Thin-film flow technology in controlling the organization of materials and their properties
Special Collection: Distinguished Australian Researchers
Clarence Chuah and Xuan Luo contributed equally to this work.
Abstract
Centrifugal and shear forces are produced when solids or liquids rotate. Rotary systems and devices that use these forces, such as dynamic thin-film flow technology, are evolving continuously, improve material structure-property relationships at the nanoscale, representing a rapidly thriving and expanding field of research high with green chemistry metrics, consolidated at the inception of science. The vortex fluidic device (VFD) provides many advantages over conventional batch processing, with fluidic waves causing high shear and producing large surface areas for micro-mixing as well as rapid mass and heat transfer, enabling reactions beyond diffusion control. Combining these abilities allows for a green and innovative approach to altering materials for various research and industry applications by controlling small-scale flows and regulating molecular and macromolecular chemical reactivity, self-organization phenomena, and the synthesis of novel materials. This review highlights the aptitude of the VFD as clean technology, with an increase in efficiency for a diversity of top-down, bottom-up, and novel material transformations which benefit from effective vortex-based processing to control material structure-property relationships.
1 INTRODUCTION
The field of nanotechnology has witnessed considerable progress in recent years, both in design and the scope of applications. As an interdisciplinary field, nanotechnology has undoubtedly led to industrial production of novel nanomaterials with designer properties such as being more robust, lighter, longer-lasting, antireflective, antimicrobial, electrically conductive, or becoming more luminous. In particular, nanoparticle (NP)-based medicine has gained traction, promising to revolutionize medical treatment with innovative therapeutics that are more potent and less toxic.[1]
Nanotechnology is revolutionary, and its hype is justified, especially for improving the quality of human life with novel consumer products through various materials and manufacturing methods. However, there are concerns on nanotechnology potentially creating delayed impacts on the environment and human health, especially where detrimental consequences are only noticed after commercialization has long begun. Thus, technologies being developed require cautionary measures to be upheld to avoid future predicaments for the environment and humanity, in tracking toward a sustainable future. This is to prevent history from repeating itself, such as petrol with lead, electronics with polychlorinated biphenyls, chlorofluorocarbons reducing upper atmosphere ozone dramatically, and the construction material asbestos, all of which led to environmental disasters.
Despite substantial research in the field and considerable progress, strategies for manufacturing nanoscale materials, through both top-down and bottom-up production processes, still face challenges. Top-down approaches for reducing materials to nanoscale dimensions often involve the use of harsh chemicals, leading to significant waste generation and raising environmental safety concerns.[2] Traditional assembly lines create products by building them up from the molecular level, the bottom-up strategy, which combines chemical synthesis and self-assembly. For current practical abilities, the main challenges are that the bottom-up strategy can be time-consuming, requiring extensive expertise and skill to control the size, morphology, and properties of the nanoscale products. Of paramount importance is the choice of synthetic method to finely control these features while circumventing uptake of impurities.[2] As such, the fundamental problem regarding the bottom-up strategy is developing the capability to exquisitely control the synthesis of the NPs while appropriately controlling size, morphology, and properties at nanoscale dimensions.
From a technological viewpoint, traditional methods abound in developing processes to control the growth and properties of materials. Such bottom up material processing at the nanoscale dimension has been developed using channel-based microfluidic devices, albeit with some limitations. The main drawback is insufficient mixing resulting from laminar flows, often requiring sample dilution or reagent homogenization. The mixing process is usually restricted to diffusion control processing under such flows, denying the possibility of harnessing the advantages of turbulent mixing available in macro-scale systems.[3] While mixing enhancements can be achieved by incorporating multiple system parameters, including energy input, the velocity of flow, and the geometry of the mixers, these methods are time-consuming, leading to cost inefficiencies. In addition, channel-based microfluidic devices can suffer from clogging, specifically in the processing of macromolecules or at high reactant concentrations. Furthermore, incorporating external fields, such as electrical, magnetic, and laser fields, to control the processing is inherently complex for channel-based microfluidic platforms.[4] Although other mechanical energy forms, including sonication and grinding or milling, are effective in materials processing, they suffer from indiscriminate events in time and place, such as in cavitation and uneven energy transfer, resulting in nonuniform products under nonoptimised processing conditions.[5] This can generate waste that, coupled with high energy usage, limits the sustainability metrics of such processing. A paradigm shift in microfluidics design is required to overcome such limits.
Thin film processing is an emerging technology where the liquid is subjected to centrifugal forces/shear stress or mechanical energy within dynamic thin films on a surface. These forces are useful in a range of thin film vortexing technologies, including for chemical synthesis and separations,[6] material synthesis,[7] material processing, lab-on-a-disc microfluidics[8] and enabling chiral selection.[9] Thin film processing offers several advantages, including accelerated reaction kinetics and improved control over chemical reactivity. The application of shear stress presents opportunities for enabling new types of chemical reactions and generating materials with new shapes, morphologies and sizes.[10] Thin film processing holds potential in situations where traditional batch processing is impractical or when conventional methods fail to provide access to unique forms of materials.[11]
Rotary devices that utilize centrifugal forces, pushing away from the rotation axis, are prime examples of how these forces can shape interfaces and effectively control material synthesis and chemical reactivity.[10] A diverse range of rotary devices have been reviewed and shown to achieve such performances, including lab-on-a-disc system,[12] spiral seperators,[13] spinning disc reactors,[14] and vortex fluidic devices.[15] This review introduces the vortex fluidic device (VFD) as a paradigm shift in flow processing, with scalability factored in under the continuous-flow mode of operation of the device, along with its utility for tuning the size, morphology, and properties of materials at the nanoscale dimension. The VFD delivers high shear as a constant form of mechanical energy, with tunable control over the processing. The shear stress inside thin fluid films varies along the length of the tube reactor and has been calculated to range from 0.5246 to 0.5574 Pa when processed at 5 krpm in a 10-mm diameter tube reactor.[16] Processing in the device is not limited by diffusion control, providing a route to kinetically trapped novel forms of nanomaterials, and processing can also be facilitated by applied external fields for which the microfluidic platform is suited with the thin film of liquid approaching uniformity of treatment as such. Continuous-flow processing of the VFD is directly scalable, unlike batch processing, which requires precise process engineering for upscaling, in overcoming uneven mixing and heat transfer. Whereas previous reviews of VFDs have focused on chemical transformations[17] and comparisons with other microfluidic devices,[18] this review aims to deliver additional information about the significance of utilizing the VFD to control material structure-property relationships at the nanoscale with emphasis on its high green chemistry metrics.
