Electrostatic and hydrophobic interaction cooperative nanochaperone regulates protein folding
Abstract
Natural molecular chaperones utilize spatially ordered multiple molecular forces to effectively regulate protein folding. However, synthesis of such molecules is a big challenge. The concept of “aggregate science” provides insights to construct chemical entities (aggregates) beyond molecular levels to mimic both the structure and function of natural chaperone. Inspired by this concept, herein we fabricate a novel multi-interaction (i.e., electrostatic and hydrophobic interaction) cooperative nanochaperone (multi-co-nChap) to regulating protein folding. This multi-co-nChap is fabricated by rationally introducing electrostatic interactions to the surface (corona) and confined hydrophobic microdomains (shell) of traditional single-hydrophobic interaction nanochaperone. We demonstrate that the corona electrostatic attraction facilitates the diffusion of clients into the hydrophobic microdomains, while the shell electrostatic interaction balances the capture and release of clients. By finely synergizing corona electrostatic attraction with shell electrostatic repulsion and hydrophobic interaction, the optimized multi-co-nChap effectively facilitated de novo folding of nascent polypeptides. Moreover, the synergy between corona electrostatic attraction, shell electrostatic attraction and shell hydrophobic interaction significantly enhanced the capability of multi-co-nChap to protect native proteins from denaturation at harsh temperatures. This work provides important insights for understanding and design of nanochaperone, which is a kind of ordered aggregate with chaperone-like activity that beyond the level of single molecule.
1 INTRODUCTION
A nascent polypeptide must correctly fold to form ordered three-dimensional structure to function.[1, 2] However, protein folding is error-prone and the misfolding of proteins results in aggregation and dysfuntion, and eventually causing a variety of human diseases including neurodegeneration diseases and cancers.[1-5] Therefore, materials to effectively regulate protein folding including assisting de novo protein folding, preventing protein misfolding and aggregation, and facilitating refolding are of significant interest. However, effectively regulating protein folding using synthetic materials is a big challenge as the protein folding is an inherent complex process which is driven by multiple molecular interactions, such as hydrophobic interaction, hydrogen bonding, and electrostatic interaction.[6-8]
Living organisms utilize a kind of special proteins named molecular chaperones to regulate protein folding by directly interacting with different client proteins including nascent polypeptides, folding intermediates, protein aggregates and native proteins via spatially ordered multiple dynamic molecular forces (e.g., hydrogen bond, electrostatic and hydrophobic interaction).[9-13] Inspired by natural chaperones, various materials including small moleculars,[14] polymers,[15] inorganic nanoparticles[16] and organic assemblies[13] have been developed to regulate protein folding. Among these materials, nanochaperones, a kind of nanomaterials with chaperone-like activity, have attracted increasing interests and grew very rapidly in most recent years due to they have shown potent in protein-related biomedical applications.[17-23] However, as an emerging research area, understanding of the structure-function relationships and the working mechanisms of nanochaperones remains limited, which hinders the rational design of new nanochaperones and limits their potential.
The concept of “aggregate science” put forward by Tang et al. utilizes a bottom-up method to investigate objects at the aggregate level to fill the gaps between molecules and aggregates,[24] providing insights to construct and unterstand nanochaperones. The dominant molecular force drives protein (mis)folding and aggregation is hydrophobic interaction, which is hardly regulated in aqueous solution.[8, 25, 26] In the year of 2013, we first time demonstrated that the mixed-shell polymeric micelle (MSPM) with controllable confined hydrophobic microdomain nanostructures on their surface are effectively in regulating hydrophobic interaction in aqueous media, opening a new venue of nanochaperone on regulating protein folding.[27, 28] This MSPM-based nanochaperone is a typically ordered aggregate with unique chaperone-like activity that beyond the level of single molecule (Scheme 1). In detail, it has a hydrophobic core that consists of hydrophobic poly(ε-caprolactone) (PCL), mimicking the backbone of natural molecular chaperones. The shell of this nanochaperone consists of hydrophilic poly(ethylene glycol) (PEG) and thermal-responsive poly(N-isopropylacrylamide) (PNIPAM). At high temperatures, the PNIPAM chains turn to hydrophobic and will collapse and form confined hydrophobic microdomains on the core of micelle which can capture and stabilize unfolded client proteins, mimicking the function domain of natural molecular chaperone. More importantly, the PEG chains are invariably hydrophilic and thus can stabilize the MSPM in aqueous media, guaranteeing the ability of nanochaperone to regulate the hydrophobic interaction during protein refolding. When the temperature cooling down, the PNIPAM chains returns to hydrophilic and stretched to release client proteins and allow refolding, closely mimicking the allosteric effect of natural molecular chaperones.[27] However, this single-hydrophobic interaction nanochaperone is unable to regulate electrostatic interaction that also plays important role in drives protein folding, which limits its chaperone activity and broaded applications. On this subject, we fabricated a new nanochaperone by introducing electrostatic and hydrophobic monomers to the thermal-responsive PNIPAM chains and succesfully used this nanochaperone to facilitate de novo protein folding and enhance its activity for protein thermal protection.[29] However, the interaction between client proteins and the hydrophobic microdomains in these nanochaperone systems relies on the randomly simple diffusion of clients, and the out-stretched hydrophilic PEG shell also impedes this interaction, which limits the efficiency of nanochaperone on regulating protein folding. Thereby, strategies to facilitate the interaction of client proteins with the hydrophobic microdomains are urgently needed to enhance nanochaperone's activity and expand their applications.
