in E16b facilitated its brittleness, restricting the relative movement of molecules and their slippages.
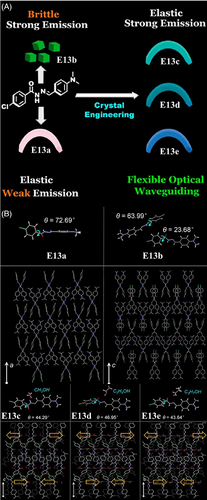
Additionally, it is interesting to note that some Schiff base organic small molecules possess both crystalline solid-state flexible properties and solution-state relevant properties such as ion sensing[52] and fluorescent probe for live cell imaging.[53] Take naphthalene-based E17 for instance,[53] the crystal could be elastically bent, which was attributed to the intermolecular C─H···π interactions and O─H···O hydrogen bonds, inferred by powder XRD and Raman studies. In the solution state, the two-photon excitation ability of E17 made it as a non-cytotoxic and photostable fluorescent probe to selectively label the mitochondria of live cells and tumor spheroids (Figure 2). The study here holds important implications for the diagnosis and treatment of mitochondrial-related diseases.
2.1.2 Multiple stimuli-responsive Schiff base elastic organic crystals
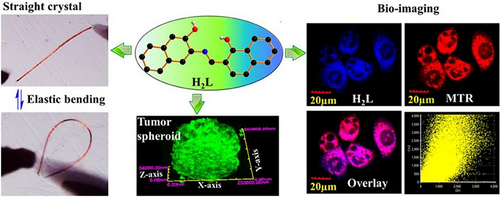
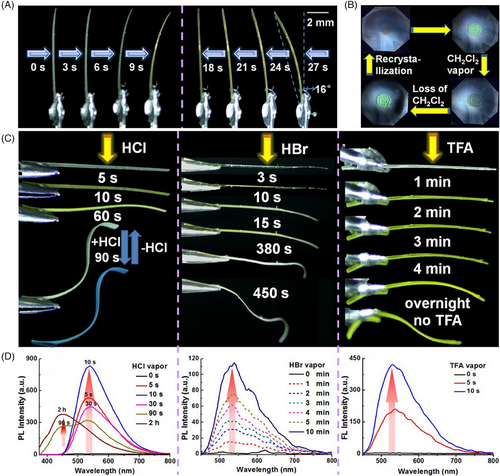
Organic crystals that show different responses when subjected to external stimuli (e.g., light, pressure, temperature, solvent, and/or humidity) are becoming candidates for smart materials, accompanied by macroscopic mechanical motion, for example, bending, splitting, curling, jumping, hopping, twisting, swimming, and self-healing.[2, 3, 18] A dual (stress and thermal) stimuli-responsive dynamic crystal E18 was serendipitously discovered by Ghosh et al. in 2015, obtained by slow evaporation of MeOH solution with 4-fluoro-3-nitroaniline and 2,6-dichlorobenzaldehyde in a 1:1 ratio.[42] The slender E18 could be bent reversibly within a threshold limit as those stress-induced Schiff base elastic organic crystals described earlier, which was attributed to their similar structural features (i.e., corrugated isotropic molecular packing with a multitude of weak/dispersive interactions) (Figure 3A,B and Scheme 2).
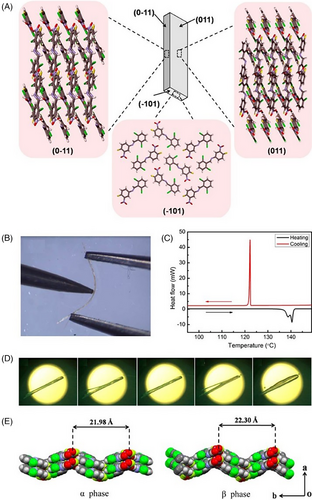
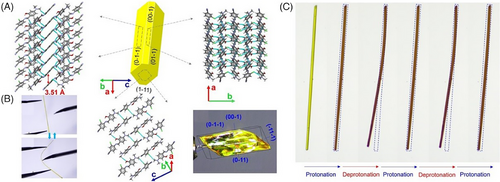
Impressively, the elastic E18 exhibited a thermosalient behavior simultaneously that required a certain anisotropy in the crystal packing, wherein the crystal underwent macroscopic motility (an initial elastic bending and then crystal splitting along the needle axis) (Figure 3D).[42] Generally, thermosalient effect occurs during a phase transition, arising from small yet obviously anisotropic changes in the crystallographic unit cell parameters despite no distinct packing difference. Several characterization methods was used to investigate the thermosalient behavior of E18, for instance, variable-temperature single-crystal XRD, Fourier transform infrared spectroscopy, powder XRD, and differential scanning calorimetry (DSC) along with hot stage microscopy, revealing the presence of martensitic transition (namely a first-order displacive and diffusion-less irreversible phase transition at 138°C from the low-temperature α-form to the high-temperature β-form followed by melting at 140°C) (Figure 3C). The phase transition was related to abrupt molecular motion with new molecular orientations in the martensitic phase β-form, giving rise to accumulated strain energy that was released rapidly at an interface to cause crystal splitting. The thermosalient phenomenon in E18 was due to anisotropic changes in lattice parameters across the martensitic phase transition, notably a preferential anisotropic expansion along the b axis (1.5% from 21.98 to 22.30 Å) (Figure 3E), which was rooted in competitive short-range π···π repulsions and long-range attractive dipolar interactions (NO2 and C─Cl) in an orthogonal direction.