2 VORTEX FLUIDIC TECHNOLOGY
From manipulating single-cell organisms to processing advanced materials and small molecule synthesis, these processing capabilities and many more can be conducted through a VFD by accurately regulating the shear force experienced by the fluid, among various other parameters that affect the fluid dynamics. As shown in Figure 1, the VFD has noticeably unique features and capabilities relative to other processing platforms, in particular the ability to vary the tilt angle of the device. Typically a VFD has a glass or quartz tube with an outside diameter (OD) of either 10 mm, 15 mm, or 20 mm, and can be spun at speeds from 1000 to 10,000 rpm while inclined at an angle θ which can be varied from 90 to 0° with recent developments toward −45° processing.[19] The inclination angle and rotational speed control the management of shear rates along the tube reactor, with the fluid dynamics being inherently complex when θ ≫ 0°.[16, 20] The formation of a thin film from the liquid in the VFD witnesses high shear from the interaction between gravitational and centrifugal forces. These forces are accompanied by the appearance of Stewartson/Ekman layers as fluid flows upward on the rotating tube's internal surface and downwards near the liquid's surface, parallel to the rotational axis of the rotating tube.
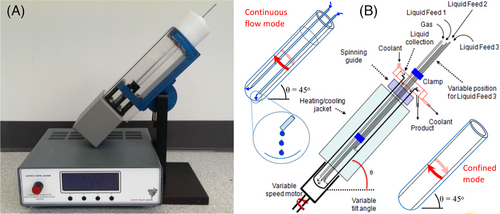
The VFD has two modes of operation, a confined mode, and a continuous-flow mode, with the former having a finite amount of liquid in a glass tube that is closed at one end and rapidly rotated. The latter has liquids exiting from the top of the tube after being fed continuously into the base or at specific positions along the inside of the tube. Although the capability for processing in continuous flow was the original purpose of the designed technology, processes in the confined mode can also be used for small quantities of liquid, with processing scalability being dependent on the amount of material required. As such, industrial applications with high volumes would require a single large unit[22] or multiple units in a parallel array for processing. In contrast, small volume niche applications, down to ∼0.5 mL for a 10-mm diameter tube, for example in medicine, require only a single VFD unit. The VFD itself is relative inexpensive, ∼$5,000 US, and is a versatile microfluidic platform for controlling chemical reactivity and selectivity, material synthesis, and probing the structure of self-organized systems, offering a range of benefits over conventional processing.[17, 19] Micro-mixing and high shear stress are imparted in the dynamic thin film on the surface of the rotating rapidly tube in the VFD, typically between 3000 and 9000 rpm. The VFD has been employed successfully for a remarkable diverse range of applications, such as in fabricating nanocarbons,[23, 24] exfoliating of graphite and boron nitride,[25] accelerating enzymatic reactions,[26, 27] manipulation of polymer networks,[28] protein purification,[16] and indeed protein folding[29]
The VFD is particularly useful for regulating the shape and size of NPs for both bottom-up and top-down processing. The rapidly evolving capabilities and innovative processing toolbox for the device allows mapping out synthetic strategies for seemingly endless research and industrial possibilities in the nanomaterials arena alone. Intense micro-mixing when liquids are added to the rotating tube in the VFD, and high shear stress associated with high mass topological fluid flows (see below) in the resulting thin film of liquid in the VFD, can be harnessed in a controlled way to discover and improve chemical reactivity beyond what is possible using batch processing where processing is limited by diffusion control. The VFD is proficient in probing the structure of self-organized systems, materials processing, instigating chemical reactions, and rapidly imparting organic modifications to a range of motifs in a controlled fashion. Despite the VFD being unlike typical microfluidics where channels are used with typically laminar fluid flow, it is acquiring distinction as a multipurpose microfluidic platform with novel operating characteristics. Modern-day chemistry focuses on the metrics of incorporating green chemistry into the science at its inception and in this context processing the VFD can be significantly improved for research and industrial applications.
