Unusual phosphorescence of boric acid: From impurity or clusterization-triggered emission?
Abstract
The unusual room-temperature phosphorescence (RTP) from the n electron-rich systems (without regular conjugated structure) has aroused great attention for structural designing and application development of RTP materials. Such emission has been ascribed to clusterization-triggered emission (CTE) via weak through-space conjugation of n electrons in the heteroatoms. However, there was suspicion on such RTP as impurity-induced result. Therefore, in-depth photophysical investigation and effective proof methods are needed to trace the origin of such RTP. Here, using the recently reported CTE phosphor boric acid as the example, a Jablonski diagram-based verification protocol was proposed to confirm the intrinsic luminescence of the n electrons-rich systems. Meanwhile, some other types of luminophores, that is, traditional phosphors, already reported impurity-induced and host-guest doping luminophores, were included for comparison. Overall, this work provides a basic paradigm for differentiating between the impurity-involved and the n electron-rich phosphors and will further deepen the understanding of nonconventional luminescence.
1 INTRODUCTION
Room-temperature phosphorescence (RTP) from purely organic materials is currently a research hotspot. Regularly, molecular design of purely organic RTP materials relies on El-Sayed rule,[1, 2] heavy atom effect,[3, 4] and a variety of triplet exciton-stabilizing strategies.[5-13] However, the role of impurity in RTP inducing has long been a controversial topic,[14, 15] such as carbazole[16, 17] and triphenylamine.[18] On the basis of impurity engineering, Ma and Tian constructed efficient two-component RTP by introducing trace components.[19] Obviously, the existence of minor impurity makes the tracing of RTP origin an important and inevitable task.
Recently, RTP from nonaromatic structures has attracted growing interests.[20, 21] It was first observed from saturated amine[22, 23] and some hyper-branched dendrimers[24] and was once regarded as impurity-induced results due to the absence of typical luminescent structures.[20, 25] Currently, the scope of materials capable of such nonconventional luminescence was greatly expanded, such as amino acids,[26] proteins,[27] and also natural products (e.g., starch and cellulose[28]). Generally, these luminophores lack aromatic structure, but are rich in heteroatoms (N/O/S). In solids or aggregated state, n electrons in the heteroatoms would be brought close proximity to yield weak through-space conjugation (TSC). Since TSC contributes to the formation of low-lying luminescent centers (relating to the clustering state of n electrons[29-33]), Tang et al. proposed a clusterization-triggered emission (CTE) mechanism for such intriguing phenomenon.[21, 34-37] However, questions about the origin of such abnormal luminescence didn't stop, partially because the photophysical process and the triplet exciton dynamics of CTE were largely different from the conventional systems. For example, our group reported CTE-induced ultralong RTP from boric acid[30] (and similar results from another group[38]), a kind of n electron-rich material that has been investigated for over 100 years.[39] It was largely different from the conventional boronic acids and esters in structure and phosphorescence mechanism,[40-44] thus was suspected as impurity-induced results very recently.[45] Moreover, although purification helps impurity identification in RTP confirmation, ppb-level impurity was once reported to be effective for RTP promoting.[46] Therefore, it is important to develop a photophysical protocol to trace the origin of RTP in the n electron-rich systems and rule out the possibility of impurity.
In this work, a verification protocol was proposed (Scheme 1) to confirm the intrinsic luminescence of boric acid. First, from the theoretical viewpoint, the position of the low-lying T1 energy level in boric acid was probed with the host-guest strategy.[47] Second, the intrinsic phosphorescence from boric acid was confirmed through multiple purification and synthetic routes. Third, excitation- and temperature-dependent phosphorescence was adopted to explore the luminescence differences between the n electrons-rich CTE phosphors and the impurity-induced systems. Last, the CTE mechanism of boric acid was further demonstrated via physical (mechanical loading) and chemical modulation of the through-space conjugation. Through the above protocol, it is evident that the luminescence of the n electrons-rich system is largely different from those of impurity-induced phosphors, and thus is truly a new luminescence system that deserves further exploration.