The Spy (spheroplast protein Y) chaperone harnesses the long-range electrostatic interaction to rapidly attract clients to its hydrophobic groove active site, thereby efficiently stabilizing unfolded protein strcuture and finally facilitating protein folding.[30-32] Inspired by this, herein we fabricate a multi-interaction cooperative nanochaperone (multi-co-nChap) by rationally introducing electrostatic interactions to the surface (corona) and confined hydrophobic microdomains (shell) of traditional single-hydrophobic interaction nanochaperone. This is achieved by conjugating a charged group to the end of the PEG to obtain PCL-b-PEG-X (X is carboxyl (-COOH) or guanidine), and co-polymerization of charged and hydrophobic monomers to PNIPAM to obtain PCL-b-P(NI-co-Ntb-co-Y) (Y is acrylic acid (AAc) or N-(3-methacrylamidopropyl) guanidinium (GUA)), then self-assembling PCL-b-PEG, PCL-b-PEG-X and PCL-b-P(NI-co-Ntb-co-Y) in water to abtain the multi-co-nChap. We demonstrate that the corona electrostatic attraction facilitates the diffusion of clients into the hydrophobic microdomains, while the shell electrostatic interaction balances the capture and release of clients (Scheme 1). By finely adjusting the cooperation of electrostatic and hydrophobic interactions, the capability of multi-co-nChap for facilitating de novo folding of nascent polypeptides and protecting native proteins from thermal denaturation is significantly enhanced. Moreover, we demonstrate that this multi-interaction cooperative strategy is universal for facilitating (re)folding of proteins with various physicochemical properties (e.g., lysozyme, carbonic anhydrase B (CAB), trypsin, and α-amylase). We believe this work will provide important insights for understanding and design of new nanochaperone systems.
2 RESULTS AND DISCUSSION
2.1 Preparation and characterization of multi-co-nChap
Amphiphilic di-block copolymers with different charges PCL78-b-P(NI60-co-Ntb18-co-GUA9), and PCL78-b-P(NI54-co-Ntb16-co-AAc8) were synthesized according to our previous report and their chemical structures were shown in Figure S1.[29] PCL99-b-PEG114, Carboxyl (-COOH) and guanidine (-GUA)-modified PCL-b-PEG, including PCL86-b-PEG114-COOH and PCL98-b-PEG114-GUA, were synthesized as described in Scheme S1 and their chemical structures were confirmed by 1H-NMR spectroscopy (Figure S2-S4). Four types of nanochaperones were prepared by self-assembling different amphiphilic di-block copolymers in water and were denoted as pn-co-nChaps with positively charged surface (corona) and negatively charged hydrophobic microdomains (shell), np-co-nChaps with negatively charged corona and positively charged shell, pp-co-nChaps with positively charged corona and positively charged shell and nn-co-nChaps with negatively charged corona and negatively charged shell (Table S1, Figure S5). The successful preparation of nanochaperones were confirmed using transmission electron microscopy (TEM) and dynamic light scattering (DLS), that all nanochaperones were spherical structures with narrow size distributions and hydrodynamic diameters around 100 nm (Figure S6).
