Biomimetic Fe7S8/Carbon electrocatalyst from [FeFe]-Hydrogenase for improving pH-Universal electrocatalytic hydrogen production
Special Collection: Aggregation-Induced Processes and Functions
Dohun Kim and Subramani Surendran contributed equally to this work.
Abstract
Efficient and cost-effective electrocatalysts that can operate across a wide range of pH conditions are essential for green hydrogen production. Inspired by biological systems, Fe7S8 nanoparticles incorporated on polydopamine matrix electrocatalyst were synthesized by co-precipitation and annealing process. The resulting Fe7S8/C electrocatalyst possesses a three-dimensional structure and exhibits enhanced electrocatalytic performance for hydrogen production across various pH conditions. Notably, the Fe7S8/C electrocatalyst demonstrates exceptional activity, achieving low overpotentials of 90.6, 45.9, and 107.4 mV in acidic, neutral, and alkaline environments, respectively. Electrochemical impedance spectroscopy reveals that Fe7S8/C exhibits the lowest charge transfer resistance under neutral conditions, indicating an improved proton-coupled electron transfer process. Continuous-wave electron paramagnetic resonance results confirm a change in the valence state of Fe from 3+ to 1+ during the hydrogen evolution reaction (HER). These findings closely resemble the behavior of natural [FeFe]-hydrogenase, known for its superior hydrogen production in neutral conditions. The remarkable performance of our Fe7S8/C electrocatalyst opens up new possibilities for utilizing bioinspired materials as catalysts for the HER.
1 INTRODUCTION
Water electrolysis to produce hydrogen has been widely studied as an environmental and sustainable energy technology.[1] In the real case, electrochemical hydrogen evolution reaction (HER) required overpotential to overcome the activation energies.[2] For minimizing input energy, the development of electrocatalysts has been considered a crucial factor in its commercialization.[3] Likewise, the grid scale of hydrogen production requires the development of highly efficient and robust catalysts.[4] So far, platinum-based catalysts have been conventionally used as HER catalysts for their high activity and durability.[5] However, due to its intrinsic scarcity in nature and high cost, the development of efficient earth-abundant metal-based catalysts is necessary for practical electrochemical water splitting.[6] Recently, transition metal-based oxide,[7, 8] phosphide,[9, 10] and sulfide[11-13] have been extensively studied for HER catalysts.
Metal sulfide catalysts have garnered significant attention as they aim to mimic the active sites of hydrogenase enzymes.[14] Hydrogenase enzymes, present in bacteria, archaea, and certain eukaryotes, exhibit an impressive turnover frequency of approximately 10,000 s−1 for proton reduction reactions.[15] This exceptional activity surpasses that of synthetic catalysts, approaching the thermodynamic efficiency limit. The active site of hydrogenase consists of an iron-sulfur cluster surrounded by functional proteins.[14, 15] The structural analysis of hydrogenase active sites has inspired the development of organometallic chemistry methods for synthesizing artificial hydrogenase systems.[16, 17] Researchers have explored the synthesis of inorganic catalysts containing components similar to hydrogenase, including Fe-S clusters.[18-20] Various iron sulfide materials, such as FeS2 (pyrite), Fe3S4 (greigite), and Fe9S10 (pyrrhotite), have been investigated for their potential to catalyze water-splitting reactions.[21-23] Previous studies have demonstrated that the pyrite structure exhibits the highest hydrogen evolution activity among the iron sulfide materials, primarily attributed to a high sulfur-to-iron ratio.[18] However, these Fe-S approaches alone have exhibited low stability and activity in HER.[24] In addition, the inferior conductivity of iron sulfide electrocatalysts hinders efficient proton/electron transfer, limiting their intrinsic performance compared to previously reported HER electrocatalysts.[16, 20, 25-27] To address these challenges, recent advancements have focused on heteroatom doping or carbon coating techniques, which have shown remarkable improvements in the efficiency and stability of inorganic iron-sulfur electrocatalysts for the HER.