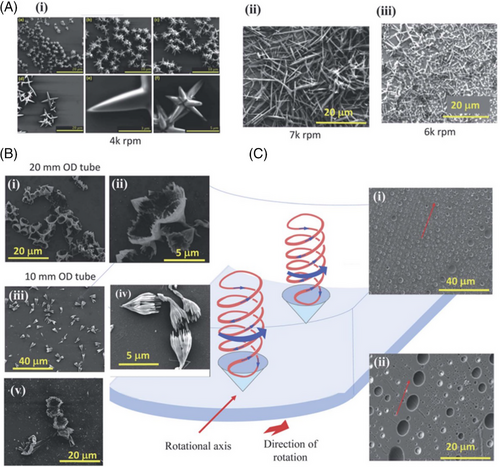
Recent fundamental studies on fluid flow in the VFD have established an understanding that the fluid exhibits resonant behaviors from the constricting boundaries of the glass surface and the meniscus, which define the liquid film thickness. To overcome the inability to directly measure the unique fluid flow in the rotating reference frame in the VFD, materials processing strategies were employed which allowed identifying specific topological mass transport regimes,[30] namely the spinning top flow normal to the surface of the tube, double-helical flow across the thin film, and spicular or spherical flow, and an area of transition whereby both effects are present. The presence of these topological fluid flows which have specific size and shape was further supported by determining mixing times, temperature profiles, and film thickness for increasing rotational speed. These flow patterns also possess distinctive signatures that make it possible to predict the morphology of nanomaterials processed in the VFD, for instance, in shear-stress induced recrystallization, crystallization, and polymerization, depending on the rotational speeds, thereby providing molds of high-shear topologies, as “positive” and “negative” spicular flow behaviors, which are further detailed below. This is shown in Figure 2 along with “molecularly-drilling” of holes in thin films of preformed polysulfone, which correspond to the spatial arrangement of double helical topological fluid flows. We note that optimal processing in the VFD for a myriad of applications is at θ 45° tilt angle and the aforementioned results for this angle uniquely provide the distinct behavioral topological fluid flow regimes, Figure 3. This model introduces a novel idea for creating unique nanomaterials and the organization of matter, now with the ability to have a high level of predictability for the optimal processing in tackling new applications of the VFD.
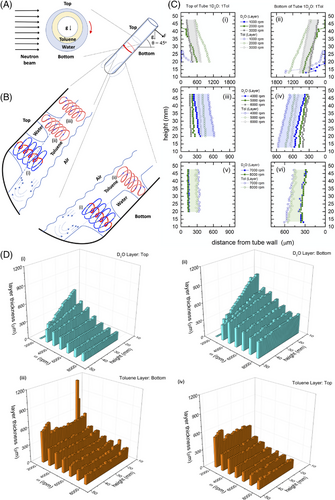
We recently established that the VFD can centrifugally separate immiscible liquids of different densities in a θ 45° inclined rotating tube without using phase transfer catalysts, microgels, surfactants, complex polymers, nanoparticles, or micromixers.[15] Depending on the properties of the two liquids, the micro to submicron size topological flow regimes in the thin films discussed previously caused substantial inter-phase mass transfer. A Coriolis force is produced from the hemispherical base of the tube which is the spinning top topological fluid flow. This is present in the less dense liquid but penetrates the denser layer of liquid, transporting liquid from the upper layer through the lower layer to the surface of the tube. In a similar way, double helical topological flow in the less dense fluid, caused by Faraday wave eddy currents being twisted by Coriolis forces, also impact of the surface of the tube. Through the self-assembly of nanoparticles at the interface of the two liquids, the lateral dimensions of these topological flows have been identified, Figure 4A. When a threshold rotational speed is achieved, 7500 rpm in a 20 mm OD tube, double helical flow also occurs in the denser layer at high rotational speeds, which results in preformed emulsions of two immiscible liquids rapidly phase separating. By altering the shape of the base of the tube while maintaining rapid mass transfer between phases, it is possible to perturb the spinning top flow relative to double helical flow while avoiding the necessity for phase transfer catalysts, Figures 3 and 4B. The results discussed here have implications for overcoming mass transfer limitations at liquid interfaces and presenting innovative technologies for extraction and separation research, all while preventing the creation of emulsions.
3 TOP-DOWN NANOMATERIAL TRANSFORMATION
The pre-eminence of utilizing a VFD for top-down nanomaterial fabrication is evident by the shear stress within dynamic the thin film in the device being effective for controlled exfoliating of 2D laminar materials, in gaining access to material for use in a wide range of applications. For instance, hexagonal boron nitride (h-BN) and single-layered graphene sheets can be effectively exfoliated from bulk material by using VFD processing.[25] For specific applications it is essential to develop a simple and scalable approach to generate 2D material without uncontrollable chemical degradation or imparting defects, and for this the VFD can be effective. Graphene synthesis from graphite oxide or graphite, for example, using solution-based methods, such as high energy wet ball milling[31] or high power sonication,[32, 33] can deleteriously affect the properties of the resulting graphene. To this end, Chen et al. developed the VFD as device for imparting tunable mechanical energy simply by varying the rotational speed, ω, of the tube (3000–9000 rpm), to control the exfoliation of oxide-free graphene and also h-BN sheets from graphite and bulk h-BN respectively, in N-methyl-pyrrolidone, Figure 5. This work is the first report on using the VFD to essentially disassemble material, and indeed for the VFD in general, and is effectively a paradigm shift for the top-down fabrication of nanomaterials.[25] Continuous flow fabrication of green graphene oxide or gGO (20 mg mL−1) was recently developed using only aqueous H2O2, achieving a yield of 40–50% at 8000 rpm with a flow rate of 0.5 mL min−1.[34] The resulting gGO exhibits distinctive characteristics with controllable defects/oxidation of ∼35% toward the edges rather than the basal plane of the sheets. These properties give rise to remarkable electrical and optical attributes, including purple to deep blue emission of narrow full width at half maximum (<35 nm).
