Stimulating and harnessing circularly polarized luminescence of helically assembled carbonized polymer dots via interfacial dynamics
Jiaying Lin and Rulin Liu contributed equally to this work.
Abstract
Stimulating and harnessing circularly polarized luminescence (CPL) is not only a sine qua non for fundamentally unveiling chirogenesis in physical chemistry, but also a pivotal prerequisite for implementation of such phenomenon in research fields including chiral optoelectronics and theranostics. Herein, red-emissive carbonized polymer dots (CPDs)-based helical structures were synthesized in this work via biomolecule-tailored organic–inorganic co-assembly strategy. The surface states-related chirality exhibits enhanced circular dichroism (CD) and CPL activities with anisotropic factors as high as gCD,max = 5.4 × 10−3 and glum,max = 1.5 × 10−2, respectively. The obtained CPL signals can be further manipulated in an excitation-dependent manner, indicating that a synergistic-competition phenomenon exists between configurational chirality and intermolecular energy-transfer dynamics, which is further supported by simulations based on density function theory (DFT). Such tunable CPL behavior triggers revolutionary designs and applications of these chiral CPDs into the realm of chirality-related biological issues and next-generation chiral optoelectronics.
1 INTRODUCTION
Chiral carbonized polymer dots (CPDs), as an emerging chiral nano-luminophore, have recently attracted considerable attention due to its versatile capabilities including low cytotoxicity, ultrasmall sizes, high surface multifunctionality, and more prominently tunable chiral absorption/photoluminance behaviors. These properties permit CPDs to become particularly suitable for potential applications ranging from chirality-based bioimaging to imaging-guided therapy, nonlinear nanophotonics, and chiral optoelectronics and spintronics.[1-5] Therefore, CPDs with circular dichroism (CD) and/or circularly polarized luminescence (CPL) activities were widely investigated in the past decades. Apart from intrinsically chiral CPDs, which show generally weak chiral signals and suffer from undefined structures for understanding the chiral origins, the rest of chiral CPDs are obtained via either: (a) ligand-induced chirality endowed by chiral organic ligands such as amino acids,[6-9] or (b) assemblies of achiral CPDs into chiral configurations by using chiral substrates.[10-16] For ligand-induced chiral CPDs, they are often limited by low dissymmetry factors (g-factors) due to inefficient nature of chirality transmission from organic ligands to inorganic cores. For the latter, nematic cellulose nanocrystals (CNC) hierarchical films, for instance, are frequently employed as chiral templates for generating photonic bandgap-based CD and CPL signals from CPDs, but most of them can only form left-handed macroscopic structures due to the intrinsic feature of CNC (Table S1).[12, 13] In fact, chirality-engineerable DNA molecules,[17] enantioselective supramolecular chiral assemblies,[18-20] and inorganic helical silica,[21] or nano-ceramics[22] are as well promising candidates for construction of inorganic building blocks into chiral assemblies at nanoscale, even though many of them have not yet been applied for CPDs. Kuzyk et al., for instance, employed DNA origami as the stereoselective scaffolds for the synthesis of gold nanoparticle helices with plasmonic CD responses in 2012.[17] Similarly, Cheng and colleagues have demonstrated the synthesis of hybrid nanohelices by grafting gold nanoparticles,[23] CdSe/CdS quantum dots (QDs),[21] or perovskite[24] onto silica helical templates transcripted from helical organogels, respectively. Further, Zhou et al.[25] reported a supramolecular co-assembly approach for the fabrication of inorganic lanthanide upconversion nanoparticles (UCNPs)-based nanohelix, in which the polarization of upconverted CPL responses can be tuned by the helicity of supramolecular nanohelix. Very recently, multicolor-emitting chiral CPDs were achieved by Ru's group[26] for which they exploited the supramolecular self-assembly of organic lipid gel (N,N-bis(octadecyl)-L/D-glutamic diamide) as host and L-/D-tryptophan capped CPDs as guests for the synthesis of CPDs-doped chiral cogels. Notably, their chiral systems presented multicolor manipulation capability, the CPL and especially CD responses, however, are still weak when compared to forementioned systems, probably resulting from the weak electrostatic interactions and low grafting density of CPDs on the gel surface. In addition, apart from 2D chiral films (CNC+CPDs[12] for instance), almost no chiral CPD systems could exhibit strong CD responses originating from surface states transitions at visible range. The observed CD activities, for the moment, are mainly caused by the π–π* transitions of the C=C and C=N bonds in aromatic sp2 domains no matter in ligand-induced chiral systems or above-mentioned chiral assemblies probably due to the mismatch between the chiral center and achiral surface groups.
