Helicene-type β-isoindigo-based boron-dipyrromethene analogs with strong near-infrared chiroptical activity
Ziwei Chen and Zhigang Ni contributed equally to this work.
Abstract
Near-infrared (NIR) chiroptical response has been less explored because it is challenging to achieve both chirality and NIR absorption/emission. Herein, we describe the design of heterohelicene-type β-isoindigo-based boron-dipyrromethene (BODIPY) analogs (β-IBs), which shift the absorption peak to 800 nm and produce significant Cotton effects (127.8 M−1 cm−1) and absorbance dissymmetry factors (|gabs| = 3.5 × 10−3). The luminescence dissymmetry factor (glum) and circularly polarized luminescence (CPL) brightness (BCPL) of up to 1.24 × 10−3 and 1.78 M−1 cm−1 were realized beyond 800 nm. These β-IBs are the first examples of helicene-type compounds with the highest gabs in the NIR region and CPL beyond 800 nm. Theoretical calculations demonstrate that the strong chiroptical activities are triggered by their large transition magnetic dipole moments. This study not only provides a new approach to the synthesis of a larger variety of unprecedented helicene-type BODIPY analogs but also demonstrates excellent NIR chiroptical properties.
1 INTRODUCTION
Helicenes are a fascinating class of chiral polycyclic aromatic hydrocarbons (PAHs) consisting of ortho-fused aromatic rings. Helicene chemistry is regarded as a cutting-edge field with numerous high-tech applications.[1-7] Of great interest is their potential applications in chiral bio-imaging and optoelectronic devices, for which strong NIR chiroptical activity is highly preferable.[8-13] However, single non-functionalized helicenes usually have low ΦFl and short excitation/emission wavelength.[14] The main strategy to improve such properties is π-extension at the periphery of the helicene backbone to construct versatile π-extension helix such as double-, multiple- and superhelicenes, and hetero- and π-annulated helicenes, which offers a flexible strategy to tune their chiroptical properties.[15-17] However, most of the π-extension helix shows the chiroptical properties at the maxima in the ultraviolet (UV)-visible region, and few examples in the “deep-red” (650–700 nm) spectral range,[18-22] with extremely few π-extended helix in the NIR (700–900 nm) region despite tremendous efforts by chemists in the past decade.[23-26] Particularly, according to the energy gap theory, ΦFL declines with the narrowing of the energy gap.[27] Therefore, the design of NIR helicenes-type compounds with a strong Cotton effect and high circularly polarized luminescence (CPL) brightness (BCPL) remains a great challenge.
Boron-dipyrromethene (BODIPY) derivatives have garnered significant attention due to their exceptional spectroscopic properties.[28, 29] BODIPYs are typically achiral molecules owing to their C2v-symmetric core, the chiroptical activity can be achieved by combining them with chiral fragments or through the introduction of axial chirality.[30] However, such chiral molecules often display weak chiroptical responses. This is because the chiral and light-emitting centers are separated within the molecule, leading to a small coupling between them.[30] A promising strategy for high chiroptical performance is to create unique, low-symmetrical BODIPY analogs to form helicene-type chirality,[31, 32] however, achieving NIR chiroptical activity remains a challenging task due to the lack of construction scaffold. In this regard, β-isoindigo, which consists of two isoindole units and was discovered in 1941,[33] is a valuable architecture despite receiving scant attention[34-36] since it offers extended π-conjugation, enhanced coordination versatility, and distorted structure, allowing for the creation of more diversified molecules. We recently used the deamination method to synthesize a series of β-isoindigo-based Aza-BODIPY analogs. However, their chiroptical activities are mainly in the “deep-red” spectral regions.[21] Scrutinizing the frontier molecular orbitals (MOs) coefficient of the core (Figure 1), HOMO at the meso-position has a larger coefficient than LUMO, it is clear that replacement of high electronegativity N atoms by C would dramatically destabilize HOMO and further addition of electron-withdrawing -CN groups would stabilize the LUMO orbitals. Extending the π-conjugation on the electron-deficient pyridine ring decreases the LUMO energies, which inevitably leads to red-shifted spectra (Figure 1). On the basis of this rational concept, herein, we present the design of heterohelicene-typed β-isoindigo-based BODIPY analogs (β-IBs) for the first time, with which we have succeeded in shifting the absorption band to 800 nm with high Cotton effect (127.8 M−1 cm−1) and absorbance dissymmetry factors (|gabs| = 3.5 × 10−3) through synergistic effects. CPL spectra are achieved at 800–900 nm ranges.