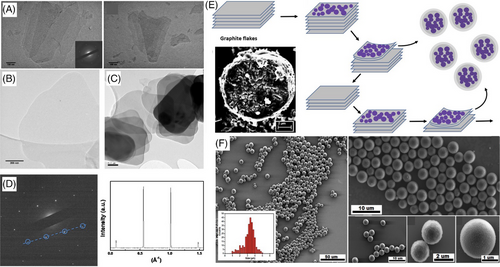
The high shear stress (mechanical energy) in the VFD is also effective in exfoliating graphene from graphite as spheres confining self-assembled fullerene C60.[35] The spheres form in essentially quantitative yield in the VFD through micro-mixing a o-xylene solution of C60 and a dispersion of graphite in DMF at room temperature, without the need for auxiliary substances and surfactants.[35] Interestingly their formation involves both top-down (graphene exfoliation) and bottom up (C60 self-assembly and simultaneous confinement) processes. The spheres are uniform in shape and have a size distribution of 1.5–3.5 μm with the ability to control their diameters by varying the VFD operating parameters, Figure 5E–J. As an electrode, the composite material has high cycle capacitance stability, with capacitance maintained at a high scan rate of 100 mV s−1 at 86.4 mF cm−2 (83.5%) and 24.7 F g−1, and high areal capacitance of 103.4 mF cm−2. These findings augur well in developing a range of all carbon material for energy storage applications. Moreover, the ability to prepare such material provides tantalizing possibilities for making composites of fullerene aggregates shrouded by other 2D materials, with different properties in general. In this context, a composite of graphene oxide shrouding self-assembled fullerene C70 has recently been reported.[36]
The precise slicing of carbon nanotubes to obtain a narrow length distribution with minimized defects except at the tube ends, holds tremendous potential for various applications, such as in solar cell technologies, sensors,[37, 38] electronic devices, and biomedical sciences.[39] Several methods have been reported for producing short carbon nanotubes using different physical,[40] electrical, or chemical strategies.[41] The dynamic thin film of liquid in a VFD can laterally slice micron length BN and carbon nanotubes (BNNTs and CNTs respectively) with[19, 23, 39] and without the use of lasers,[42] independent of the number of concentric rings making up the tubes. Notably, the process reduces the level of defects for sliced CNTs and occurs without the need for chemical stabilizers and surfactants, with scalability of the process using the continuous mode of operation of the VFD. In contrast, controlled lengths of shorter nanotubes were found to be more substantial in the confined mode for single-walled carbon nanotubes (SWCNTs), with potential for drug delivery applications due to the suitable length scale with narrow size distributions, Figure 6A–C.[23] Indeed, the availability of short single, double, or multiwalled carbon nanotubes (MWCNTs) down to 80 nm using VFD processing, where the side wall defects are minimal, as evident from Raman spectroscopy, further highlights the unique capabilities of the VFD and the prospect of uptake of the material in applications when specificity of the length scale is imperative.
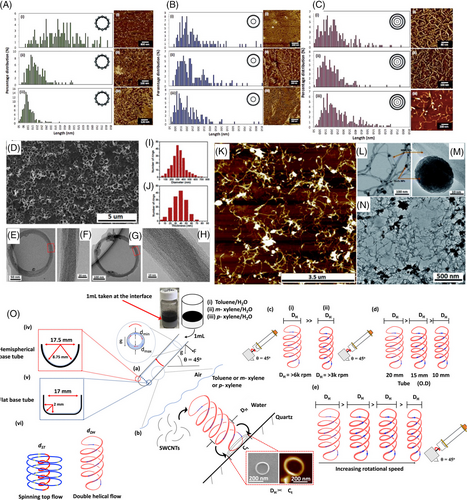
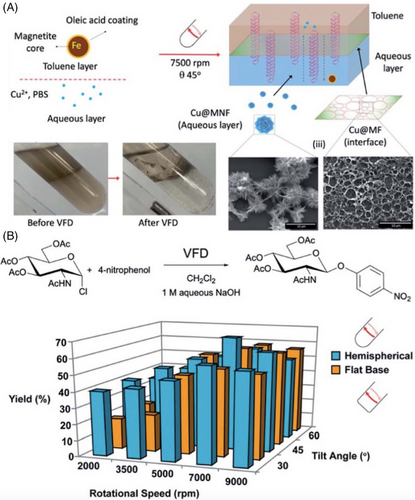
As well as slicing CNTs, the VFD is also effective in fabricating coiled SWCNT nano-rings in high yield (80%) under continuous flow, with the processing devoid of surfactants, and without toxic chemicals.[43] The coiled nano-rings were fabricated with wall thicknesses from 3 to 70 nm, with 300 nm being the average diameter, Figure 6D–J. Importantly the rings are formed with more uniformity and without variation relative to the use of batch-to-batch processing. Magnetic force microscopy established that the thickness of the VFD fabricated SWCNT nano-rings affects their magnetic properties with the magnetic interactions stronger for the thicker nano-rings due to them being more efficiently packed, thus allowing electrons to tunnel more efficiently. Compared to straight SWCNTs, the curvature of coiled SWCNT nanoring influences their properties, endowing the coiled structures with potential in a range of applications, for example, electronic circuits and polymer composites as sensing devices, noting the scalability of their VFD mediated fabrication. In recent developments, there has been significant progress in understanding the high-shear topological fluid flow within submicron dimensions in the VFD, Figure 6O.[30, 15] This understanding has enabled the control of the ring size of coiled SWCNT toroids based on the solvent systems (water with toluene, m-xylene or p-xylene), diameter of the VFD tube (10, 15, and 20 mm outside diameter) and the shape characteristics of its reactor base (hemispherical or flat-base), all achieved under confined mode VFD processing.[44] By carefully manipulating these parameters, selective control over the diameter of the resulting toroids of SWCNTs down to about 35 nm in diameter has been established.