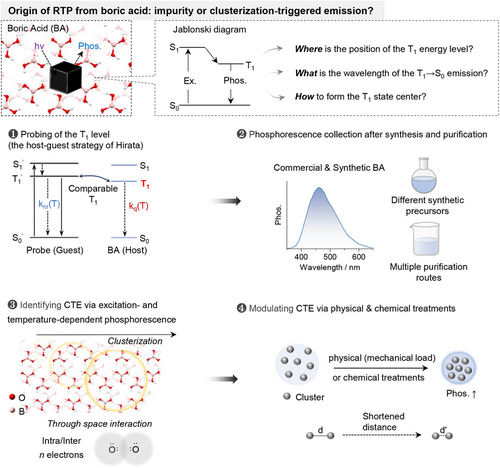
2 RESULTS AND DISCUSSION
2.1 Probing of the relative position of the T1 energy level of boric acid
Based on the Jablonski diagram, the position of the T1 energy level of boric acid (BA) was first probed using the host-guest protocol proposed by Hirata.[14] Theoretically, in solid phosphor, the triplet deactivation pathways mainly include radiative transition (kp), non-radiative relaxation (knr) and intermolecular interaction-induced quenching (kq, e.g., triplet migration, Figure 1A).[14] Although knr and kq are both temperature-dependent, knr is mainly dependent on the structure and rigidity of the phosphor thus is dominated in the lower temperature region (77 K-room temperature), while kq is related to triplet migration thus is more appreciable in the higher temperature region (Figure 1B).[6] Therefore, when doping a guest phosphor into the host matrix, the relative positions of the T1 level between host and guest can be derived from the corresponding temperature dependences of kq. For example, comparable T1 energy levels will lead to triplet energy migration between the guest and host (Figure 1C), thus similar temperature dependence of kq and similar slopes of the two in the higher temperature region. In contrast, largely mismatched T1 levels will result in blocked triplet energy migration (Figure 1C), thus suppressed temperature dependence of kq and distinctly lower slope. In this manner, the relative position of the T1 energy level of the BA can be probed.
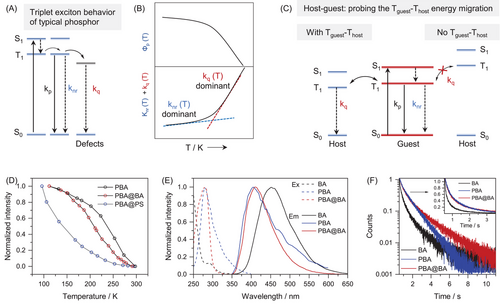
On the basis of the above theoretical model, phenyl boric acid (PBA), a well-known phosphor with blue phosphorescence at molecularly dispersed state,[44, 47] was explored as the probe, since it could be doped into BA with clear host-guest interaction.[47] Meanwhile, polystyrene (PS) was also chosen as the control host, as its phosphorescence is absent in the visible region (high T1 energy). As shown in Figure 1D, the temperature-dependent phosphorescence of PBA@BA was similar to that of PBA only, while PBA@PS exhibited distinctly different temperature dependence (Figures S1–S3). Such evidence clearly demonstrated triplet energy migration between PBA and BA, and thus comparable T1 energy levels (left part of Figure 1C).
The energy migration between PBA and BA was further confirmed via their phosphorescence excitation/emission spectra and lifetime investigations. As shown in Figure 1E, the excitation/emission spectra of PBA@BA exhibited mainly the characteristics of PBA. Meanwhile, the phosphorescence spectra of PBA and BA are overlapped. Moreover, the phosphorescence decays of PBA@BA and PBA are largely matched, while no decaying component of the BA host could be observed in PBA@BA (Figure 1F). These spectra features clearly indicated the possibility of triplet migration between PBA and BA in the host-guest system of PBA@BA, which further verified the similar T1 energy levels of PBA and BA.
2.2 RTP identification from commercial and synthetic BA samples
On the basis of the above theoretical protocol, PBA was further doped into both commercial and synthetic (through HCl-assisted hydrolysis of methylborate[45]) BA. As shown in Figure 2A, the temperature-dependent phosphorescence variations of the two samples almost resembled each other, together with also identical excitations and emissions (Figure 2B). Such spectra features demonstrated that triplet migration existed in both samples, that is, there are no appreciable difference between the commercial and synthetic BA samples. The phosphorescence of BA should be originated from its intrinsic T1 energy level.
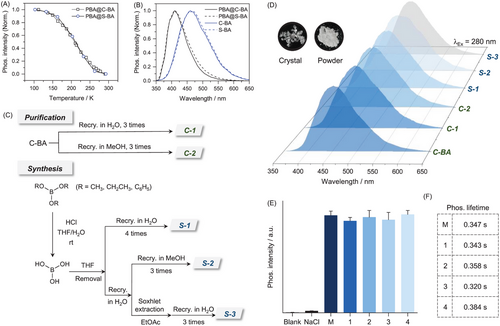
To directly identify the intrinsic phosphorescence of BA, extensive purification was adopted for both commercial and synthetic BA samples (Figure 2C). Specifically, synthetic BA was obtained through HCl-assisted hydrolysis of methyl-, ethyl-, and phenyl-borate (Figures S4-S8). Generally, to rule out possible organic and inorganic impurities in commercial (highest purity available, 99.999%, Sigma-Aldrich) and the crude synthetic BA samples, extensive recrystallization with H2O and methanol (MeOH) was carried out for at least three times. Besides, BA was extracted with ethyl acetate (EtOAc) to further eliminate potential inorganic impurities in H2O and THF (as well as in the precursors). Although the solubility of BA in EtOAc is extremely low, tedious soxhlet extraction with EtOAc as the solvent was also carried out (2 days) to ensure the consistency of the purified procedures that depicted previously.[45] The obtained synthetic BA samples were characterized with NMR, FT-IR spectra, single-crystal X-ray diffraction (XRD) and elemental analysis (EA, Figures S9-S12 and Table S3-S4). All the above obtained samples exhibited similar RTP performances, including emission wavelengths, intensity, and lifetime (Figure 2D). Moreover, the intensity and lifetime of synthetic BA was almost unchanged during multiple rounds of recrystallizations (Figure 2E and 2F, Figure S6). Besides, the possibility of the impurity caused by the reaction between BA and oxygen/water in the atmosphere was further excluded through stability testing (Figure S7). Therefore, these results indicated that the RTP of BA was likely to be intrinsic rather than impurity-induced.