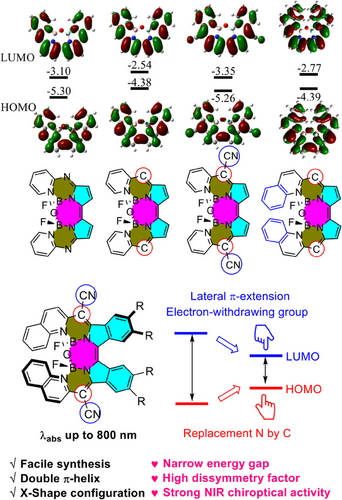
2 RESULTS AND DISCUSSION
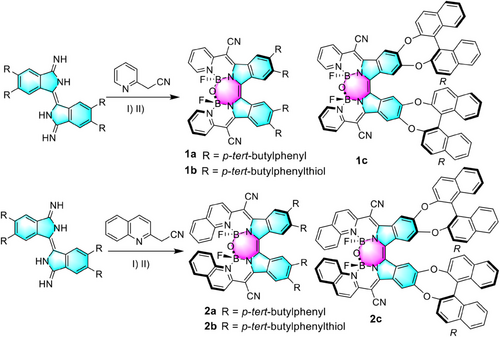
The synthetic procedures are shown in Scheme 1. The diimino-β-isoindigo derivatives were prepared from a 4,5-disubstituted phthalonitrile with an overall 50% yield.[35] The diimino-β-isoindigo was reacted with 2-(pyridin-2-yl)acetonitrile or 2-(quinolin-2-yl)acetonitrile to afford the ligand and subsequently coordinated with BF3·OEt2 with two-step yields of up to 23%. The spontaneous hydrolysis of adjacent B–F bonds in the constrained cavity by a tiny amount of water produces the B–O–B bridges thus forming highly distorted structures. It is anticipated that the quinolinyl group will cause a red-shifted wavelength and further deform the structure to create the staggered conformation. Additionally, the addition of the binaphthol group at the periphery is expected to induce a chiral ligand, resulting in the formation of chiroptical activity without chiral separation. These compounds demonstrated excellent solubility in a variety of solvents such as dichloromethane and tetrahydrofuran, and great stability in natural conditions.
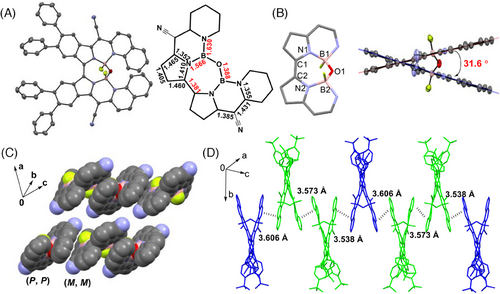
To demonstrate the helical architecture, single crystals of racemic 2a were grown by the slow diffusion of hexane into a THF/CH3CN solution. The crystal structure analysis revealed that 2a crystallizes in the monoclinic space group C2/c as a 1:1 racemic mixture, with two crystallographic isomers facing opposite orientations in each unit cell. The linked C-C bond distance between the isoindolinyl unit is 1.381 Å, which is characteristic of a typical double bond (Figure 2A). Notably, the central seven-ring containing the B-O-B bond exhibits an average torsion angle of 34.6°, indicating significant distortion. The highest torsion observed between C1-N1-B1-O is 62.7°, further indicating substantial deformity, which is challenging to achieve in a pure sp2 hybridized carbon ring (Figure 2B). The X-shaped helical structure with a large dihedral angle of 31.6° is accurately confirmed (Figure 2C). (P,P) and (M,M) enantiomers with homochiral stacking are revealed in the packing structure. Multiple close π−π stackings between intermolecular quinolyl units are observed, forming a slipped-stack arrangement with distances in the range of 3.538-3.606 Å (Figure 2C,D). These findings provide clear evidence for the helical architecture and shed light on its unique structural characteristics.