2.2 multi-co-nChap facilitating de novo folding of proteins
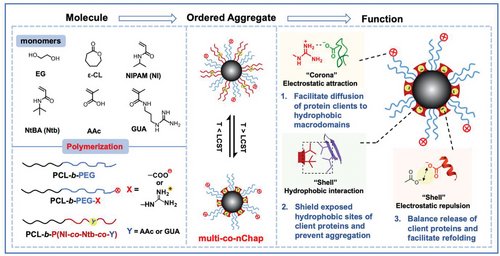
Facilitating de novo protein folding is significant for the production of recombinant proteins.[33-36] In our previous study, we demonstrated that the shell electrostatic interaction plays important roles in assisting refolding of denatured proteins.[29] However, a high ratio of P(NI-co-Ntb-co-GUA)/PEG (e.g., 6/1 (w/w)) is necessary for the capture of clients into the hydrophobic domains for efficient refolding, which limits the stability of nanochaperone in aqueous media. We hypothesize that introduction of corona electrostatic attraction can facilitate capture of clients into the hydrophobic microdomains, and thus can efficiently assist refolding of nascent polypeptides using nanochaperones with a decreased ratio of P(NI-co-Ntb-co-Y)/PEG. To verify this hypothesis, we investigated the capability of np-co-nChaps (x%, x = 0, 5, 10, and 15, whereas x represents the weight ratio of PCL-b-PEG-COOH in nanochaperone (Table S1)) with a total P(NI-co-Ntb-co-GUA)/PEG ratio of 1/1 (w/w) on refolding of positively-charged denatured lysozyme. As shown in Figure 1A, the refolding yield of lysozyme with nanochaperones is higher than that of the control group (denatured lysozyme alone), indicating the effective assistance of the nanochaperone in protein refolding. However, the refolding yield of lysozyme is only 51.6 ± 3.2% with the assistance of np-co-nChaps (0%), which is much lower than that with the assistance of nanochaperone with the P(NI-co-Ntb-co-GUA)/PEG ratio of 6/1 (w/w) (97% as reported previously).[29] In comparison, after introducing electrostatic attraction to the surface of nanochaperones, the refolding yields are significantly increased that 62.9% ± 6.6%, 85.2% ± 4.3%, and 69.4% ± 9.3% of denatured lysozyme were refolded with the assistance of np-co-nChaps (5%), np-co-nChaps (10%), and np-co-nChaps (15%), respectively, clearly verifying our hypothesis. It is noted that, the refolding capacity of np-co-nChaps is firstly enhanced and then declined as the ratio of PCL-b-PEG-COOH increased and with an optimal ratio of 10%, indicating an appropriate surface electrostatic attraction is crucial for efficient protein refolding. The structure of the refolded lysozyme with the assistance of np-co-nChaps (10%) was further characterized using circular dichroism (CD) spectroscopy. The results showed that the refolded lysozyme had nearly the same spectra as that of native lysozyme (Figure 1B), and there was no significant difference between the secondary structure of the refolded lysozyme and native lysozyme (Figure 1C), confirming the refolding capacity of np-co-nChaps.
According to the above findings, we could foresee that the pp-co-nChaps that with positively charged surface and positively charged hydrophobic microdomains would prevent the capture of positively-charged lysozyme into the hydrophobic microdomains, and thus losing the capability to facilitate de novo protein folding. The results of Figure 1D verified this hypothesis that the refolding yields of lysozyme were 16.9% ± 0.5%, 13.2% ± 2.9%, and 16.1% ± 3.9% with the assistance of pp-co-nChaps (5%), pp-co-nChaps (10%), and pp-co-nChaps (15%), respectively, which were much lower than that of groups of control (27.7% ± 1.5%) and pp-co-nChaps (0%) (52.2% ± 8.5%). Considering the effects of the surface electrostatic interaction of nanochaperone on the capture of client proteins and consequently affects protein folding, we further investigated the adsorption and desorption of client proteins with nanochaperones using quartz crystal microbalance (QCM) by monitoring frequency changes of nanochaperone-coated chips as a function of time (Figure 1E). As shown in Figure 1F, the QCM results showed a two-step change of the resonant frequency (F) of np-co-nChaps (0%) and np-co-nChaps (10%). The resonant frequency firstly decreased upon injecting the denatured lysozyme, suggesting the denatured lysozyme had been adsorbed to nanochaperones. Subsequently, the resonant frequency increased after injection of the solution of refolding buffer, indicating the desorption of lysozyme from np-nChaps. Such a two-step change of the resonant frequency is in consistent with our previous study, confirming the denatured client proteins are first quickly captured by nanochaperones and then refolded into native states and detached from nanochaperones.