Herein, we designed a carbon matrix-incorporated iron-sulfur cluster (Fe7S8/C) inspired by the structure of hydrogenase to enhance the stability and activity of iron sulfur-based catalysts. Similar to hydrogenase, protein assemblies surround the iron-sulfur metal site and serve as an effective matrix for facilitating electron charge transfer during electrocatalytic reactions. To stabilize the iron sulfide nanoparticles, we utilized polydopamine molecules as a matrix. Polydopamine, structurally resembling natural melanin (eumelanin), contains various functional groups such as catechol, hydroxyl, amine, and imine. Catechol compounds, including DOPA, can covalently or non-covalently attach to diverse surfaces, resembling the adhesive proteins found in mussels. The Fe7S8/C electrocatalyst prepared in this study exhibits low overpotentials of 90.6, 45.9, and 107.4 mV in acidic, neutral, and alkaline conditions, respectively. Notably, the iron species undergoes a change in oxidation state to monovalent during the HER, a catalytic mechanism similar to that of hydrogenase. In hydrogenase, a carbonyl or cyanide group adjacent to the active iron site induces low valency in a low spin state, promoting efficient hydrogen production. Similarly, the presence of monovalent iron species in the Fe7S8/C catalyst enhances its electrocatalytic activities toward HER. This significant improvement achieved in our work highlights the potential of biomimetic materials as efficient electrocatalysts for HER across all pH conditions.
2 RESULTS AND DISCUSSION
2.1 Structural characterization
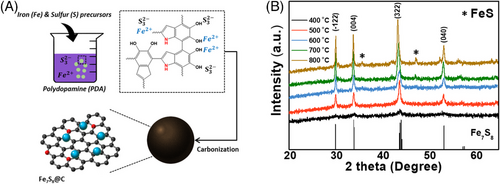
The Fe7S8/C samples were prepared by a facile wet-chemical method followed by calcination (Figure 1A). The X-ray diffraction (XRD) pattern of the prepared Fe7S8/C nanoparticles annealed at each temperature shows well-defined and high crystallinity peaks, which correspond to the standard JCPDS No. 98-015-1766 pattern of pyrrhotite 4C Fe7S8 (Figure 1B). The XRD pattern of the sample annealed at a low temperature of 400°C showed a broad peak due to the amorphous structure of the sample conjugated by the accretion of polydopamine. The crystallinity of the sample increases corresponding to the annealing temperature due to the contraction of the carbon layer. The XRD result confirms the formation of stable pyrrhotite 4C Fe7S8/C nanoparticles throughout the annealing process. The minor XRD peaks arising at 800°C annealing temperature can be due to the recrystallization of Fe and S samples as insignificant impure phases of monoclinic FeS (JCPDS: 98-008-7500), as shown in Figure 1A. The samples prepared at 600°C do not exhibit such insignificant impurity peaks which indicate the recrystallization of impure FeS phases at temperatures higher than 700°C. X-ray photoelectron spectroscopy (XPS) was performed to analyze the chemical valence states for the prepared Fe7S8/C electrocatalyst. The deconvoluted Fe 2p spectra in Figure S1A reveal two peaks for Fe2+ and Fe3+ at 711 and 713 eV for Fe 2p3/2, and at 724 and 726 eV for Fe 2p1/2, respectively. Figure S1B for S 2p spectra exhibits two peaks around 164 eV and 165.2 eV for S 2p3/2 and S 2p1/2, respectively. An additional peak at 168.8 eV corresponding to S-O bonds arises due to the passivation of the electrocatalysts with air exposure. Besides, the C 1s spectra, shown in Figure S1C, reveal four major peaks at 284 eV, 285.2 eV, 286 eV, and 288 eV corresponding to sp2 bonded C=C bonds, C–C bonds, passivated C–O bonds, and the carboxyl groups (O–C=O) in the prepared electrocatalysts. O 1s spectra in Figure S1D depict three signals at 530 eV, 531.5 eV, and 532.4 eV corresponding to adsorbed OH, C–O–C bonds, and carboxyl groups, respectively.