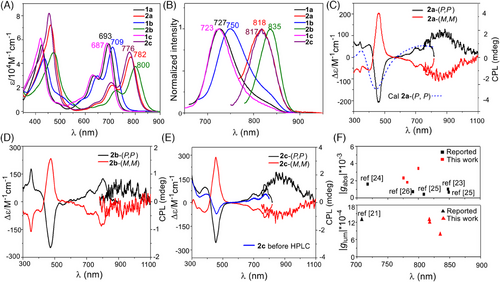
The photophysical properties in dilute dichloromethane solutions were investigated and summarized in Figure 3 and Table 1. The β-IBs display an intense absorption in the NIR regions with high-energy bands at 400–500 nm regions (Figure 3A). Specifically, 1a-1c with pyridyl unit exhibits main peaks at 693 (ε = 59500 M−1 cm−1), 687 (53800 M−1 cm−1) and 709 nm (56600 M−1 cm−1). The extended homologs 2a-2c with quinolinyl units show a red-shifted absorption band at 782 (45500 M−1 cm−1), 776 (49700 M−1 cm−1) and 800 nm (37600 M−1 cm−1) with generally broad profile covering the 650–850 nm (Figure 3A). It's interesting to note that the molar absorptivity in the NIR region for these β-IBs is higher than that of D9H, H7H (composed of 138 and 158 conjugated carbon atoms, for D9H, λabs = 730 nm, ε = 10000 M−1 cm−1) and even NP, the largest π-conjugated compound made up of 258 sp2 carbon atoms.[37-39] On the basis of time-dependent density functional theory (TD-DFT) calculations, the low-energy band with similar shapes originates from the intense 0–0 band of the S0–S1 transition state with HOMO → LUMO contribution. A shoulder on the high-energy side of the maxima band is ascribed to the 0–1 vibrational transition. In the 400–500 nm region, the β-IBs exhibit comparable or even more intense absorption bands, which can be attributed to the transitions to higher Sn (n = 6,7) excited states (Figure S1 and Table S1). The incorporation of a fused benzene ring at the pyridine ring stabilizes LUMO, thus 2a-2c has a red-shift absorption peak than 1a-1c (Figure S2). These β-IBs display a broad NIR fluorescent peak ranging from 727 to 750 nm for 1a-1c and 818 (2a), 835 (2b), and 817 nm (2c), demonstrating multicolor fluorescence properties (Figure 3B). To the best of our knowledge, the emission peak beyond 800 nm is one of the longest-wavelength emissions reported among helicene molecules. These molecules undergo light emission with quantum yields (ΦFl = 0.03 − 0.15) and with a fluorescence lifetime (τ = 0.60 − 1.00 ns). The value of fluorescence quantum yield is higher than those of most previously reported NIR helicene-typed molecules.
|
λabs [nm] |
εmaxa [104] |
λem [nm] |
SSb [cm−1] |
ΦPLc |
τd [ns] |
kre [108 s−1] |
knrf [108 s−1] |
OBGg [eV] |
EHh [eV] |
ELi [eV] |
EHj [eV] |
ELj [eV] |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 693 | 5.95 | 727 | 674 | 0.15 | 0.53 | 2.83 | 16.04 | 1.67 | −5.16 | −3.57 | −5.19 | −3.15 |
| 1b | 709 | 5.38 | 750 | 771 | 0.04 | 0.70 | 0.57 | 13.71 | 1.61 | −5.16 | −3.62 | −5.16 | −3.15 |
| 1c | 687 | 5.66 | 723 | 918 | 0.10 | 0.84 | 1.19 | 10.71 | 1.64 | −5.17 | −3.63 | −5.24 | −3.17 |
| 2a | 782 | 4.55 | 818 | 562 | 0.07 | 0.73 | 0.96 | 12.74 | 1.47 | −5.21 | −3.75 | −5.19 | −3.27 |
| 2b | 800 | 3.76 | 835 | 524 | 0.09 | 0.52 | 1.73 | 17.50 | 1.43 | −5.25 | −3.80 | −5.17 | −3.30 |
| 2c | 776 | 4.97 | 817 | 647 | 0.03 | 1.00 | 0.30 | 9.70 | 1.49 | −5.28 | −3.76 | −5.24 | −3.31 |
- a Extinction coefficient (M−1 cm−1) of the maximum absorption band.
- b Stokes Shift.
- c Fluorescence quantum yield determined using the absolute method.
- d Fluorescence lifetime measured in time-correlated single-photon counting operation mode.
- e Radiative rate constant calculated using: kr = ΦPL/τ.
- f Non-radiative rate constant calculated using: knr = (1 − ΦPL)/τ.
- g Optical band gap (OBG) determined from the absorption spectrum offset in CH2Cl2.
- h Estimated from the oxidation potential and calculated using: E = −(4.8 + Eox1) eV.
- i Estimated from the reduction potential and calculated using: E = −(4.8 + Ered1) eV.
- j Calculated at the B3LYP/6-311G(d) level.