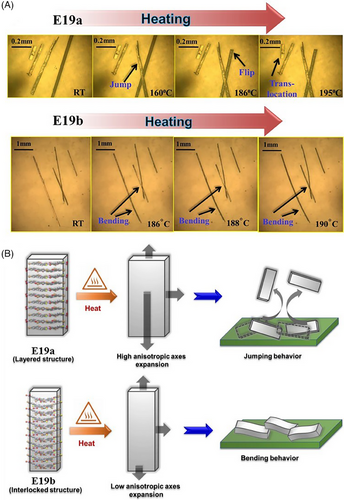
Attempts to extend this work to other Schiff bases such as 2-hydroxy-3,5-dibromobenzylidine-4-fluoro-3-nitroaniline, afforded new pressure and temperature-induced dual responsive molecular crystals—two polymorphs (thick needle plates E19a and thin acicular E19b).[54] The rigid layered structure in E19a answered for its brittle behavior while the criss–cross interlocked arrangement in E19b together with multiple weak and dispersive interactions allowed for elastic bending. When heated gradually, both E19a and E19b presented thermosalient behaviors along with martensitic phase transitions (at 188°C and 187°C, respectively) in virtue of anisotropic crystallographic face expansion: progressive thermal responses including jumping, flipping, and translocation in the former and thermal bending in the latter case (Figure 4). For the layered E19a with the limited number of weak/dispersive interactions, gradual heating led to a sudden accumulation of strain during the phase transition that released quickly at an interface and then translated into the intense mechanical motion manifested as jumping. In contrast, thin needle-shaped E19b showed a thermosalient bending behavior, presumably as a result of its interlocked crystal packing with multiple weak/dispersive interactions that were capable of absorbing the strain slowly and prevent abrupt mechanical motions.
However, 5-chloro substituted E20 was similarly elastically bendable but non-thermosalient though it was isostructural with E19b, which can be explained by the greater effective interaction strength among adjacent corrugated sheets, dissipating the generated strain energy more efficiently during heating.[54] Specifically, multiple interactions (e.g., type I Cl···Cl, type I F···F, type II Cl···Cl, type II Br···Br, and Br···O interactions) accounted for binding neighboring sheets in E20 whereas only two kinds of interactions (type I F···F and type II Br···Br) mainly sustained neighboring sheets in E19b. Accordingly, molecular structures, morphologies, intermolecular interactions and the underlying crystal packing jointly determine the mechanical properties of molecular crystals.
In Hao's group, multiple stimuli response phenomena were found in two polymorphs (E21a and E21b) as well: mechanical bending, heating-induced jumping and acid-induced bending (Figure 5).[41] Such two polymorphs were prepared by solvent diffusion in dichloromethane and petroleum ether (yellow E21a from nearly saturated CH2Cl2 solution; red E21b crystallizing in low concentration). Under external force, E21a showed a better ability of elastic deformation in comparison with E21b with anisotropic interactions and relatively rigid π-stacking columns. Moreover, the two polymorphs displayed jumping motions upon heating, occasionally accompanied by rolling and flipping, which may be owing to the sudden release of accumulated stress induced by anisotropic expansion. Variable temperature powder XRD and DSC measurements indicated that the jumping motions in E21a and E21b were not the result of thermosalient effects unlike those in E19a and E19b, but the consequence of inhomogeneity engendered by different degrees of lattice deformation on different regions because of the uneven stimulation. When fumed by volatile acids such as formic acid and then exposed to air, both polymorphs bent and straightened alternately quickly for many times, which was on account of the repeated protonation/deprotonation process that altered molecular conformation and structure.
Through rational molecular design and structural modification, more diverse kinds of flexible organic crystals with multiple mechanical responses would be developed. Multiple stimuli-responsive performances manifest the possibility of the exploitation and utilization of multifunctional smart organic crystals, which show potential applications in flexible sensors, flexible smart actuators, and more.
2.2 Multifunctional applications of Schiff base elastic organic crystals
2.2.1 Schiff base elastic organic crystals for optical waveguides
Although there has been an increasing number of reports on elastic organic crystals, those suitable for photoelectric applications are still very limited. Recently, high elasticity and bright emission were demonstrated in two Schiff base crystals (E22 and E23) both in the macroscopic and microscopic regimes.[55] Their mechanical flexibility may arise from weak/dispersive intermolecular interactions, for example, C─H···Cl, π···π stacking, Cl···Cl, and Br···Br. Both red-emitting E22 and green-emitting E23 exhibited optical-path-dependent low-loss active waveguides with Fabry–Pérot-type optical modes in their straight crystals and even in extremely bent ones. Such bent crystal waveguides conduct as useful optical elements in the construction of microcircuits (Scheme 3).
When E23 with chlorine atoms was replaced by dimethylamino-substituted E24, a highly elastic organic crystal for flexible optical waveguide yielded upon solvent diffusion layering hexane on the top of CH2Cl2 solution with E24.[56] Single-crystal XRD analysis revealed that intramolecular hydrogen bonds O─H···N between CH═N and OH groups resulted in a relatively planar conjugated molecular skeleton for π-stacking, which combined with the close and cross arrangement, endowed the crystal E24 with not only bright orange–red emission but also high elasticity (Figure 6A–E). E24 crystal displayed a high fluorescence quantum yield of 0.43, and could work as a low-loss optical waveguide even at a highly bent state, confirmed by the optical loss coefficients at 615 nm (0.270 dB mm−1 for the straight state and 0.274 dB mm−1 for the bent state) that were comparable to or even better than those of ever reported organic crystals and some polymers (Figure 6F–I).