The morphological characterization of the prepared Fe7S8/C@600 was identified using scanning electron microscopy (SEM) analysis. The obtained SEM image reveals a uniform distribution of Fe7S8/C spherical nanoparticles embedded within a three-dimensional porous carbonized polydopamine matrix (Figure 2A). This arrangement enhances mass transport and augments the catalytic surface area. The interconnected porous network facilitates increased electrolyte ion access to electroactive sites, improving the catalyst's active surface area and consequently enhancing its electrocatalytic activity. The inset of Figure 1A shows the high-magnification SEM image of Fe7S8/C. Substantially, the transmission electron microscopy (TEM) image in Figure 2B reveals network-like clusters of Fe7S8/C spherical nanoparticles similar to the SEM image. The HRTEM images in Figure 2C reveal the interface of carbon structures and crystalline Fe7S8 structures. The inset of Figure 2C shows well-defined lattice fringes indicating the interplanar spacing of about 0.2 nm corresponding to (322) lattice planes of Fe7S8 supported by the FFT image also reveals the bright spot for (322) lattice planes in the reciprocal lattice as observed from XRD analysis. In Figure 2D, the selected area electron diffraction (SAED) image shows dot diffraction patterns implying the high crystallinity nature of the prepared Fe7S8/C@600 sample.
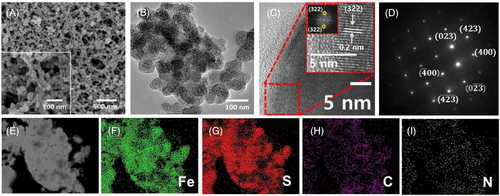
Energy-dispersive X-ray spectroscopy (EDS) analysis was conducted to assess the chemical composition of the prepared Fe7S8/C@600 sample, as shown in Figure 2E–J. Elemental mapping images show the co-existence of the Fe, S, N, and C in the Fe7S8/C@600 sample. Therefore, the structural and morphological analysis explicates the phase pure formation of interconnected network-like Fe7S8/C spherical nanoparticles, which can enhance electrocatalytic activity.
2.2 Electrocatalytic activity
The electrocatalytic activity of Fe7S8/C electrocatalysts was characterized by the linear sweep voltammetry (LSV) technique using a rotating disk electrode in a three-electrode system. To optimize the annealing temperature, which enhances HER activity, LSV was conducted for the samples prepared at different annealing temperatures (400–800°C), as shown in Figure 3A. Comprehensively, the LSV curve of the sample annealed at 600°C showed the largest positive shift toward the equilibrium potential of 0 V (vs. reversible hydrogen electrode [RHE]) compared to the samples annealed at 400, 500, 700, and 800°C.
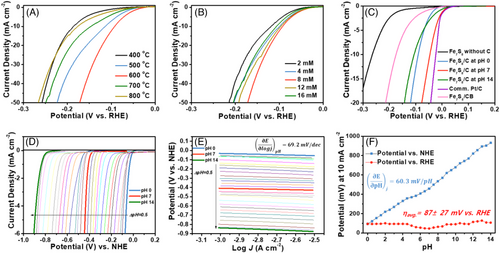
Here, the Fe7S8/C catalyst annealed at 600°C, with its optimized amorphous-carbon interface, exhibits superior electrocatalytic hydrogen production compared to other annealing temperatures for Fe7S8/C. Consequently, the Fe7S8 sample annealed at 600°C strikes an optimal balance in terms of crystallinity, indicating the presence of an amorphous carbon interface.[28] Thus, the Fe7S8/C annealed at 600°C demonstrates heightened electrocatalytic performance for the HER due to its mixed crystalline-amorphous phases, surpassing samples annealed at lower and higher temperatures. Further, to estimate the optimal synthetic condition, the LSV curves of the Fe7S8/C catalyst prepared by varying the molar ratio of Fe precursor from 2 to 16 mM were obtained (Figure 3B). The sample prepared from 8 mM Fe precursor showed improved electrocatalytic hydrogen production. Additionally, the Fe7S8/C@600 electrocatalysts were distinguished from exhibiting superior HER performance across all pH conditions, and commercial Pt/C samples as shown in Figure 3C. In addition, the electrochemical HER performances were performed by mixing the Fe7S8 samples prepared in the absence of dopamine with carbon black (Fe7S8/CB). The Fe7S8/CB samples reveal higher electrocatalytic activity than Fe7S8 without C indicating significant conductivity effects of carbon coating (Figure 3C). However, the Fe7S8/C samples reveal higher HER activity than Fe7S8/CB due to the significant electrocatalytic effects of N doped C matrix. The results reveal higher HER electrocatalytic activity under various pH conditions for Fe7S8/C compared to Fe7S8 without C and Fe7S8/CB electrocatalysts, and commercial Pt/C under neutral pH conditions.