Aside from SWCNT transformations, the VFD thin film microfluidic platform can also decorate MWCNTs with superparamagnetic magnetite (Fe3O4) NPs.[45] This process was a three-step-in-one operation: (i) Bulk iron metal was ablated using a pulsed laser at 1064 nm to generate iron oxide superparamagnetic NPs in situ, (ii) MWCNTs were sliced, and finally, (iii) the surface of the MWCNTs was decorated with the NPs, as shown in Figure 6K–N. Generating this composite material directly from stock MWCNTs with minimal processing time and without the use of harsh chemicals further demonstrates the versatility of the VFD. The composite material was utilized for supercapacitor measurements as an electrode. At a scan rate of 10 mV s−1, high areal capacitances and gravimetric of 1317.7 mF cm−2 and 834 F g−1, respectively, was established, being superior to those reported using similar materials prepared using other methods.[45] Moreover, at the specific power of 2085 W kg−1, the VFD fabricated material also had a substantially higher specific energy of 115.8 W h kg−1, thus demonstrating potential as a material for next-generation energy storage devices.
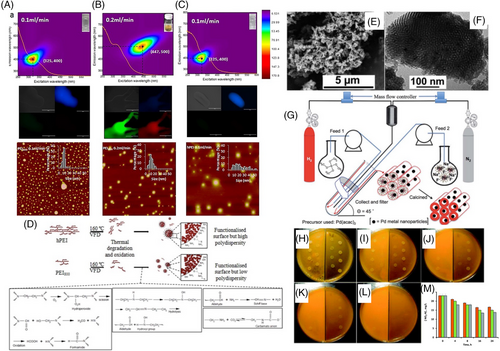
Luo et al. reported a tunable and effective procedure for preparing fluorescent polyethyleneimine-based NPs with controlled average particle sizes, as shown in Figure 7A-C, using the VFD operating under continuous mode.[28] Due to the high shear mechanical energy alongside the elevated temperature of the air (∼160°C) within the VFD, chain scission and polymer degradation cause the fragmented chains to reorganize into self-passivated NPs. Varying the flow rate of the liquid entering the VFD allows tuning the fluorescent properties of these NPs, all formed in the absence of any auxiliary reagents. The most favorable outcomes were achieved at flow rates of 0.1 mL min−1 (lex = 325 nm, lem = 400 nm) and 0.2 mL min−1 (lex = 447 nm, lem = 500 nm). 2D fluorescence maps revealed a more concentrated signal region, indicating a relatively higher level of sample homogeneity and a 1.7-fold increase in fluorescence intensity compared to samples prepared using the confined mode of operation of the VFD. Notably, processing conditions at 0.1 mL min−1 resulted in more significant degradation of the polymer structure and a greater incorporation of -OH functionality than conditions at 0.2 mL min−1, primarily due to the extended residence time associated with the lower flow rate of 0.1 mL min−1. Enhancement of the auto-fluorescence and fluorescence intensity over an extensive range of excitation wavelength was possible by incorporating amide groups. Notably, cytotoxicity was significantly reduced in the resulting NPs after processing in the VFD compared to as-received materials, thus allowing the possibility of medical applications.
4 BOTTOM-UP NANOMATERIAL TRANSFORMATION
Further advantages of the VFD include its ability to create self-assembly structures such as micelles,[46] mesoporous nanomaterials,[47, 48] hydrogels,[49] fullerene and its composites,[50, 35, 30] and vesicles[51] and the potential to control the shape and size of NPs during formation and for regulating the thickness and strength of coating at the nanoscale.
The VFD has also been utilized as an innovative strategy for preparing mesoporous silica prior to the required calcining to remove the polymer template.[46] Tong et al. reported that the processing time of the VFD was less than an hour, with 5 h being the overall duration for the synthesis involving 60 mL of solution, contrasting to the 48 h required for the traditional materials involving hydrothermal batch processing.[47] Moreover, the VFD process operated at ambient pressure and temperature, significantly reducing energy consumption, and remarkably the pore size can now be controlled to fine-tune the properties of mesoporous silica. This is under continuous flow VFD processing such that there is potential for scaling up the synthesis of the pre-calcined material with uniformity of the product along with controlled pore wall thickness (at ∼5 nm) and pore size (from 2.8 to 3.8 nm). The latter relates to the micelles being stretched by the shear forces in the thin film within the VFD. Consequentially, the increase in pore wall thickness increased the thermal stability of the calcines material, which is essential for high-temperature applications.
Tong et al. also developed an effective method for the nucleation and growth of palladium NPs in situ in the above micelle templated mesoporous silica synthesis, also as a continuous flow VFD process, then for calcining under batch processing, Figure 7E–G.[48] A mixture of Pluronic P-123 and palladium(II) acetylacetonate mixture was continuously fed into the tube reactor spinning at 5000 rpm and then aqueous sodium fluoride was added. The solution mixture was then fed back into the VFD through one jet feed, and tetramethoxysilane (TMOS) simultaneously fed into the VFD through another jet feed, under a N2/N2 atomosphere, with the H2 for reducing Pd(II), Figure 7G. The resulting Pd/SBA-15 composite calcined material is effective in a wastewater treatment process for removing nitrate-nitrogen, Figure 7M, with the maximum adsorption value for nitrate-nitrogen at 9.5 mg L−1 after 16 h, upward of 41% removal efficiency, with 36% removal efficiency upon recycling. Because Pd/SBA-15 is easily separated from the effluent, the relatively low nitrate-nitrogen adsorption is significant. Of particular note is that the Pd NPs in the composite material are entirely embedded within the pores rather than on the surface of the mesoporous silica particles, which is important in avoiding leaching of the NPs and for taking advantage of any catalytic process being enhanced within the confined space within the pores.