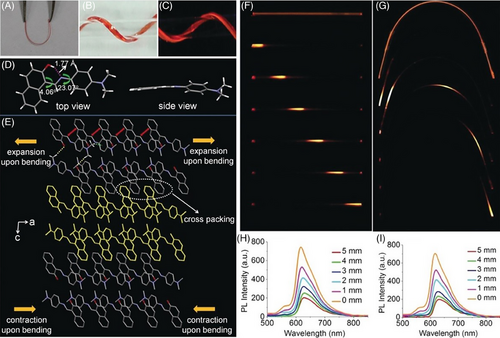
Expanding the applicable temperature range of flexible materials is essential for extending application regions to extreme environments such as the outer space. Considering that the elasticity of organic crystals is usually examined at room temperature, in-depth investigation of the influence of low/high temperature on elasticity is a step forward for applications. When subjected to low-temperature experiments and bent in liquid nitrogen (−196°C/77 K), the E24 crystal lost its elasticity and broke immediately. However, E25 with similar one-dimensional (1D) π···π stacking showed elasticity not only in low-temperature liquid nitrogen but also at high temperature before 150°C (Figure 7A).[57] To further understand the distinct mechanical properties under different temperatures between E24 and E25, their detailed crystallographic analyses at 100 K were compared with those at room temperature. It was found that weaker cold contraction and relatively isotropic structure contributed to maintaining the molecular conformation and packing features (i.e., hydrogen-bonding networks with crosswise arrangement of molecular chains based on strong π···π interactions), which was crucial for the elasticity in E25 at ultralow temperatures. Interestingly, its elasticity disappeared above 150°C, accompanied by the changes of crystal color and emitting light, which was derived from an endothermic crystalline-phase transition (Figure 7B). Based on such irreversible transforming process, free shaping of crystals was achieved, namely various shaped crystals could be obtained through heat-setting strategy (Figure 7C).
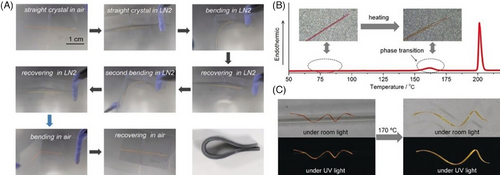
In general, freezing organic crystals lose the elasticity easily for the reason of molecular distortion and anisotropic contraction of crystallographic axes. Taking into account that the flat and rigid conformation together with interlocked crosswise packing structures would be conducive to maintaining the elasticity of crystals at low temperature, another Schiff base E26 with excellent ultralow temperature elasticity was successfully designed (Figure 8A), exhibiting an intense green fluorescence peaked at 520 nm (ΦF = 0.50), which offered a great opportunity to explore flexible optoelectronics applications in extreme environments such as flexible optical waveguide.[58] The relevant experiments revealed the self-waveguide ability of the bent crystal between −196°C and 170°C without changing its emissive and elastic property (Figure 8B–E). This work proposed a strategy to design functional organic crystals with ultralow temperature elasticity, and meanwhile expanded the application region of flexible crystalline materials to extreme circumstances.
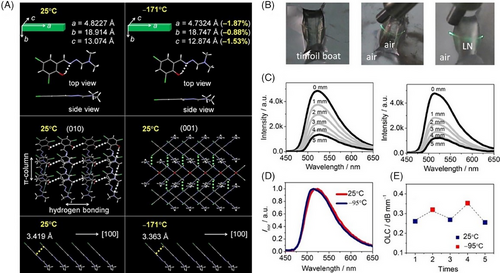
Along with this line, a new flat and rigid π-conjugated molecule E27 was synthesized, which could be elastically bent over a wide temperature range between −196°C and ≈200°C, being larger than those in most of flexible materials including polymers such as rubbers (Figure 9A).[59] Crystal structure analyses gave an insight into the mechanism behind the wide-range temperature independent elasticity: (1) the linear and fully conjugated skeleton conducive to the formation of packing structures in a parallel fashion along the crystal growth direction [100] through intermolecular π···π interactions, identified as one of the features that are correlated with crystal elasticity; (2) the double intramolecular hydrogen bonds O─H···N preventing temperature-induced molecular distortions and improving the stability of molecular arrangements; (3) the intermolecular hydrogen bonds C─H···O acting as a buffer to dissipate the internal elastic energy during the bending process. Intriguingly, thin prismatic E27 crystal presented temperature-dependent optical properties, namely the emission wavelength can be finely regulated from 547 to 577 nm once being heated from 20°C to ≈200°C, and similar case was observed on optical waveguides of both straight and bent crystals, which provided a guide to designing lightweight optoelectronic devices such as flexible waveguide tuners (Figure 9B–E).