Voltammetry was used to investigate the electrochemical properties (Figure S3), cyclic voltammetry, and differential pulse voltammetry redox potentials as well as the experimental HOMO and LUMO energy levels determined by the first oxidation and reduction potentials (Ox1 and Red1) are summarized in Table 1. 1a-1b and 2a-2b exhibit two reversible one-electron oxidation and reduction process. Both Ox1 and Red1 shifted positively when the aromatic ring moieties became larger from pyridine (1a: 0.36 V and −1.23 V, 1b: 0.36 V and −1.18 V vs. Fc+/Fc) to quinoline (2a: 0.41 V and −1.05 V and 2b: 0.45 V and −1.00 V). The potential difference between Ox1 and Red1 of 2a (1.47 V) and 2b (1.43 V) decreases relative to 1a (1.67 V) and 1b (1.61) (Table 1). 1c and 2c show two irreversible oxidation processes, indicating the strong electronegativity of oxygen atoms significantly affects the electrochemical properties. These electrochemistry results are in good agreement with the observed drastic changes in the absorption spectra.
Chiral resolution of enantiopure of 1a, 2a-2c was achieved using high-performance liquid chromatography (Figures S4 and S5). Circular dichroism (CD) spectra of the optically resolved compounds except 2c display a perfect mirror image in the range of 300–900 nm with opposite Cotton effects (Figure 3C–E). The absolute configurations of the enantiomers are determined by comparing the experiment data with those simulated by the TD-DFT method: the Cotton effects corresponding to the main absorption band are positive for (P,P) and negative for (M,M) (Figure 3C and Figure S6). In the case of 1c and 2c, the R-binaphthol groups at the periphery of the β-IB core induce the formation chiral cavity, thus displaying optically active before chiral resolution (Figure 3E and Figure S7). The mixture 2c is composed of 67% (P,P)-enantiomer and 33% (M, M)-enantiomer, resulting in a 34% enantiomeric excess. This represents an intriguing attempt to produce enantiomeric compounds. The R-binaphthol group has chiroptical activity at the UV region, which disrupts the mirror image at higher energy in the CD spectra.
The Cotton effects Δε at low energy were determined to be 83.3 M−1 cm−1 for 2a, 127.8 M−1 cm−1 for 2b, and 118.6 M−1 cm−1 for 2c. The absorption dissymmetry factor |gabs| of 1a was found to be 0.2 × 10−3, while for 2a-2c it reached a higher value of 3.4 × 10−3 (Figure S8 and Table 2). These values represent the largest Δε and |gabs| among the NIR region of 700–900 nm (Figure 3F). It is worth noting that the chiroptical responses covered a wide range of 300–900 nm, which is extremely rare in the literature for helicene-type compounds.[26] This suggests the unique chiroptical properties of these compounds. Moreover, the CD intensities of 2a and 2c in CH2Cl2 persisted at ambient temperature even after a month, indicating the configurational stability of the compounds (Figure S9). The S1–S0 transition-related mirror-image in the CPL spectra was also present, with luminescence dissymmetry factors (glum) reaching up to 1.24 × 10−3. To the best of our knowledge, these β-IBs are the first helicene-type compounds with CPL wavelength over 800 nm (Figure 3F). The CPL performance has been assessed using the CPL brightness (BCPL). Based on the calculation formula BCPL = ε × ΦFl × glum/2, the BCPL values of 2a-2c are calculated as 0.93–1.78 M−1 cm−1, representing high BCPL values of helicenes in the NIR region. These compounds are promising for CPL-related biomedical applications due to their high BCPL value, exceptional stability, and tunable emission features.
|
λabs [nm] |
Δεa S0-S1 |
Δεb S0-Sn |
|gabs|c [10−3] |
|glum|d [10−3] |
BCPLe [M−1 cm−1] |
|μ|f [10−18] |
|m|f [10−20] |
cosθf |
gcalcg [10−3] |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 693 | 11.9 | 84.5 | 0.20 | – | – | 9.48 | 0.21 | 0.42 | 0.37 |
| 2a | 782 | 83.3 | 206.1 | 1.83 | 1.12 | 1.78 | 7.24 | 2.93 | 0.16 | 2.55 |
| 2b | 800 | 127.8 | 251.5 | 3.40 | 0.78 | 1.32 | 7.12 | 3.23 | 0.12 | 2.24 |
| 2c | 776 | 118.6 | 290.8 | 2.39 | 1.24 | 0.93 | 6.92 | 3.13 | 0.20 | 3.68 |
- a Δε = mdeg/(3300*l*c), unit: [M−1 cm−1] in the S0-S1 transition.