Under all pH conditions, the current density associated with the HER exponentially increased when the potential was swept from 0.15 to −0.3 V vs. RHE. In addition, iron sulfide nanoparticles without dopamine additives were also tested to investigate the dopamine-derived polymerization effect. The overpotential necessitated by the Fe7S8/C was significantly lower than that of the pristine Fe7S8 sample prepared without carbon (η = 263 mV), substantiating the role of carbon in promoting improved charge transfer and catalytic activity. Additionally, the Fe7S8/C catalyst was observed to be highly active under the neutral (η = 45.9 mV vs. RHE), base (η = 107.4 mV vs. RHE), and acid condition (η = 90.6 mV vs. RHE). To our knowledge, the value of overpotential (η) is the lowest among the reported iron sulfur-based electrocatalysts (Tables S1–S3).
The results reveal higher electrocatalytic activity for Fe7S8/C prepared in the presence of dopamine with C and N elements. Due to different ionic components, acidic and alkaline electrolytes exhibit different non-Faradaic reaction mechanisms. The latest literature reported that the Volmer reaction (H3O+ + e− → Hads + H2O in acidic / H2O + e− → Hads + OH− in alkaline) barrier was higher in acidic conditions rather than alkaline conditions.[29] Therefore, the reactant adsorption on the catalytic surface easily occurs in alkaline electrolytes, resulting in lower overpotential in the non-Faradaic region. However, the Faradaic reaction, including the Heyrovsky reaction and Tafel reaction (which occurred only in acidic conditions), occurred in acidic conditions rather than in alkaline conditions. For these reasons, lower overpotential in pH = 14 at low current density while at high current density, Fe7S8 shows lower overpotential in pH = 0. The turnover frequency (TOF) values in Figure S2 indicate the rate of charge transfer reactions at the electrode surface for hydrogen evolution. Notably, the Fe7S8/C electrocatalysts exhibit significantly higher TOF values in neutral electrolytes across a range of HER overpotentials compared to their performance in acidic or alkaline electrolytes.
To further examine the electrocatalytic reaction of the Fe7S8/C catalyst, electrochemical impedance spectroscopy (EIS) analysis was conducted at HER condition (−0.1 V vs. RHE). The Nyquist plot from the EIS measurement is displayed in Figure 4A.
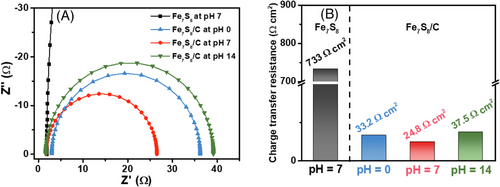
Compared to pristine Fe7S8 without carbon (733 Ω cm2), the Fe7S8/C sample showed a much smaller semicircle, corresponding to lower charge transfer resistance (Rct) values of 33.2 Ω cm2 at pH 0, 24.8 Ω cm2 at pH 7, and 37.5 Ω cm2 at pH 14, respectively (Figure 4B). The charge transfer resistance obtained from the fitted equivalent circuit is closely related to kinetic barrier energy for faradaic reactions (HER) at the interface between electrocatalysts and the electrolyte. The charge transfer resistance is also inversely proportional to the exchange current for the faradaic reaction. Therefore, the lower charge transfer resistance implies the enhanced HER performance with a lower overpotential. The electrochemical surface area analysis (ECSA) infers the higher active surface available for charge transfer reactions over prepared Fe7S8/C electrocatalysts. The scan rate versus current plots were derived from the cyclic voltammetry (CV) curves obtained for various scan rates in the non-Faradic region, as shown in Figure S3A–C. The double layer capacitance (Cdl) values for the prepared Fe7S8/C samples annealed at 500, 600, and 700°C were estimated to be 0.45, 1.31, and 0.62 mF, respectively (Figure S3D). The corresponding ECSA values of Fe7S8/C samples annealed at 500, 600, and 700°C are 11.25, 32.75, and 15.5 cm2, respectively. The higher ECSA values for Fe7S8/C annealed at 600°C indicate highly exposed active sites for electrocatalytic reactions compared to samples annealed at 500 and 700°C.