Silica hydrogel is readily formed in the VFD in water under continuous flow as a benign process, without the need for organic solvents or any other acids or bases, with significantly reduced processing times relative to batch processing.[49] Briefly, TMOS was fed into the VFD tube through one jet feed and MilliQ water was fed through another, with the flow rate in the range between 0.8 and 1.2 mL min−1. This process is also effective in incorporating curcumin nanoparticles, with the silica hydrogel converting to a xerogel 3.5 h post-VFD processing. The use of VFD under scalable continuous flow conditions results in much faster kinetics, beyond the limits of diffusion control. The functional composite material showed improved inhibition of bacterial growth compared to bulk curcumin, Figure 7H-L. Here the xerogel silica network provides shielding for the curcumin particles for then slow leaching of the curcumin which becomes bioavailable. The xerogel silica has potential for drug delivery applications, noting that the overall process of incorporating drugs in situ is simple, without the need for auxiliary substances. In addition to in situ synthesis, Vortex fluidic-mediated shearing can also effectively manipulate the entanglement of three-dimensional self-assembled supramolecular gel networks.[52] This was achieved through the disruption of fluorous bis-urea derived gels and studied the local structure and aggregation with small angle neutron scattering.
5 INNOVATIVE VFD TRANSFORMATIONS
With its unique operating characteristics, vortex fluidic technology warrants consideration for preparing/transforming nanomaterials, as a paradigm shift for preparing material with novel properties, for niche innovative research and industrial applications. For instance, it has been shown that enzymatic reactions could be accelerated using a VFD, with pressure waves effective in accelerating enzymatic reactions.[26] Despite chemical transformations being catalyzed with outstanding regio- and stereo-specificities, extended reaction times limit numerous enzymes. However, Britton et al. found that the above pressure waves generated at specific rotational speeds allow an enzyme to respond, providing an acceleration landscape, Figure 8. Enzymatic efficiency (kcat/Km) and rate constants (kcat) have been increased, with an average 15-fold enhancement for deoxyribose-5-phosphate aldolase, an a seven-fold average acceleration displayed by four other enzymes. The VFD increase the mass transfer such that the chattering events for the enzyme are more likely to be successful, with negative pressure collapsing the transition, that is, mechanically changing the secondary structure of the enzyme. More recently, the fabrication of hybrid laccase-Cu3(PO4)2 nanoflowers via an intermediate toroidal structure is dramatically accelerated in the VFD. This innovative approach leads to the formation of the composite material with enhanced catalytic activity (1.8 fold) compared to free laccase under diffusion control.[27] Following the fabrication process, the hybrid nanoflowers are subsequently integrated as a coating on the side wall of the reactor surface. The resulting coating exhibits exceptional stability and reusability. This remarkable durability enables a significant 16-fold enhancement in catalytic rates compared to the control conditions.
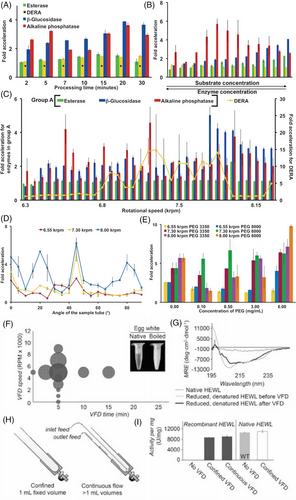
The VFD has also been utilized to procure a rapid protein refolding technique, with yields for proteins being increased for simple cell lines (Figure 8I), not to mention lowering costs, reducing streams of waste, and significantly reducing the time associated with protein expression for an extensive range of research and industrial applications, as reported by Yuan et al.[16] HEWL (hen egg white lysozyme) isolated from inclusion bodies without VFD processing exhibits a complete absence of lysozyme activity. In comparison, the application of VFD treatment to recombinant lysozyme results in the recovery of more than 82% of its activity, irrespective of whether it is conducted in confined or continuous flow modes. Particularly noteworthy is the potential scalability and parallelization of the continuous flow approach, making it an attractive choice for industrial applications demanding the treatment of exceptionally large volumes.
The shear stresses from fluid films micrometer wide have been observed in refolding lysozyme in hen egg-whites, Figure 8. Recombinant lysozyme in hen egg-whites and caveolin-1, and a protein much larger in size, cAMP-dependent protein kinase A, all of which require only minutes for processing, thereby being much faster than overnight dialysis by conventional means. In recent advancements, the ability to manipulate the unfolding and refolding of β-lactoglobulin has been established using the technology, aided by the monitoring of aggregation-induced emission luminogen (AIEgen), tetraphenylethene maleimide (TPE-MI), which reacts with exposed cysteine thiol when the protein unfolds, as established with an increase in fluorescence intensity.[53] The AIEgen have been intensively explored in the biomedical field[54] and in this case they serve as a monitoring tool, enabling real-time observation of the folding behavior of the protein during the processes. Furthermore, solutions with larger volumes (up to L) can be accommodated, with the VFD scaling up through multiple units for parallel processing or the continuous mode, consequently dramatically lowering financial costs and time for refolding inactive proteins on an industrial scale.