- b The S0-Sn transition. (n = 7 for 1a and n = 6 for 2a-2c).
- c Corresponding S0-S1 transition |gabs| of the (M,M)-configuration calculated as gabs = Δɛ/ɛ.
- d glum = 2(IL − IR)/(IL + IR).
- e BCPL = ɛ × ΦPL × |glum|/2.
- f Calculated at the TD-B3LYP-D3/6-311G(d) level. |μ| unit: [esu cm], |m| unit: [erg G−1].
- g Calculated using: g = 4cosθ|m|/|μ|.
TD-DFT calculations were carried out to analyze the transitions for the chiroptical bands (Table 2). The dissymmetry factors were theoretically determined using the simplified equation gabs = 4cosθ|m|/|μ|, where m, μ, and θ are the transition magnetic dipole moment (TMDM) and transition electric dipole moment (TEDM), and the angle between them, respectively. Therefore, a high gabs value can be achieved by having a large cosθ and a large ratio of |m|/|μ|. The quinolinyl-fused 2a-2c exhibit slightly smaller |μ| values but substantially larger |m| values (|m| = 0.21 × 10−20 erg G−1 for 1a and |m| = 2.93 ∼ 3.23 × 10−20 erg G−1 for 2a∼2c), which demonstrate the higher |gabs| value of 2a-2c than that of 1a (Table 2 and Figure 4A). The transition dipole moment density was assessed in order to rationalize the chiroptical activity. For μ and m, the y- and z- components have dominant contributions, respectively. Thus, we analyzed the y-component of the TEDM density and the z-component of the TMDM density. The μ density is mainly located on the inner helicene backbone and cyano group, with a minor contribution of the π-extension part. In contrast, the m density is distributed over the whole molecular framework. The large |m| is explained by the contributions of the helicene backbone as well as the additional fused benzene rings (Figure 4B). Thus, the π-extension of 2a-2c significantly affects |m|, has less effect on |μ|, and finally increases the dissymmetry factor. Essentially, π-extension can increase the size of the molecular system, thus decreasing the light-matter size mismatch. In most small molecules, at least when considering dipole-allowed electronic transitions, the |m| is overwhelmed by the |μ|, and their non-optimized alignment (θ) results in very small g-factors: the ‘molecular’ g-factor. The |μ| involves the translation of charge density, while the |m| requires a rotation of charge density. This combination (translations and rotation) is responsible for creating the helical interaction of the light. Therefore, in order to increase the g-factor, improving the coupling between the element of chirality and the chromophore is an important handle.40 In addition, the peaks at high-energy at the 400–500 nm range showing the larger CD signal (2a, Δε = 206.1 M−1 cm−1) were assigned to the high-energy S0→S6 transition for 2a, the TEDM direction of S0→S6 transition contributed HOMO−3→LUMO, HOMO−4→LUMO and HOMO→LUMO+1 is from the substituent to the β-IB core (Table S1), having a change compared with S0→S1 transition, while the TMDM direction remained the same (Figure 4B). As a result, the cosθ value has doubled, leading to a large Δε value.

3 CONCLUSION
In summary, we present a facile synthesis toward β-isoindigo-based double π-helix BODIPY analogs, with the goal of creating efficient NIR chiroptical molecular materials and elucidating chiral variation. The excellent chiroptical responses by CD spectra were revealed across the visible and NIR region with the Cotton effects of 127.8 M−1 cm−1 and |gabs| of 3.4 × 10−3, achieving the highest performance in the 700–900 nm region. Moreover, these β-IBs demonstrated efficient CPL activity beyond 800 nm with glum and CPL brightness up to 1.24 × 10−3 and 1.78 M−1 cm−1. These impressive characteristics highlight the potential application of β-isoindigo-based π-helix as chiral bio-imaging and photodetector materials. TD-DFT calculations revealed that the high chiroptical properties are connected to the substantial TMDM promoted by π-extension. We believe that the current study provides a simple synthetic approach and a comprehensive insight for managing NIR chiroptical characteristics, and inspires further investigation on the design of NIR-CPL materials.
ACKNOWLEDGMENTS
The authors thank the National Natural Science Foundation of China (Nos. 21871072 and 22003014) for financial support. DFT calculations were performed at the Computational Center for Molecular Design of Organosilicon Compounds, Hangzhou Normal University. The author thanks Dr. Yuan Cheng from the Instrumentation and Service Center for Molecular Sciences at Westlake University for the assistance in CPL measurement.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Supporting Information of this article.