Herein, we present a facile approach to obtain enantioselective chiral CPD nanohelix via co-assembly of chiral hydrogels (Fmoc-[L/D]Glu [Fmoc: N-fluorenyl-9-methoxycarbonyl, Glu: glutamic acid] and [L/D]Lys [Lys: lysine]) and achiral near-infrared-emissive CPDs (Figure 1), inspired by the virtue of co-assembly strategy. By tuning the amounts of organogels and CPDs, and gelation time, the optimized chiral CPD systems can modulate CD and CPL performances in the vicinity of characteristic absorption/emission bands originated from the surface states of CPDs with anisotropic factors as high as gCD,max= 5.4 × 10−3 and glum,max = 1.5 × 10−2, respectively. More interestingly, due to intermolecular energy-transfer dynamics between the chiral organogels and luminophore groups of CPDs,[27-29] the obtained CPL signals can be manipulated/inverted in an excitation-dependent manner, which exhibits a strong correlation to red-edge effect as indicated by molecular modeling, proving for the first time, surface-states-determined CD and CPL activities. This strategy offers not only a straightforward method for achieving enantioselective CPD-based nanocomposites with enhanced chiroptical activities but also provides new insights into the interfacial emission properties of host–guest nano-luminophores in the realm of stereo-synthesis, chiroptics, and spin-defined devices.

2 RESULT AND DISCUSSION
2.1 Structural and optical characterization of excitation-independent CPDs
To obtain surface states-determined chiroptical property, excitation-independent CPDs with NIR emission were synthesized via the hydrothermal approach as described in Experimental Procedures and Figure S1, in which growth mechanism is consistent with previous reports.[30-32] Transmission electron microscopy (TEM) image in Figure 2A demonstrates the monodisperse size of CPDs with spherical morphology. Typically, the as-synthesized CPDs show a statistical average size around 3.3 nm, as illustrated in Figure 2A. In addition, the lattice spacing is characterized by high-resolution TEM as inserted in the figure in which the 0.21 nm spacing refers to the graphene (100) planes. In order to clarify the chemical structure of CPDs, X-ray photoelectron spectra (XPS) analysis was utilized as shown in Figure 2B–E, which corresponds to C 1s, N 1s, O 1s, and S 2p orbitals, respectively (see Figure S2 for the survey spectrum). C 1s spectrum exhibits four subpeaks after fitting: strong C–C with red line at 284.8 eV; mild blue line for C=C at 283.8 eV as well as magenta for C–N/C–S at 285.7 eV; weak C=O at 287.6 eV (Figure 2B). N 1s also shows three peaks involving strong pyridinic N peak at 399.5 eV, two weak peaks correspond to pyrrolic N around 397 eV, and graphitic N around 402 eV (Figure 2C). Two types of O are observed in Figure 2D for O 1s spectrum, which can be assigned to S–O bond with 530.8 eV and S=O bond with 533 eV. Finally, Figure 2E investigates the S 2p orbital. There are four different peaks corresponding to S–C and C–SO3 bonds both in 2p1/2 and 2p2/3 orbitals. These results confirm the abundant sulfonic acid group around surface of as-prepared CPDs. In addition, the Raman spectra exhibit two evident peaks around 1348 and 1587 cm−1, which assign to disordered structures or defects (D band) and the graphitic carbon domains (G band), respectively, with intensity ratios (ID/IG) of 0.94. Figure 2G is the Fourier transform infrared (FTIR) spectra for an as-prepared sample. It reveals the C–H vibration mode at around 3000 cm−1, as well as lower energy stretching vibrations of C=C at 1477 cm−1, C=O/C=N at 1583 cm−1, C–N at 1307 cm−1 and C–N–C at 1060 cm−1. It should be noted that the observed FTIR peaks located at 1140 cm−1 for C–SO3 and 731 cm−1 for C–S vibration confirmed the presence of sulfonic acid group and thiol group onto the surface of pristine CPDs.