[29, 37, 38] Notably, the decrease of the resonant frequency (ΔF) of nanochaperones in the first step was significantly enhanced after introducing surface electrostatic attraction that the saturated ΔF was −40 Hz and −180 Hz for np-co-nChaps (0%) and np-co-nChaps (10%), respectively. In contrast, the saturated ΔF was only −7 Hz for pp-co-nChaps (10%), indicating introduction of surface electrostatic repulsion significantly prevented adsorption of client proteins. Moreover, the ΔF saturated more quickly for np-co-nChaps (10%) (6 min) than that for np-co-nChaps (0%) (25 min), indicating that the surface electrostatic attraction accelerates binding of clients to nanochaperones. As such, these results verified our hypothesis that the surface electrostatic attraction facilitates the interaction between client proteins and nanochaperones. Moreover, the resonant frequency of np-co-nChaps (10%) gradually increased and finally saturated at about ΔF = −20 Hz in the second step, indicating the shell electrostatic repulsion provided by the positively-charged monomers of hydrophobic microdomains was strong enough for the sufficient release of refolded native lysozyme from the nanochaperones. Taken together, we could conclude that the multi-interaction cooperative effects significantly enhance nanochaperone's activity for assisting de novo protein folding.
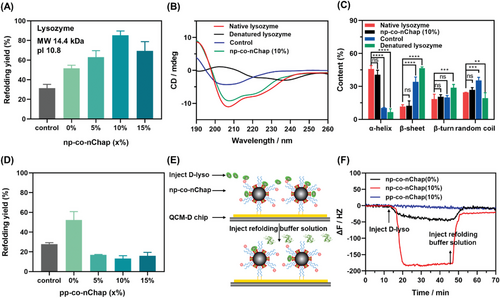
Next, we evaluated whether our strategy is universal for proteins with various physicochemical properties.. According to the findings above, pn-co-nChaps should efficiently facilitate refolding of negatively charged proteins. To verify this, two negatively charged enzymes including α-amylase (pI = 5.8, Mw = 51-54 kDa) and carbonic anhydrase B (CAB) (pI = 5.9, Mw = 28.9 kDa), and another two positively charged enzymes (e.g., trypsin (pI = 10.5, Mw = 24 kDa) and bromelain (pI = 9.55, Mw = 33 kDa)) were employed, and the protein refolding yields assisted by pn-co-nChaps or np-co-nChaps were evaluated by measuring the recovered enzyme activity. The results confirmed that the pn-co-nChaps had a better capability to assist the refolding of negatively charged α-amylase and CAB than that of traditional nanochaperone (0% group) (Figure 2A,B). Especially, the refolding yield of α-amylase with the assistance of pn-co-nChaps (5%) was 3.2-fold higher than that of pn-co-nChaps (0%) (52.7% ± 2.7% vs. 16.7% ± 1.6%). Similar results were also obtained for the refolding of positively charged trypsin and bromelain using np-co-nChaps (Figure 2C,D). The successful refolding of proteins was also confirmed by the CD results (Figure S7). Moreover, this multi-co-nChap could be also used for protecting native proteins from denaturation during long-term storage of proteins which is extremely important for practical applications. As shown in Figure S8, the remaining enzymatic activity of CAB was only 51.9% ± 8.5% left after being stored alone for 25 days, while were 72.1% ± 3.8%, 82.4% ± 3.5%, 82.9% ± 5.7%, and 91.4% ± 3.3% left with the assistance of pn-co-nChaps (0%), pn-co-nChaps (5%), pn-co-nChaps (10%), and pn-co-nChaps (15%), respectively. Therefore, these results clearly confirmed the universality of the design strategy of utilizing multi-interaction cooperative effects to enhance the chaperone activity of nanochaperones.