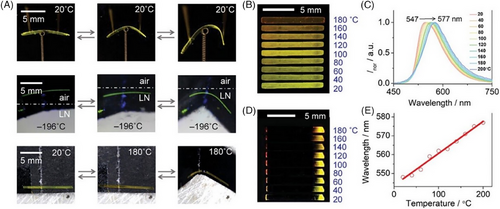
Whereafter, low-temperature-resistant flexible organic crystals based on two chiral Schiff bases (E28a and E28b) were reported in Zhang's group, obtained by solvent diffusion method layering cyclohexane on the top of saturated dichloromethane solution.[1] The two enantiomeric crystals were elastically flexible at ambient conditions and even at low liquid nitrogen temperature (77 K), which could be traced down to their crystal structures driven by non-covalent supramolecular interactions (i.e., intramolecular hydrogen bonds between hydroxyl and CH═N groups, and intermolecular C─H/Br···π interactions). E28a and E28b crystals exhibited strong green fluorescence with the maximum at 520 nm, a lifetime of 1.57 ns and an absolute quantum yield ΦF = 0.20, whose corresponding chiral optical properties were investigated by circular dichroism spectra and circularly polarized luminescence (CPL) spectra. CPL was caused by preferential emission from the excited state of chiral luminescent groups, and its intensity could be assessed by dissymmetry factors gPL that were ±6.0 × 10−3 from 450 to 700 nm for the two enantiomers (Figure 10A,B), being comparable with the reported CPL intensity of chiral materials at room temperature. The combination of low-temperature flexibility and intense emission with CPL activity in E28a and E28b prompted the exploration of flexible optical waveguide applications in extreme environments. The two chiral crystals retained the ability of good optical waveguide in both straight and bent states even at low temperature. Take E28a, for example (Figure 10C–H), the optical loss coefficients were determined to be 0.076 and 0.189 dB mm−1 for straight crystals, and 0.085 and 0.204 dB mm−1 for bent crystals at 298 and 77 K, respectively. This work was regarded as the first report on low-temperature-resistant flexible organic crystals with CPL properties serving as flexible optoelectronic elements and media for optical communication in extreme environments, which extended the application realm of flexible crystalline materials to extreme circumstances.

When extending light transduction from organic single crystal to hybrid crystalline materials, five cladded crystalline actuators were fabricated by mechanically coupling suitable polymers with elastic organic crystals E26 and E29–E32.[60] To be specific, five elastic Schiff base crystals were first coated by a mixture of poly(styrene sulfonate) (PSS) and poly(diallyldimethylammonium) (PDDA) for mechanical reinforcement, and then were mechanically coupled to the layer of humidity-responsive polymer polyvinyl alcohol (PVA). Hygroresponsive bilayer elements responded reversibly to a wide range of aerial humidities with relatively low fatigue resulting from the reversible hydration and rehydration of well-known hygroactivated PVA through hydrogen-bonding interactions (O─H···O), attaining control over the crystal shape and mechanical deformation of hybrid materials by humidity, and ultimately light output. It was demonstrated that these hybrid crystalline materials could act as active optical waveguides with the precise spatial control of light output by variations in aerial humidity. The approach stated here offered a simple way for contactless and controllable mechanical reconfiguration of molecular crystals, and opened up a prospect for the application of hybrid hygroresponsive crystalline materials as humidity-controlled actuating elements.
Thereafter, magnetic field, instead of the humidity stimuli described above, was introduced to effectively control the shape and motion of flexible organic crystals, and thus optical waveguides. Elastic crystals E25 or E29 were first coated by two polymers (positively charged PDDA and negatively charged PSS), and then combined with iron(III) oxide magnetic nanoparticles (MNPs).[61] The resulting magnetic hybrid materials as optical waveguides responded to magnetic field remotely and rapidly when a permanent magnet approached, whose light output can be precisely controlled. The novel method for regulating mechanical behaviors and optical properties of elastic crystals by magnetic fields, goes beyond the boundary of the known external stimulus means.
Recently, a two-dimensional (2D) compound from the MXene family, Ti3C2Tx (T = surface terminal groups including O, F, or OH), was chosen to slightly modify the flexible hybrid organic crystalline materials based on centimeter-long slender E29.[45] By combining elastic organic crystals E29 with charged polymers as adhesive counter-ionic components (e.g., PDDA/PSS/PVA) and MXenes as thermal absorbers, the ensuing multilayered hybrid organic crystalline materials showed the ability to mechanically respond to infrared light, whose deformation and optical waveguide can be precisely controlled by modulating the position, duration and power of infrared light excitation in the systems of both individual hybrid crystals and ordered 2D crystal arrays. Adding infrared light to the pool of available excitation stimuli, for instance, ultraviolet (UV) or visible light, stress, temperature, humidity, and magnetic field, will expand the prospects for constructing mechanically compliant organic crystals toward flexible optoelectronics.
2.2.2 Schiff base elastic organic crystals for optoelectronic devices
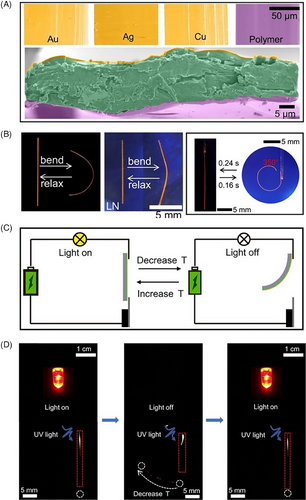
In addition to the great application potential in optical waveguides, Schiff base elastic organic crystals can be applied to flexible optoelectronic devices. Elastic E29 crystal mentioned above was selected as optically transducing medium to construct a novel sandwich-type hybrid structure by combining with the polymer poly(urethane urea) for mechanical reinforcement and metals (Au, Ag, or Cu) as electrically conductive components (Figure 11A).[62] The resulting hybrid materials were favorable for both light and electricity, and meanwhile responded to temperature variations by deformation (Figure 11C,D). In silver-coated crystals, the electrical conductivity was able to reach up to 21.0 S μm‒1, and remained stable over prolonged cyclic operation, decreasing less than 1% even after 10,000 thermal cycles. In the gold-coated case, the trilayer hybrid elements responded rapidly and repeatedly to temperatures by curling or uncurling in about 0.2 s with fast deformation and recovery rates of 2187.5° s‒1 and 1458.3° s‒1, respectively (Figure 11B). The mechanical and thermal robustness favored these conductive flexible hybrid organic crystals as candidates for various applications in the field of flexible organic optoelectronics such as thermal switching of optical/electrical circuits.