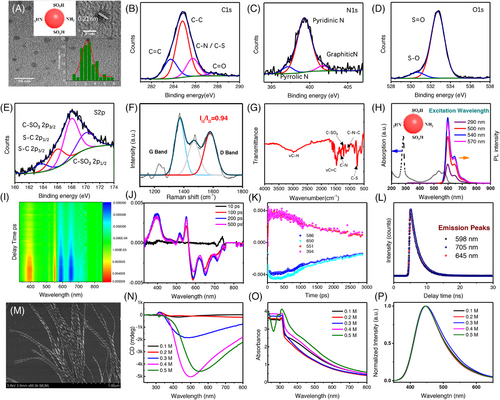
Besides the structural characterization, the optical properties of CPDs with NIR emission are also investigated. Steady-state UV-Vis absorption and photoluminescence (PL) measurements in Figure 2H confirm the excitation-independent emission property of the CPDs. The PL emissions invariably exhibit a strong peak at 600 nm together with a weak peak at 650 nm and a shoulder around 720 nm with respect to 290–570 nm excitation only except the PL intensity increases gradually. The emission covers the red and NIR region with a PL quantum yield as high as 86%. The magnetic circular dichroism (MCD) spectra (Figure S3) in parallel demonstrates that the featured absorption peaks around 540 and 590 nm showed much weaker response compared to the MCD signal originated from carbon core in UV region. As the strong π–π* transitions of the C=C and C=N bonds in aromatic sp2 domains generally locate close to UV region, we assert the observed NIR emission is probably attributed to the surface states. In order to better investigate the carrier dynamics and emission process, further characterizations are conducted in-assisted with transient spectroscopy. Figure 2I shows the 2D contour maps of transient absorption spectra for CPDs under 540 nm laser pumps. The corresponding wavelength and temporal evolution are also exhibited in Figure 2J,K. Evident bleaching signals are shown at 586 nm, as a result of ground-state bleaching (GSB) signal in CPDs according to steady-state absorption behaviors. GSB also accompanies with the photo-induced excitation state absorption at 550 nm and around short wavelength region at ∼400 nm. There are also two bleaching peaks with the stronger one located at 552 nm and weaker one at 712 nm, which are attributed to the stimulated emission based on their PL performance. The ultrafast dynamics for GSB proclaim the multiple relaxation process for the hot carriers after excitation. The decay of bleaching peak at 586 nm could be fitted into three exponent functions. The fastest decay time of 1.2 ps is generated from optical phonon scattering within ultrafast pumps. Subsequently, the carriers would undergo an electron–phonon coupling with 125 ps for heat transfer from excited carrier to lattice by trapping from surface states. The longest decay time of 2.2 ns demonstrates a phonon–phonon coupling for heat dissipation process based on non-radiation recombination. In addition, the time-resolved photoluminescence spectra (TRPL) is employed for investigating the fluorescence lifetime for CPDs. It is easy to note that the delay time for 600, 650, and 720 emission peaks exhibits similar lifetimes, namely 2.33, 2.37, and 2.41 ns, respectively, after exponent fitting as shown in Figure 2L. These results indicate the three PL emissions all originated from the same radiative recombination channel, which is attributed to the surface states of the CPDs.
2.2 Enantiomeric synthesis of CPD helix via supramolecular co-assembly
With NIR-emissive CPDs, supramolecular co-assembly strategy is then applied for the fabrication of CPD-based nanohelix. First, the molecular assembling dynamics of chiral hydrogels (Fmoc-(L/D)Glu+(L/D)Lys) is investigated. Figure 2M shows the SEM images of typical organogels (0.4 M) consisting of Fmoc-L-Glu+L-Lys, named as L-gels hereafter, in which they form helical nanostructures in 7 days, with diameters varying from 16 to 44 nm (30.3 nm as an average) and pitch length varying from 59 to 96 nm (79.4 nm as an average) (see Figure S4 for gels made of Fmoc-D-Glu+D-Lys molecules, named as D-gels hereafter). Notably, the pitch of a single fiber is uniform in nature; therefore, the overall helicity of the molecular assembly can be finely tailored via the chirality of building molecules with nearly the same sizes.