Besides protein refolding, the VFD provides a novel processing methodology for phase demixing for the separation of proteins, as established for an aqueous two-phase system (ATPS) of aqueous potassium phosphate and polyethylene glycol, for a mixture of C-phycocyanin (C-PC) Spirulina maxima.[29] The process increased the efficacy of separation by 22% compared to conventional ATPS methods, with a 1.18-fold increase in C-PC purity in contrast to allophycocyanin, the primary contaminant protein, and a 9.3-fold increase in phase demixing efficiency, Figure 9. This VFD-based methodology has potential for rapid phase demixing associated with purifying biologically active proteins. A mechanistic understanding of phase separation in VFD was established in 2022.[15] This is driven by micron to submicron size topological flow regimes in the thin films in VFD which induces high inter-phase mass transfer and facilitates the protein exchange. Briefly, this is achieved through a hemispherical base tube which creates a Coriolis force as a spinning top fluid flow in the less dense liquid which penetrates the denser layer of liquid, delivering liquid from the upper layer through the lower layer to the surface of the tube. In the same way, Faraday wave induced double helical flows penetrate both phases, also facilitating mass transfer across the phase boundary. Therefore, protein separation can be achieved instantaneously as a mixture with no evidence of damage to the proteins under the shearing.
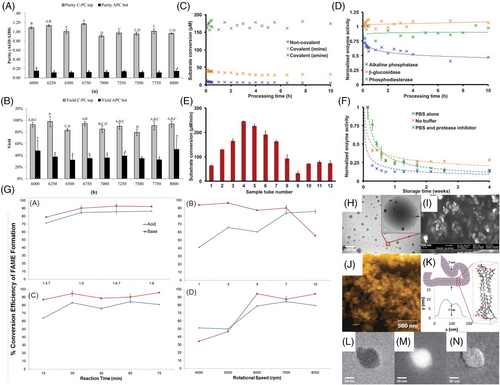
Furthermore, Britton et al. reported utilizing thin film processing in a continuous mode as a versatile strategy for immobilizing enzymes, with glutaraldehyde and noncovalent bioconjugation immobilizing enzymes onto the surface of the tubes.[55] The stock protein solution was able to consecutively coat more than ten reactors even after being recycled, with the approach requiring only a nanogram of protein for each tube. This technique reduced the number of necessary proteins and a piranha-cleaning solution and other reagents by up to 96% by confining the reagents in thin films during immobilization, with no loss in catalytic activity after being processed for 10 h, Figure 9. Nature's machinery can generate complex molecules by incorporating biocatalysts into multistep processes, offering the potential for proteins with low expression to facilitate biocatalysts for complex substrate transformations in pharmaceutical manufacture. Another example of lysing/disrupting cells using VFD is for the biodiesel production.[56, 57] The performance of the processing technology, both in confined and continuous flow mode, was evaluated by assessing the efficiencies of fatty acid extraction and the subsequent conversion of fatty acids to fatty acid methyl ester. Average extraction efficacies were 41% and conversion efficiencies >90% with the processing technology showing a broad tolerance to parameter settings.
The VFD is also capable of probing the structure of self-organized systems.[58] As reported by Mo et al., vesicles 107 ± 19 nm in diameter, shown in Figure 9, are found on the self-assembly of phenolic oxygen centers with tetra-para-phosphonomethyl calix[4]-arene bearing n-hexyl moiety attachments, were useful within a macrocycle cavity by binding to carboplatin under the shear stress induced by the dynamic thin films within the VFD.[51] Carboplatin was retained inside the calixarene's hierarchical cavity structure, and the diameters of the vesicles were maintained after shearing. The loading efficiency and release profile of the carboplatin were investigated, with the drugs loaded under shear. The vesicles are stable at a normal tissue pH with preferential release of the drug cargo at pH 6, which is the typical pH for cancerous tissues. Thus they have the potential for anticancer drug delivery applications. The hierarchical structured vesicles lower the concentration of IC50 10-fold, increasing carboplatin efficacy 4.5-fold for ovarian cancer cells and increasing cell percentage during DNA replication (S-phase) of the cell cycle. The vesicles mimic calixarene lipids and have potential application in drug delivery. This would only increase once a targeting agent was tagged alongside, such as fluorescent molecules with aggregation-induced emission characteristics.
Recently, aggregation induced-emission luminogens (AIEgens) with high emission efficiency in the aggregated state, excellent photo-stability, and increased sensitivity have become one of the most promising nanoprobes for both material and process characterizations due to their flexibility, versatility, and robustness when compared to other strategies.[59] The most frequently used approach to preparing AIEgen particles is precipitation. Without proper mixing under shear, AIE particles will be distributed in various sizes, affecting their ultimate brightness and applications. In the inaugural attempt for VFD-mediation of AIEgen size, controlling the size and shape of AIEgens was possible, impacting their fluorescence (FL) properties.[59] By increasing both water fraction (in tetrahydrofuran) and rotational speed (up to 5000 rpm) during the preparation of a particular AIEgen, TPE, the particle size was controlled and significantly reduced, with the smaller particles increasing the brightness. The ability of the VFD to produce AIEgens < 10 nm in size with tunable FL intensities directly from a 90% solvent/antisolvent (SA) ratio at the VFD rotational speed of 5000 rpm is shown in Figure 10. In traditionally prepared TPE particles, a 40-time increase in the fluorescent maxima had been observed at the SA ratio of 95% compared to that of TPE particles prepared at the SA ratio of 80%. At SA ratios < 80%, the associated emissions had been zero. Surprisingly, it was found that VFD-derived solutions of TPE particles were fluorescent at the SA ratios < 80% (down to the SA ratio = 40%). At constant rotation speeds above 1000 rpm, the SA ratio increased to fluorescent maxima and maximum relative intensity. The highest maximum relative intensity for VFD-derived TPE particles was about 190 times greater for the SA ratio and rotation speed of 90% and 5000 rpm, respectively. Although other approaches, including multichannel and microfluidic methods, had been unable to produce AIEgens < 80 nm in size, use of the VFD provides a unique strategy to tune the size and control the FL property of AIEgens, which are important properties for different applications. As an advantage of size reduction, the direct diffusion of NPs (nano-sized) will occur which can cross a cellular membrane and enter a cell.[60] This opens new opportunities for biological and material studies.