Stimuli-responsive materials have been established as a niche class for molecular logic gates at the core of organic electronic devices, which are able to produce multiple readouts in response to different external stimuli. Marandi et al. reported the multi-stimuli-responsive modulation in mechanical properties of naphthalidenimine-based Schiff base E33.[63] The corrugated crystallographic packing, together with almost planar geometry due to intramolecular hydrogen bonds O─H···N between hydroxyl group and azomethine nitrogen, was beneficial to the moderate elasticity of E33 crystal with a strain load of ∼0.6%. Its mechanical properties could be tuned by simple chemical treatments, namely from moderate elasticity to brittleness or high elasticity treating by metal ions (e.g., Cu(II)) or acid vapors (e.g., glacial acetic acid) that bonded on the surface of the crystals, respectively. The binding of Cu(II) ions and protonation of imine nitrogen atoms were confirmed by scanning electron microscopy with energy-dispersive X-ray analysis and high-resolution X-ray photoelectron spectroscopy (XPS). Taking the chemical treatments as inputs and multi-stimuli responses of the crystals as outputs, a molecular logic circuit was encoded.
2.2.3 Schiff base elastic organic crystals for biomimetic soft robots
Natural mechanical softness as a common feature of most biological tissues have triggered research efforts into an exploration of artificial soft materials for biomimetic soft robots. As noted above, some polymers with special functions can be used to regulate mechanical properties of elastic organic crystals by depositing onto the surfaces of crystals, even simulating biological behaviors. Intriguing organic polymer–crystal hybrid dynamic elements were fabricated by mechanical coupling between hygroresponsive and thermally responsive polymers and elastic organic crystals: centimeter-long E29 crystals were first coated on all faces by a mixture of PSS and PDDA to improve the interlayer adhesion, and then one of wide faces was deposited by a mixture of a common hygroscopic PVA, PSS for sensitivity reinforcement to humidity and glutaraldehyde as a cross-linking agent (Figure 12A).[64] The coated crystals retained their elasticity and responded reversibly to both temperature and humidity with relatively high cyclability and durability, caused by the repeated adsorption and release of water molecules by polymers through hydrogen-bonding interactions (Figure 12B).
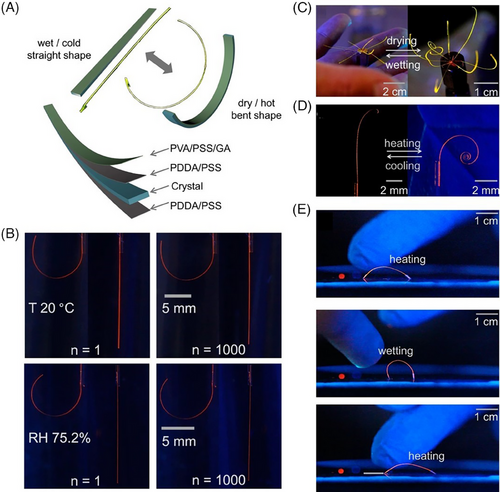
Reversible deformations in the resulting hybrid dynamic elements could translate momentum into macroscopic motions, which inspired the construction of actual prototypical soft robots (Figure 12C–E).[64] The organic crystal–polymer hybrid elements were exploited to mimic not only plant tendrils but also inflorescences, induced by the differential strain that developed at phase boundaries. Furthermore, simple walking robots were built, which were able to walk on a smooth silicon wafer once alternatively exposed to humidity and heat. By integrating the hygroresponsive and thermally responsive functionalities of polymers into elastic crystals, biomimetic functions of molecular materials in the crystalline state have been realized. In principle, any elastic crystal as active element could be combined with responsive polymers as the driving element to construct hybrid dynamic smart elements, provided that polymer solutions are able to deposit and adhere firmly onto the surface of elastic crystals without dissolving it. The hybrid strategy described here has greatly expanded the scope of possible applications of molecular crystals, and brought them closer to the fields of soft robotics, flexible devices, and bionics.
3 SCHIFF BASE PLASTIC ORGANIC CRYSTALS
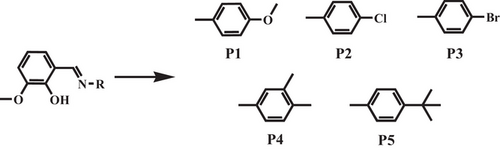
Although there have been increasing reports on flexible organic crystals, most of them were found by serendipitous discoveries rather than rational design. Earlier studies on plastically bendable crystals indicated that bending proceeded by segregation of the bent section into flexible layers sliding on top of each other via reorganizing weak intermolecular interactions (such as π-stacking and vdW forces) in the perpendicular direction, thereby engendering domains with slightly different lattice orientations.[9] In light of this, Reddy's group capitalized on the crystal engineering strategy to design plastically flexible crystals (P1–P3) by introducing active slip planes in the structures of unsymmetrical ovanillin-based Schiff bases with multiple types of vdW groups (e.g., ─OMe, ─Cl, ─Br) and great conformational flexibility.[43] Such non-interfering spherical vdW groups, combined with π-stacking among aromatic groups in the orthogonal direction, favored the formation of active slip planes with the least attachment energy, thus preserving short-range molecular motions during the bending process and facilitating impressive plastic flexibility of Schiff base crystals. However, Schiff base compounds (P4 and P5) with purely alkyl substituents (─Me or ─t-Bu) failed to form slip planes and did not show plastic deformation, which was probably due to the interference from electronegative groups (formation of sp3C─H···O hydrogen bonds between ─OMe groups). The study here emphasized the potential of weak dispersive interactions for regulating mechanical behaviors of ordered molecular materials by supramolecular design of shape complementarity/synthons, though they have been long regarded as poorly dependable to construct predetermined structural architectures (Scheme 4).