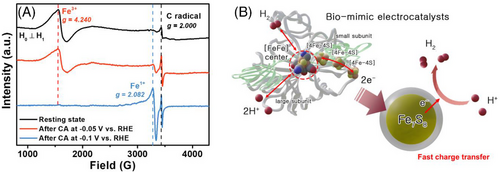
The g value of the catalyst was calculated by 71.4484 v/B (mT). In the resting state, the Fe3+ and carbon radical signals were at g values of 4.24 and 2.00, respectively. After CA testing at the potential of −0.05 V vs. RHE, the Fe1+ signal was detected at a g value of 2.082 (B = 3308 G) in the vicinity of the carbon radical signal (g = 2.00 and B = 3444 G), while the intensity of the Fe3+ signal had slightly decreased (g = 4.24 and B = 1624 G) and the carbon radical signal had increased. When the potential was increased to −0.1 V vs. RHE, the Fe1+ signal further increased and the Fe3+ signal further decreased, as shown in Figure 5A. These results suggest that iron species are involved during the HER and that the iron valency changes from 3+ to 1+. This behavior can be found in the iron-sulfur cluster in natural hydrogenase, called [FeFe]-hydrogenase, which is a well-known hydrogen-evolving catalytic reaction mechanism.[35, 36] Such enzymes have a unique organometallic cofactor, called the H-cluster, composed of a canonical [4Fe-4S] cluster, as shown in Figure 5B. This structural arrangement makes the active site a blocked Lewis pair system, prompting efficient and reversible heterolytic H+/H− coupling. In the [FeFe]-hydrogenase, [Fe-Fe] center with Fe-S clusters as active sites can catalyze the reduction of protons with extremely high efficiency in some anaerobic microorganisms.[37]
During redox reactions under the influence of electrolytic ions and applied potentials, Fe atoms can undergo significant changes in their valence states. These reduction performances are pivotal in inducing a shift from Fe2+ to Fe1+ valence states, ultimately enhancing electrocatalytic activity.[38] It is worth noting that Leonard et al. have observed this Fe2+/Fe1+ valence state change through operando XAS studies during CO2 reduction conducted on porous Fe-nitrogen-carbon (Fe-N-C) materials.[39] Thus, the presence of lower valence states (2+/1+) in Fe is suggested to occur during electrochemical reduction processes.
Figure S4A–C shows the long-term stability tests for the Fe7S8/C samples under neutral, acidic, and alkaline pH conditions which reveal negligible differences after 12-hour tests. The XPS analysis in Figure S5A shows that under acidic and neutral conditions negligible changes were noted to the characteristic peaks of Fe 2p relating to the as-prepared sample. However, under alkaline conditions, Fe 2p spectra revealed a partially oxidized state compared to as prepared samples. The small positive shift can be attributed to the partially adsorbed intermediates during HER activity. Besides, the S 2p spectra reveal almost similar peak intensity for S-O, S 2p3/2, and S 2p1/2 for the samples after the stability test under acidic and neutral pH conditions, as shown in Figure S5B. The S 2p spectra for alkaline pH conditions show decreased peak intensity for S 2p3/2 and S 2p1/2. Hence, the alkaline pH condition for Fe7S8/C annealed at 600°C reveals relatively higher chemical oxidation state changes than acidic or neutral electrolytes. However, the XRD results in Figure S5 after the stability test under acidic, alkaline, and neutral pH conditions reveal insignificant changes to the crystal phase of Fe7S8. The XRD analysis confirms that the bulk Fe7S8 did not show any structural variations and the strong amorphous peak around 24° corresponds to the carbon cloth substrate. Besides, morphological analysis by FESEM after stability tests under different pH conditions has revealed insignificant changes due to the partially adsorbed reaction intermediates (Figure S6A–D).