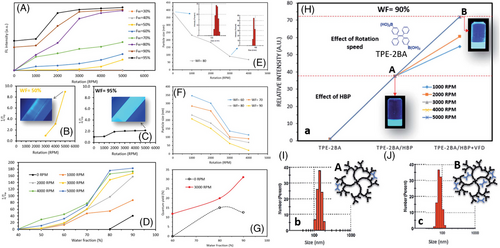
In another study, TPE-2BA AIEgen, a derivative of TPE with two boronic acid groups, was physically coupled with a commercially available hyperbranched polymer (HBP; bis-MPA polyester-64-hydroxyl; generation 4) using VFD.[61] Significant differences in FL intensity were found for the AIE–HBP (HBP concentration = 1 mM) at different SA ratios, as shown in Figure 10. The FL intensity increased 32-fold with the SA ratio = 90% compared to the SA ratio of 40%. Negligible changes in particle size in the VFD-driven AIE-HBP particles were reported compared to those prepared without VFD. The formation of AIE-HBP under shear stress increased AIE molecules’ penetration within the HBP structure, leading to significantly brighter AIE-HBP particles. It was reported that at the SA ratio = 90%, the average particle size for the traditionally prepared AIE-HBP was approximately 150 nm, with a relative FL intensity of 38 times greater than that of TPE-2BA alone. When the VFD was used, the particle size of the AIE-HBP was reduced to approximately 80 nm, and the relative FL intensity became 73 times greater. The formation of a smaller AIE-HBP complex containing more AIE molecules within the HBP molecule structure might be the reason for that observation. The authors concluded that, with the employment of a VFD, it was possible to form an AIE-HBP complex less than 100 nm in size under optimized conditions. This later resulted in the fabrication of fluorescent hydrogels with enhanced mechanical properties, and no sign of failure was observed in the hydrogel containing AIE-HBP (1 mM) over 800% strain.
6 CONCLUSION
Since the inception of the VFD in 2011, the thin film microfluidic platform has impacted on numerous diverse fields, thereby highlighting favorable support for vortex-based processing. These distinct applicable transformations suggest that the resulting Faraday waves (pressure waves) and the Coriolis from the base of the hemispherical tube of the VFD can impart on a wide variety of transformations with diminution of laborious handling. The Faraday waves generate eddies which are twisted by the Coriolis from the curved side wall of the tube into double helical flows, and these alone can be harnessed for certain applications. The Coriolis from the base of the tube takes on the shape of spinning top, typhoon like structure, which is effective for other applications, for example in the exfoliation of 2D material and exfoliation with scroll formation. Importantly with the knowledge base of the high shear topological fluid flows in the VFD there is now a heightened awareness of the predictability of processing parameters in tacking new applications of the VDF, which are seemingly endless. This review has highlighted the aptitudes of the VFD, including but not limited to the top-down transformation of graphene sheets being exfoliated, bottom-up fabrication of materials and accelerating enzymatic reactions through mechanical fluctuations in the secondary structure of the enzyme, and folding of proteins, and tuning properties of aggregation-induced emission nanoparticles. Through this novel device, we aim to reformulate how matter could be organized in precise ways using fluid flow mechanical induced effects, in striving toward accessing advanced materials, all the while circumventing any adverse effects of the engineered particles on the environment and human health. This includes adhering to the principles of green chemistry, from the inception of science to the product being in the marketplace, importantly reducing the use of toxic materials and the production of waste in the processing.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the Postgraduate Research Scholarship and Flinders University Research Investment Fund 2022, and the Australian Research Council (grant numbers: DP200101105 and DP200101106).
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Biographies

Clarence Chuah received his biomedical engineering bachelor's degree in 2019 from Flinders University in Australia under the supervision of Prof. Youhong Tang. His research interests mainly pertain to the investigation of micromixing luminescent materials in the thin film microfluidic platform known as the vortex fluidic device.

Xuan Luo completed her MSc in Biotechnology and obtained her PhD in Nanobiotechnology in 2019 from Flinders University. Subsequently, she joined Prof. Colin Raston's laboratory as a postdoctoral research associate in the College of Science and Engineering. Her current research encompasses biomedical and biosensing applications that involve the integration of the various versions of the vortex fluidic device. This includes the development of arrays, bioassays, as well as process intensification. The technology leverages the thin film intensified process as an alternative strategy to accelerate reaction kinetics in the biomedical fields and beyond.

Javad Tavakoli completed his PhD in Biomedical Engineering at Flinders University in 2018 and joined Prof Youhong Tang's research group as a postdoctoral research associate in the College of Science and Engineering where he collaborated with Prof. Raston on using VFD platform to tune the AIEgen's properties. Javad joined the University of Technology in Sydney in 2020 as a Chancellor's Research Fellow and he is currently a senior lecturer at the UTS School of Biomedical Eng. His new and expanding areas of interest include the use of additive manufacturing to engineer novel 3D tissue and organ-on-a-chip models for tissue engineering studies.