2.3 multi-co-nChaps protect proteins from thermal denaturation at harsh temperatures
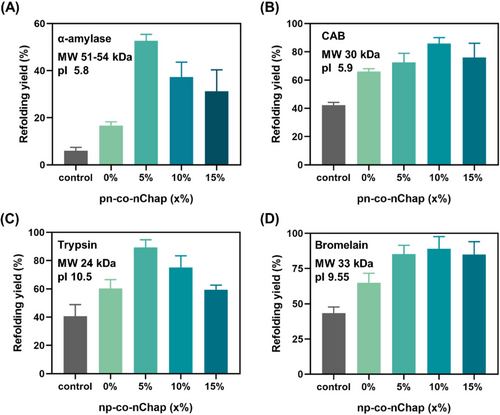
The native proteins tend to rapidly unfold and aggregate at harsh temperatures, and will finally lose their bioactivities.[39, 40] In our previous study, we found that the sufficient binding of protein clients by nanochaperone is crucial for protecting proteins from aggregation at harsh temperatures.[18] However, a large electrostatic attraction hydrophobic macrodomains (e.g., P(NI-co-Ntb-co-AAc)/PEG = 6:1 for lysozyme protection) is required for this sufficient binding, which in turn limits the release and refolding of protein clients, as well as limits the stability of nanochaperone in aqueous media. According to our findings above, we hypothesize that the surface electrostatic attraction can facilitate capture of clients into the hydrophobic microdomains of nanochaperone with a total P(NI-co-Ntb-co-Y)/PEG ratio of 1/1 (w/w), by which can address the above limitations. To verify this, we heated proteins with various physicochemical properties including lysozyme, trypsin, and CAB at harsh temperatures that higher than their denaturation temperature of clients in the absence or presence of nanochaperones, and then cooled down the solution to 4°C to allow renaturation. As expected, introduction of electrostatic attraction to the surface of nanochaperones significantly enhanced their capability to assist refolding of thermally denatured proteins. Moreover, the results indicated that the multi-co-nChaps were more powerful at harsher conditions. For example, the recovered enzymatic activity of lysozyme was 70.6 ± 2.4% in the presence of nn-co-nChap (15%) after heating at 80°C for 30 min, which is 1.3-fold higher than that of nn-co-nChap (0%) (53.04 ± 5.7%) and 1.6-fold higher than that of phosphate buffer saline (PBS) (control) (43.5% ± 3.8%) (Figure 3A). In comparison, when heating at 90°C for 30 min, the recovered enzymatic activity of lysozyme in the presence of nn-co-nChap (15%) (70.6% ± 2.4%) was 3.3-fold higher than that of nn-co-nChap (0%) (21.4% ± 1.1%), and 7.1-fold higher than that of PBS (control) (10.0 ± 1.3%). Notably, the recovered enzymatic activity of lysozyme after heating at 90°C for 30 min in the presence of nn-co-nChap (15%) was much higher than that of our previously reported nanochaperone that without surface electrostatic attraction but with a total P(NI-co-Ntb-co-AAc)/PEG ratio of 6/1 (w/w) (35 ± 2%).[18] Similar results were also obtained for the refolding of trypsin by nn-co-nChaps (Figure 3B) and CAB by pp-co-nChaps (Figure 3C). Moreover, the DLS results showed that there were no large protein aggregates after heating the nanochaperone/protein mixture at 90 or 80°C for 30 min (Figure 3D-F), indicating the unfolded clients were sufficiently captured by nanochaperones. It is noted that, the hydrodynamic diameters of multi-co-nChaps had negligible changes upon heating (Figure S9A), indicating the multi-co-nChaps were thermally stable. However, the natural Hsp70 would denature upon heating as indicated by the circular dichrograms (CD) results (Figure S9B). As such, these results confirmed that the additional surface electrostatic attraction could enhance nanochaperone's activity for protecting proteins from thermal denaturation at harsh temperatures, as well as the preponderance of synthesized nanochaperones in protecting proteins from thermal denaturation compares to natural chaperones.