In terms of chiroptical properties, take L-gels as an example, the overall chiral morphology could contribute a CD response in a range of 300–600 nm (with generally positive signs from 300 to 350 nm and large negative signs from 350 to 600 nm) depending on the concentration of the gels, which is overwhelmingly associated to the geometrical shapes of the organogels (Figure 2N,O). Further, PL spectra with peaks around 450 nm confirm that the assembling behavior of the organogels results in the same emission property as shown in Figure 2P. Therefore, to avoid reproducibility issues and maintain relatively strong chirality, we choose L/D gels with 0.4 M as standard concentration for co-assembly with CPDs hereafter. Then, intrinsically achiral CPDs with various amounts (0.1–1.0 mg/mL) were applied for co-assembly with forementioned organogels. As CPDs have an average size of ∼3.3 nm (Figure 2A) and only a very limited amount (<1.0 mg/mL) was added, the co-assemblies of CPDs with organogels as a result maintains the overall helical nanostructures (average diameters of 28.6/29.2 nm in a range of 21–36/19–38 nm, and average pitch lengths of 81.7/84.3 nm in a range of 62–104/65–116 nm for L and D co-assemblies, respectively) as illustrated in Figure 3A,B), indicating the addition of CPDs does not necessarily change the supramolecular assembly behavior of the organogels but endows achiral CPDs with enhanced chiroptical activities in the vicinity of their featured surface states absorption bands (550–650 nm), as exhibited in Figure 3C–F. Although some imperfections were observed as well in the CD measurements probably due to strong absorptions and inhomogeneity, the observed CD signals show basically mirrored lineshapes as L/D organogels were used, respectively. To further quantify and compare the induced chiroptical activities of L-/D-gels+CPDs co-assembly systems, noted as L-/D-CA hereafter, gCD-factor, defined as Δε/ε = ΔA/A, where ΔA is the absorbance difference between left- and right-handed circularly polarized lights, is calculated for each wavelength accordingly. As the amount of achiral CPDs increases, the characteristic absorption peak intensity of L-/D-CA increases as expected, and the gCD values reach a maximum of 5.4 × 10−3–4.2 × 10−3 (Figure 3G–I) for the sample of 0.7 mg/mL CPDs, respectively, indicating an optimized ratio of CPDs (0.7 mg/mL) to organogels (0.4 M) within such system, which is, so far unprecedented, the highest gCD value originating from surface states of CPDs fabricated by supramolecular assembly strategy (Table S1).
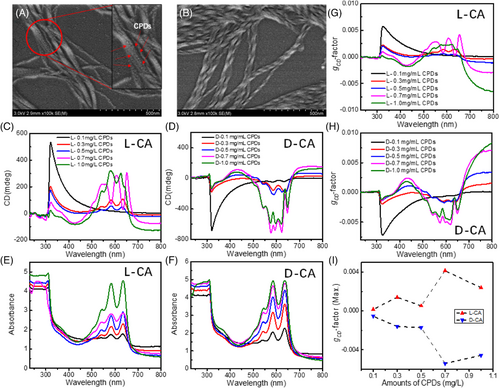
In addition, gelation time is investigated in parallel to figure out the best reaction time for the formation of CPD helix. Take D-CA for instance, the sample could show twisted-ribbon-like shape within 1 day after mixing as indicated in Figure S6, and longer gelation time to 7 days will result in more uniform helical structures, which possesses strongest CD signal and gCD-factor as previously observed. Further elongation of gelation time (14 and 21 days), however, does not necessarily improve the CD performances (Figure S6A–C) in which the corresponding SEM images (Figure S6D–G) point out that excess gelation time would increase the possibility for unexpected aggregations and even untwist the helices, leading to an undesired decrement on chiroptical activities. Therefore, within this synthesis, a mixture of 0.4 M chiral gels and 0.7 mg/mL intrinsically achiral CPDs with 7 days gelation can basically bring in uniform helical structures with optimized optical chirality.
To deepen further the chirogenesis, only two possible chiral origins could be intuitively involved in this system as stated in previous section: (a) ligand-induced chirality, and (b) chiral assemblies of achiral CPDs. However, it is obvious that for ligand-induced chirality, the gCD values are generally close to 10−4 or even less (Table S1) and more importantly, such strategy can only transmit chirality of organic ligands to π–π* transitions in aromatic sp2 domains of the CPDs; while chiral assemblies of achiral CPDs at nanometric or microscopic level could achieve gCD values as high as 10−3. Therefore, although we did not clearly observe TEM images (probably because of poor negative staining and low contrast of CPDs, Figure S7) showing the helical attachment of CPDs onto the gels, the above-mentioned SEM images, CD/UV measurements, and CPL studies (discussed in next part) prompt us to assert that the helical co-assembly structure is the main reason for the induced chirality.