2.3 Excitation- and temperature-dependent phosphorescence spectra of BA
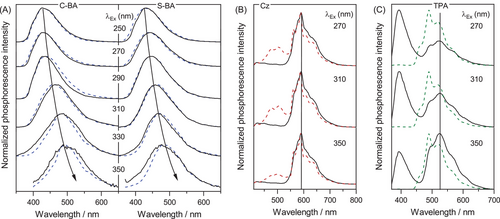
To gain further insights into the luminescence mechanism, excitation- and temperature-dependent phosphorescence of BA was explored. As shown in Figure 3A, at a given excitation, the normalized phosphorescence spectra at 77 K (dash line) and room temperature (solid line) almost resembled each other. Besides, appreciable excitation-dependent phosphorescence emissions were observed both at 77 K (Figure S13) and room temperature (Figure 3A), which has been widely accepted as the general characters of CTE.[34, 37] In contrast, the temperature-dependent phosphorescence from two impurity-induced systems, namely carbazole (Cz)[16] and triphenylamine (TPA),[18] exhibited no such bathochromic shift upon varying the excitation (Figure 3B and 3C). Besides, another feature of CTE, namely dual excitation peaks, could also be identified in the excitation spectra of BA (Figure S14), in which the peak at longer wavelength was ascribed to CTE luminophores.[34] In fact, for other typical CTE phosphors, such as D-xylose[32] and cellulose,[28] excitation- and temperature-dependent phosphorescence was very much similar to those of BA (Figures S15-S16). Therefore, the phosphorescence from BA can be ascribed to the CTE mechanism.
2.4 Physically regulated phosphorescence through mechanical loading
Since CTE originates from weak through space conjugation of n electrons, it is supposed that mechanical loading could increase the overlap of the electron clouds between heteroatoms and thus possibly facilitating phosphorescence enhancement.[48-50] To further confirm the CTE-induced phosphorescence from BA, the effect of applied pressure on phosphorescence intensity was investigated. Meanwhile, two traditional phosphors, namely PBA and 1,4-phenylenebisboronic acid (P2BA), two impurity-induced phosphors, that is, Cz and TPA, and two host-guest luminophores, that is, PBA@BA and P2BA-doped BA (P2BA@BA), were included for comparison. As shown in Figure 4A, the phosphorescence intensity of BA was boosted upon mechanical loading (Figure 4A and Figure S17), with almost unchanged lifetime (Figure 4B). In contrast, the brightness and the lifetime of PBA decreased significantly, while P2BA exhibited poor pressure susceptibility (Figure S18). Meanwhile, the phosphorescence of the impurity-induced and the host-guest luminophores did not change significantly after mechanical loading (Figure 4A-B and Figures S19-S21). These features indicated that the phosphorescence mechanism of BA was different from those of conventional, impurity-induced and host-guest phosphors. Most importantly, in the PBA@BA and P2BA@BA systems, PBA and P2BA can be regarded as “impurity” of BA, but the pressure-induced phosphorescence changes were largely different from that of BA, irrespective of the doping amounts (Figure S22).