2.4 multi-co-nChaps regulate the hydrophobic interaction during refolding of thermal denatured proteins
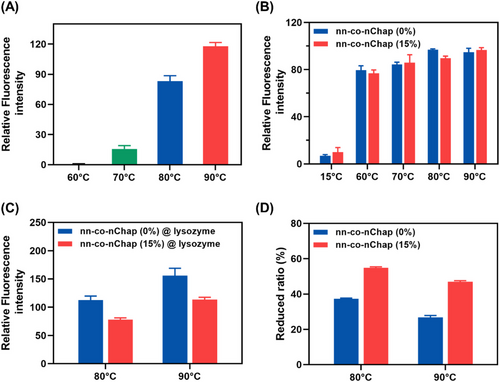
The dominant molecular force drives protein aggregation during heating is hydrophobic interaction.[8] Here, we further investigated the capability of the multi-co-nChaps on regulating the hydrophobic interactions during refolding of thermal denatured protein using 8-anilino-1-naphthalenesulfonic acid (ANS) as the hydrophobicity probe. The fluorescence intensity of ANS increases when it binds to the hydrophobic sites of a substance.[41-43] As shown in Figure 4A, the relative fluorescence intensity of ANS (ANS RFI) in the mixture solution of ANS and native lysozyme (ANS@lysozyme) slightly increased at the temperatures of 60 and 70°C while significantly increased at 80 and 90°C that were higher than the denaturation temperature of lysozyme, indicating a plenty exposure of the hydrophobic sites of lysozyme at 80 and 90°C. Similarly, when the temperatures are above the lower critical solution temperature of P(NI-co-Ntb-co-AAc), a significant increase of ANS RFI was observed for the solution of ANS@nn-co-nChap (0%) and ANS@nn-co-nChap (15%), indicating the formation of the “exposed” hydrophobic microdomains in aqueous media (Figure 4B). Then, native lysozyme was incubated with nn-co-nChap (0%) and nn-co-nChap (15%) at 80°C or 90°C, respectively, and the ANS RFI of the above solution was measured. As shown in Figure 4C and Table S2, the ANS RFI of the solution of ANS@lysozyme@nn-co-nChap (0%) and ANS@lysozyme@nn-co-nChap (15%) was 112.7 ± 7.1 and 77.9 ± 3.1 after heating at 80°C and was 156.1 ± 12.9 and 113.6 ± 3.8 after heating at 90°C, respectively. It is particularly noted that, these ANS RFIs were obviously lower than that of the direct addition of the ANS RFI of the solution of ANS@lysozyme and ANS@nn-co-nChap (Figure S10, Table S2), indicating the binding of the hydrophobic sites of denatured protein by nanochaperones, which shields the exposed hydrophobic interactions in aqueous media. Furthermore, the ANS RFI of ANS@lysozyme@nn-co-nChap (15%) was 54.9% ± 0.5% and 47.0% ± 0.5% lower than the sum of ANS RFI of ANS@lysozyme and ANS@nn-co-nChap (15%) when heating at 80 and 90°C, respectively, which were higher than that of ANS@lysozyme@nn-co-nChap (0%) (37.3% ± 0.3% at 80°C and 26.7% ± 1.1% at 90°C) (Figure 4D), indicating the nn-co-nChaps (15%) was more powerful for shielding the exposed hydrophobic interactions of denatured proteins. This reduction of the ANS RFI of ANS@lysozyme@nn-co-nChap compared to the sum of ANS RFI of ANS@lysozyme and ANS@nn-co-nChap could also be attributed to the fact that binding of client proteins to the hydrophobic domains of nanochaperones protects client proteins from unfolding, and thus preventing the exposure of hydrophobic sites. However, in any case, the above results clearly confirmed that the multi-co-nChaps could more effectively regulate the hydrophobic interaction during protein refolding in aqueous solution than our previously reported nanochaperones.
3 CONCLUSION
To conclude, we demonstrated here a novel strategy for design of nanochaperones with enhanced activity to regulate protein folding by rationally introducing electrostatic interactions to both the surface (corona) and confined hydrophobic microdomains (shell) of traditional single-hydrophobic interaction nanochaperone. Similar to natural chaperones that utilize spatially ordered multiple molecular forces to regulate protein folding, the multi-co-nChaps utilize the corona electrostatic attraction to assist the diffusion of clients into the hydrophobic microdomains, while utilize the shell electrostatic interaction to balance the capture and release of clients to facilitate protein folding. We demonstrated that the multi-co-nChaps are powerful in facilitating de novo protein folding and protecting native proteins from thermally induced misfolding and aggregation. Moreover, we first time demonstrated the design principles of MSPM-based nanochaperones from the concept of “aggregate science”, which we think provides important insights for understanding the structure-function relationships and working mechanisms of nanochaperones. We believe this work will provide directions for the design and optimization of nanochaperone systems and will find wide applications in many fields including production of recombinant proteins, protein storage, protein misfolding diseases treatment and protein delivery.
ACKNOWLEDGMENTS
The authors acknowledge financial support from the National Natural Science Foundation of China (grant numbers: 51933006, 52373153, and 52293383), National Key Research and Development Program of China (project number: 2022YFA1205702), and Haihe Laboratory of Sustainable Chemical Transformations (project number: YYJC202102).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the authors upon reasonable request.