4 SCHIFF BASE FLEXIBLE ORGANIC CRYSTALS
4.1 Schiff base flexible organic crystals integrating elasticity and plasticity
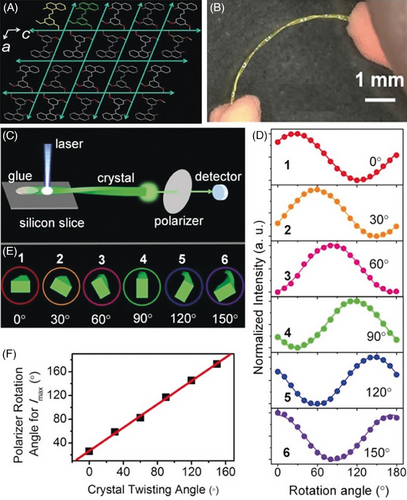
Flexible organic crystals, capable of elastic and plastic deformations simultaneously, would show much richer potential applications. Although the integration of elasticity and plasticity in a crystal is possible in theory, it is not easy to realize in practice. Liu et al. attempted to control deforming types by varying modes of external forces (i.e., bending or twisting forces) for impacting molecular packing in different crystallographic directions. To that end, a Schiff base F1 was synthesized through a one-step condensation reaction, and then crystallized by slow evaporation of a mixed solution of dry dichloromethane and hexane.[65] The needle-like crystal with two pairs of parallel bending faces displayed elastic bending under bending forces and plastic twisting under twisting forces synchronously, even twisted helical crystal could still be bent elastically. The mechanisms behind it were reversible changes of π···π stacking along the long axis of crystal, and irreversible molecular slippages in the thickness and width directions of the crystal accompanied by the destruction and rearrangements of hydrogen bonds, respectively. During the process of twisting, π···π stacking governing elastic bending is less affected due to small variations of π-overlap degree and interaction distances, revealing the mutual compatibility of elasticity and plasticity. Plastically twistable nature of the crystal F1 was successfully applied as polarization rotators to tune polarization direction of the light emitted from crystal by controlling the twisting degree (Figure 13). Subsequently, methyl substitution on E27 gave a new linear and flat π-conjugated Schiff base molecule F2,[66] whose crystal not only retained low-temperature elasticity and optical transmission capacity of its precursor E27, but also achieved reconfigurable plastic-twisting deformations and reusability of light-polarization rotations as in the case of F1. The integration of low-temperature-resistant 2D elastic bending and reconfigurable plastic-twisting deformations into one organic crystal expanded the perspectives of the emerging crystal flexibility (Scheme 5).

The modes of applied forces led to distinct mechanical deformations in F1, so would different kinds of external stimuli. Based on an asymmetric azine F3 consisting of a fluorescent benzothiazole group and an alkaline imidazole group,[67] multi-stimuli-induced mechanical responses were realized: (1) elastic bending under external force; (2) plastic bending accompanied by fluorescence enhancement via UV light irradiation or acid vapor fuming; (3) reversible fluorescence switching by alternant grinding and solvent gases fuming (CH2Cl2 or CH3OH) (Figure 14B). Thereinto, extended π-conjugation and molecular layer slippage accounted for photomechanical plastic bending. Upon UV light irradiation, a gradual fluorescence switch-on phenomenon occurred, namely non-emissive crystal F3 was converted into orange-emitting one with a lifetime of 1.59 ns and a quantum yield of 0.4% (Figure 14A). During the acid-induced process, the crystal underwent a reversible fluorescence color transition between green and blue (Figure 14C,D), and the plastic bending in this case was ascribed to the synergistic effect of intermolecular interactions including hydrogen bonds and protonation-induced push–pull electronic effects.
In addition, polymorphs often exhibit different mechanical behaviors for the reason of distinct structural features, for instance, molecular conformations, non-covalent interactions and underlying crystal packings. Based on π-conjugated Schiff base F4, three polymorphic forms with red emission were obtained by a layering method (i.e., bulky brittle F4a, needle-like plastic F4b, and elastic tetrahydrofuran solvate F4c that became plastic F4d after desolvated with the color change from red to yellow).[68] Single-crystal XRD analysis presented the structural features responsible for their different mechanical properties performed by mechanical testing such as tensile experiments and three-point bending, namely, the layered structure in F4b providing sufficient space for molecular sliding along the crystallographic [001] and [010] directions, and the criss–cross arrangement in F4c with parallel and vertically staggered molecular chains through intramolecular hydrogen bonding and intermolecular π···π interactions serving as a buffer to disperse the elastic energy. Both F4b and F4c acted as good candidates for flexible optical waveguides with low optical loss not higher than 0.262 dB mm−1.