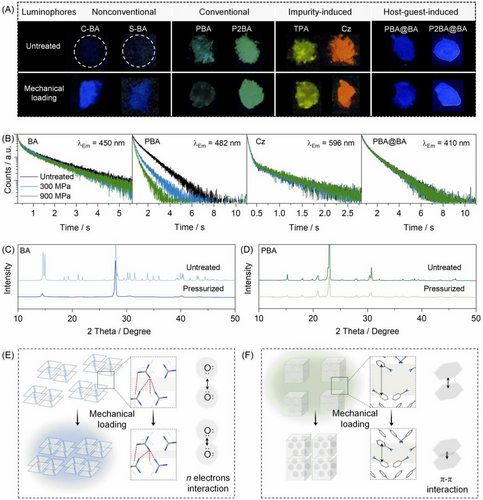
The above pressure-induced phosphorescence changes were further studied with XRD. As shown in Figure 4C,D, the XRD patterns of BA and PBA changed after mechanical loading, while those of the impurity-induced and host-guest luminophores remained almost unchanged. Therefore, the crystalline structures of BA and PBA were destructed. In this manner, adjacent O atoms in BA may be brought closer proximity to strengthen the weak intermolecular Van-der Waals interaction,[32, 51] thus facilitating electron communications between the n electrons of O atoms for efficient phosphorescence (Figure 4E). In contrast, phosphorescence of PBA is ascribed to triplet stabilization through intermolecular locking in the aggregate state.[43, 44] Under mechanical loading, the π-π interactions may be strengthened, leading to the dissipation of triplet excitons and phosphorescence quenching (Figure 4F).[52]
2.5 Chemically regulated phosphorescence of boric acid
Although mechanical loading was an effective way for modulating the phosphorescence of CTE luminophores, the exerted pressure could not reach the Young's modulus of typical materials, thus there is no remarkable change in the distance of oxygen clusters within the lattice structure. Since CTE relies on weak through space conjugation of the n electrons, shortening the distances of oxygen clusters may produce more significant phosphorescence enhancement, thus providing another way to validate the intrinsic mechanism of phosphorescence from BA. As shown in Figure 5A, BA, metaboric acid (HBO2), and boron trioxide (B2O3) all possess the BO3 unit. According to their crystal data, there are plenty of effective O···O interactions (the distances of adjacent O atoms shorter than two-fold of the van-der Waals radii of O atom,[51] 2dO (vdW) = 3.04 Å) that may contribute to CTE. For phosphor containing isolated BO3 unit (e.g., triphenyl borate), no sign of CTE could be observed (Figure S23). Most importantly, in a 3 × 3 × 3 unit cell, the number of effective O···O interactions [d(O···O) < 2dO (vdW)] follows the order of H3BO3 (8) < HBO2 (10) < B2O3 (21) (Figure 5B). Therefore, more complicated and rigidified oxygen clusters may be developed in the order of H3BO3, HBO2 and B2O3, leading to more effective through space interaction and stabilization of the triplet excitons.
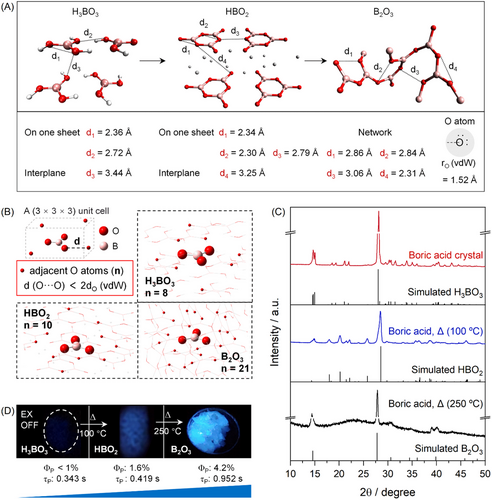
To prove the above theoretical predictions, crystalline boric acid was subjected to dehydrating to obtain HBO2 (100°C, 1 h) and finally B2O3 (250°C, 5 h),[53] as revealed with the corresponding XRD patterns (Figure 5C). To our delight, significantly enhanced phosphorescence brightness and elongated lifetime from BA to HBO2 and B2O3 could be observed (Figure 5D), accompanied with similar multicolor emission (Figures S24–S25). Therefore, the phosphorescence of CTE luminophores could also be turned through chemical regulation of the weak conjugation of the n electrons.
3 CONCLUSION
In summary, here we proposed a verification protocol to confirm the intrinsic phosphorescence of boric acid, which was substantially different from the conventional, impurity-induced, and host-guest phosphors. First, the relative position of the T1 energy level of BA was probed using the host-guest strategy proposed by Hirata.[14] Second, precursor-independent synthesis and extensive purifications were carried out to verify the RTP of BA. Third, excitation- and temperature-dependent phosphorescence was adopted to illustrate the difference between CTE and impurity-induced phosphors. Last, physically- and chemically regulated phosphorescence was successfully achieved in BA, which further confirm its CTE nature. It should be noted that the CTE mechanism was also been validated with single molecular electronics.[54] The protocol was not just limited to BA, but in fact most phosphorescent n electron-rich systems. Moreover, identification the origin of CTE is thus helpful for more rational strategies to adjust the emission wavelength and brightness,[38, 55-57] and thus more rationale applications.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (grant number: 22325403) and the Fundamental Research Funds from Sichuan University (grant number: 2022SCUNL104). Detailed characterizations supported by the public Platform of Analytical and Testing Center, Sichuan University are greatly appreciated.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.