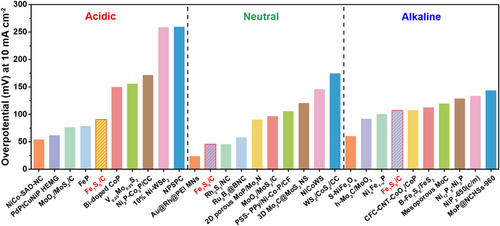
During the HER process, the oxidation state of Fe is partially changed with H+ adsorption.[63] From the effective [FeFe]-hydrogenase system, we designed the biomimetic Fe7S8/C electrocatalyst and revealed superior HER performance compared to state-of-the-art transition metal-based electrocatalysts under all pH conditions. As shown in Figure 6, our Fe7S8/C electrocatalyst showed a lower overpotential than other reported transition metal-based HER electrocatalysts.
3 CONCLUSION
In summary, we developed the highly effective HER electrocatalyst of Fe7S8/C from the co-precipitation technique. This work highlights the bio-mimetic electrocatalyst of Fe7S8/C, which could enhance the HER activities under all pH conditions. Such an iron-sulfur cluster surrounded by a carbon matrix provided iron species in a low spin state, and this effect promotes hydrogen production by inducing iron active sites like natural hydrogenase. Fe7S8/C was optimized by controlling the annealing temperature of the sulfur-polydopamine complex, the molecular concentration of iron precursors, and the pH condition of the electrolyte. Among these various samples, the 8 mM Fe7S8/C annealed at 600°C sample exhibited the lowest overpotential of 45.9 mV in neutral pH conditions. In addition, the Fe7S8/C also showed a low overpotential of 90.6 and 107.4 mV in acidic and alkaline conditions, respectively. The EIS results showed the charge transfer resistance of the Fe7S8/C electrocatalyst in various pH conditions. The charge transfer resistance was shown to be small in the order of neutral, acidic, and alkaline conditions, which revealed electrocatalytic activities depending on pH conditions. Furthermore, CW-EPR analysis confirmed the change in iron valence from 3+ to 1+ during the HER process, mirroring the behavior of natural hydrogenase enzymes. Our research demonstrates the potential of bio-inspired Fe7S8/C electrocatalysts to enhance HER performance across all pH conditions and opens avenues for their application in diverse electrochemical energy conversion systems.
4 EXPERIMENTAL SECTION
Preparation of Fe7S8 cluster and Fe7S8 surrounded by a carbon matrix (Fe7S8/C): Dopamine (2 mg) was used as a monomer and mixed in tris-(hydroxymethyl) aminomethane solution (10 mM, pH 8.5). Iron precursors in FeSO4•7H2O (2 mM, 4 mM, 8 mM, 12 mM, and 16 mM) and the sulfur precursor in (NH4)2S3 (8.5 mM) were introduced into the dopamine solution. And the obtained Fe7S8-polydopamine complex was annealed under an inert atmosphere. To optimize the annealing condition, the annealing temperature was controlled from 400°C to 800°C.
Physicochemical characterizations: Powder XRD was carried out on a D-8 Advance X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) to confirm the structure of Fe7S8/C. The chemical states of the prepared samples were examined by high-performance XPS (Multilab 2000). The morphology and the SAED patterns were obtained using a high-resolution transmission electron microscope (JEM-3000F; JEOL) with an acceleration voltage of 300 kV. EPR measurement was performed using Bruker EMX/Plus spectrometer equipment with a dual-mode cavity (ER 4116DM). The temperature was controlled by liquid He quartz cryostat (Oxford Instruments ESR900) with a controller of temperature and gas flow (Oxford Instruments ITC503). The measurement was performed under a microwave frequency of 9.64 GHz (perpendicular mode), modulation amplitude at 10 G, modulation frequency of 100 kHz, and 0.94 mW microwave power.
ACKNOWLEDGMENTS
This research was supported by the Outsourced R&D Project of Korea Electric Power Corporation (KEPCO) (Grant number: R23XO04), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2021M3H4A6A01045764, 2020M3H4A3106313, 2021R1C1C1004264, and 2021R1A4A1032114), the Korea Institute for Advancement of Technology (KIAT) grant funded by the Ministry of Trade, Industry, and Energy (MOTIE), Korea (P0025273), the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20224000000320), and the KENTECH Research Grant funded by the Korea Institute of Energy Technology, Republic of Korea (KRG2022-01-016). This research was also supported by the Regional Innovation Strategy (RIS) through the National Research Foundation (NRF) funded by the Ministry of Education (MOE) (2021RIS-002).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