Naturally, changes in chemical composition will greatly alter the mechanical and physicochemical properties of crystals. Slow diffusion of hexane into dichloromethane solution yielded sheet-like crystal of Schiff base F5 and needle-like crystal of its boron complex, containing a heptafluorobutyl chain and naphthalidenimine core.[69] While the precursor F5, possessing a tight packing without any slip-planes, was non-fluorescent and brittle, the incorporation of boron imparted interesting optical and mechanical functionalities, facilitating the formation of a third aromatic ring that was favorable for C─H···F interactions and π···π stacking. In the naphthalidenimine–boron complex, molecules were arranged in an isotropic mode with slip planes along the a and c crystallographic axes, contributing to uniform distribution of applied stress during the bending process. It was interesting that the boron complex exhibited size-dependent dual mechanical bending behaviors, namely thick crystals were observed to bend plastically whereas thin ones displayed elasticity, which was largely associated with the number of existing slip planes. Moreover, the boron complex supported multiple modes of light propagation, and thereby can be employed as flexible optical waveguide in both active and passive modes. This work highlighted the influence of subtle modifications in the molecular structure on the mechanical and optical properties of crystals.
4.2 Mechanical micromanipulation of Schiff base flexible organic crystals toward organic photonic integrated circuits
Optical waveguide properties are able to be readily characterized for organic crystals from millimeters to centimeters in length. Nevertheless, it is difficult to precisely manipulate micro-sized organic crystals in three-dimensional (3D) space, which becomes one of the major obstacles toward downsizing organic crystalline waveguides for implementation in optical micro- or nanocircuits as an integral part of miniature photonic and optoelectronic devices. The development of mechanical micromanipulation (mechanophotonics) techniques based on atomic force microscopy (AFM) facilitated the construction of organic photonic integrated circuits, for instance, bending, slicing, cutting, lifting, aligning, transferring and integrating, which created dimensionally and geometrically well-defined organic crystals presenting photonic attributes such as optical waveguides, resonators, directional couplers, interferometers, modulators, splitters, add/drop filters, delay lines, and microlasers.[70-72]
In 2020, Chandrasekar's group demonstrated that AFM could be utilized to reshape and relocate single crystals at the microscale in order to attain precise spatial control over their light output.[46] Using an AFM cantilever tip, acicular flexible microcrystals of three N-benzylideneanilines (F6–F8) were bent to an arbitrary angle, cut into shorter crystals of arbitrary length, sliced out from a bundle into individual crystals, and moved across and above a solid surface (Figure 15A), which conducted as active optical micro-waveguides once excited by laser, with the total intensity of transduced light being dependent on the optical path length. The approach described here effectively overcame the difficulties (“thick fingers” problem) commonly encountered with reshaping and positioning of small delicate objects. The validity of the non-destructive micromanipulation method was also confirmed in a twisted microcrystal F9 with an orange fluorescence,[44] which presented mechanical flexibility perpendicular to the twisted (010) and (001) planes on account of hydrogen-bonding interactions (e.g., N─H···O, C─H···O, and C─H···Br) and π···π stacking. The twisted microcrystals could efficiently guide its fluorescence in both straight and bent geometries with low optical loss of ≈0.03016 dB μm−1, and mechanically powered rolling locomotion of its optical waveguide cavity on the substrate was observed, rotating the output signal's linear polarization (Figure 15B–D).

An extension of this work was carried out based on a naphthalideneaniline Schiff base system (F10) that exhibited bright green fluorescence and high mechanical flexibility possibly imparted by intermolecular hydrogen bonds and π⋯π interactions.[73] F10 microcrystals transduced its fluorescence effectively in both straight and bent geometries. The greater crystal-surface adhesion energy than crystal-shape-regaining energy endowed the microcrystals with plastic-like behavior (i.e., pseudo-plasticity), and facilitated the micromechanical fabrication of a triply bent waveguide with the aid of AFM cantilever tip, which was later integrated with a singly bent one on the convex area to create a unique organic photonic integrated circuit (Figure 16A). The resultant circuit delivered direction-specific and excitation-position-dependent long-pass-filtered narrowband optical signals with different split ratios due to the self-absorbance of fluorescence signal in the ≈475–550 nm region.
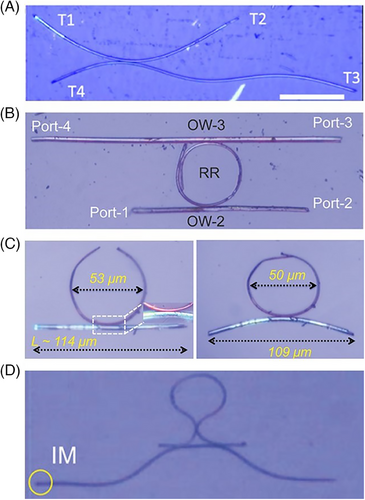
Structural variations on aniline resulted in a series of flexible organic microcrystals (F11–F14),[74-78] whose pseudo-plasticity on the substrate was successfully used to fabricate a variety of mechanically reconfigurable organic photonic integrated circuits by using the same flexible microcrystals or two different hybrid ones (Figure 16B–D). The precise AFM-micromanipulation technique allowed these microcrystals to be utilized as microscale photonic components toward flexible organic photonic integrated circuits, like ring-resonators,[74, 76] directional couplers,[75] and optical interferometers with fiber loop mirrors.[77] The growth in the field of microscale organic photonics significantly rely on the availability of mechanically compliant crystals and precise AFM-micromanipulation technique, the integration of which would contribute to the construction of new and complex organic photonic integrated circuits to route diverse optical signals effectively.
5 CONCLUSION AND OUTLOOK
This review has dealt with the design and applications of Schiff base flexible organic crystals covering elastic organic crystals, plastic organic crystals, and flexible organic crystals integrating elasticity and plasticity, which were synthesized via a one-step condensation reaction and then crystallized by slow evaporation, solution diffusion, etc. Thereinto, Schiff base elastic organic crystals usually show a similar corrugated packing patterns with interlocked structures and isotropic weak/dispersive interactions as “structural buffering” while plastic deformation greatly depends on active slip planes and anisotropic crystal packing through perpendicular strong and weak interactions.
The examples given here illustrate that slight variations in molecular structures, including chemical composition, molecular conformation, the substitution of groups, the insertion of solvent molecules and so on, exert a great influence on non-covalent intermolecular interactions and packing structures, and ultimately affect mechanical properties of molecular crystals, which are challenges faced in molecular structure design for flexible organic crystals. Although it is not easy to control over their mechanical properties directly from molecular structures, the introduction of some functional groups into molecules is conducive to achieving the flexibility of organic crystals such as halogen atoms or hydroxyl groups, which are involved in the formation of non-covalent interactions (halogen bonds or hydrogen bonds), dissipating the generated strain energy during deformation. The hydroxyl group readily forms an intramolecular hydrogen-bonding ring with the neighboring CH═N group, generating a relatively planar conjugated molecular skeleton for π-stacking, which is beneficial to bright fluorescent emission.
Flexible organic crystals based on Schiff bases show the ability to respond to external stimuli (e.g., light, pressure, temperature, magnetic field, solvent, humidity), and are becoming candidates for dynamic smart materials, accompanied by macroscopic mechanical deformation and/or motion, for example, bending, jumping, flipping, translocation, rolling, splitting, and twisting. Their potentials have been emphasized in the multifunctional applications such as fluorescence, polarization rotators, optical waveguides, optoelectronic devices, biomimetic soft robots, and organic photonic integrated circuits. Among them, relatively planar and rigid π-conjugated structures make them easier to retain intense fluorescent emission and high flexibility due to weak and isotropic contraction and the maintenance of molecular conformation when frozen at a low temperature, and thereby achieve low-temperature flexible optical waveguides. Additionally, AFM-micromanipulation technique is able to be utilized to reshape and relocate single crystals at the microscale in order to attain precise spatial control over their light output, allowing these microcrystals to be used as microscale photonic components toward flexible organic photonic integrated circuits.
It is worth mentioning that the universality of hybrid strategy has been confirmed by combining flexible organic crystals, polymers for mechanical reinforcement and compounds with special functions such as humidity-responsive polymer PVA, MNPs, thermal absorbers MXenes, and electrically conductive metals (Au, Ag, or Cu). This approach offers a simple and general route to controllable mechanical reconfiguration of flexible organic crystals, and will expand the scope of possible applications of molecular crystals in the fields of flexible optoelectronics, soft robotics, and bionics.
Although much exciting progress has been made in the area of Schiff base flexible organic crystals, there are still some challenges to be faced. First, many flexible organic crystals were discovered serendipitously, and reliable/general guidelines remain to be developed and improved. Moreover, in contrast to flexible optical waveguides, the exploration of flexible optoelectronic smart devices, especially microscale ones, has only just begun, and even less is known about biomimetic soft robots. However, with increasing research interest toward this area, we believe that more intriguing and meaningful work will emerge in the near future, for instance, (1) further exploration of the atomic-scale mechanisms of flexible organic crystals using micro-focused synchrotron radiation with excellent precision and high spatial resolution, and accordingly getting a deeper understanding of the relationship among molecular structure, crystal packing and mechanical property; (2) the development of 2D flexible crystalline materials with the availability of two pairs of bendable faces in orthogonal directions (common flexible organic crystals usually show a single pair of bendable parallel faces and bend along a specific direction on account of their inherent structural anisotropy); (3) flexible organic micro/nano-crystals toward smart optoelectronic devices (macro-crystals are limited in practical device applications due to sizes, defects and other factors); (4) the search for organic flexible ferroelectrics.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (Nos. 22205105, 62288102, 61874053, 22075136), the National Key Basic Research Program of China (2020YFA0709900), Research Innovation in University of Jiangsu Province (KYCX21_0772) and the open research fund from the State Key Laboratory of Luminescent Materials and Devices (South China University of Technology).
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
Biographies

Xue-Hua Ding received her PhD degree (2015) from the Nanjing University of Posts and Telecommunications under the supervision of Professor Shi Wang and Professor Wei Huang. Afterward, he spent time at the Nanjing Tech University as a postdoctoral researcher. Now she is an associate professor in the Institute of Advanced Material, Nanjing Tech University. Her main research interest is supramolecular crystalline materials.

Jin-Yi Lin received his bachelor degree from the Fujian Normal University (2008) and PhD degree (2014) from the Nanjing University of Posts and Telecommunications under the supervision of Professor Linghai Xie and Professor Wei Huang. In 2011, he was a research assistant in Singapore University of Technology and Design. Afterward, he spent time at the Centre for Plastic Electronics of Imperial College London and University of Oxford as a postdoctoral researcher. Now he is a full professor in the Institute of Advanced Material, Nanjing Tech University. His main research interest is supramolecular plastic optoelectronic.

Wei Huang received his PhD from the Peking University in 1992. In 2001, he was appointed as a chair professor at Fudan University, where he founded and chaired the Institute of Advanced Materials. After that, he became the vice president of Nanjing University of Posts & Telecommunications, and then president of Nanjing Tech University. He was elected as Academician of Chinese Academy of Sciences in 2011. His research interests include organic/plastic/flexible electronics, nanomaterials and nanotechnology, etc.