2.3 Study on CPL activities of CPD-nanohelix
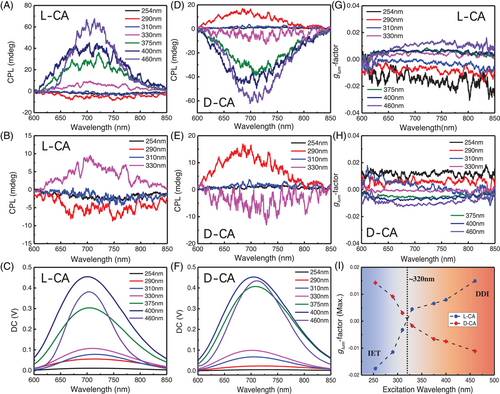
We then investigated the chiroptical activities at excited state by measuring the CPL spectra of the co-assemblies. Indeed, L-/D-CA exhibited opposite CPL responses, as presented in Figure 4. Take L-CA for instance, the observed CPL signals at ∼700 nm, which are attributed to the CPDs, vary largely when tuning the excitation wavelength as indicated by the PL spectra (Figure S8). Interestingly, it starts with a very weak CPL signal along with a slightly negative bias when the excitation is 254 nm (Figure 4A–C). Then, the signal is negatively enhanced to −5 mdeg as the excitation wavelength increases to 290 nm. Afterwards, the overall line-shape would experience a series of positive increments to reach as high as 65 mdeg as the excitation wavelength varies from 310 to 460 nm, and witness a CPL signal inversion at around 320 nm. Such CPL enhancement/inversion phenomena is reproducibly observed for D-CA sample as well (Figure 4D–F) and interestingly, the measured luminescence dissymmetry ratio, defined as: glum = 2(IL − IR)/(IL + IR), where IL and IR are the luminescence intensity of left and right CPL, demonstrated such tendency again in both L-/D-CA systems with a highest glum close to 1.5 × 10−2/−1.1 × 10−2, respectively (Figure 4G–I, Table S1), that is to say, the maximum value of |glum| would basically increase as the incident light moves from short wavelengths to longer ones, indicating the chirality-related emission from L-/D-CA is strongly excitation-dependent, as summarized in Figure 4I. Further, the obtained glum stemmed from such co-assembly's strategy, to the best of our knowledge, shows the highest glum value reported so far when compared to other related works about chiral carbon dots based on ligand-induced chirality or supramolecular assemblies. Therefore, we do believe such chiral CPD systems with excitation-sensitive CPL properties and highly enhanced glum values would become an essential highlight for next-generation chiral optoelectronics’ designs.
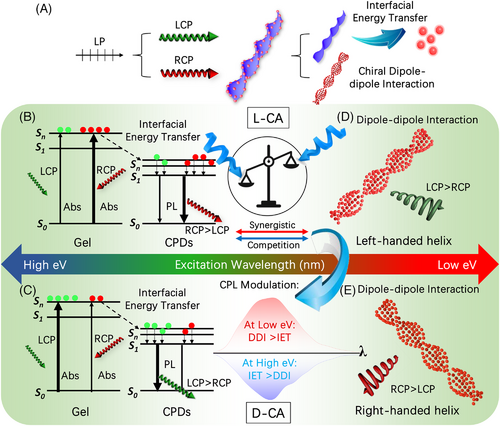
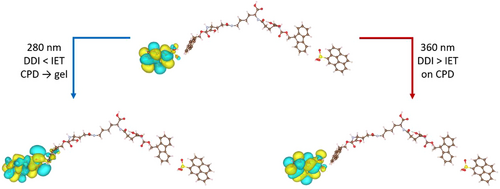
The above-mentioned findings spontaneously motivate us to look at the chirogenesis of the CPL signals and how excitation wavelength determines the CPL performance of such system. As L-/D gels can basically express CPL activities with an energy higher (∼465 nm) than that of CPDs (Figure S9), a rational understanding is[13, 28, 29, 34] when the chiral co-assemblies are irradiated by a linear polarized (LP) light, which is generated by a xenon lamp and polarized by a polarizer from the CPL instrument, one can basically consider the incident light as two circularly polarized lights that are oppositely polarized, as illustrated in Figure 5A. Apparently, the chiral system would then be excited by the two circularly polarized lights at the same time. As both chiral gels and achiral CPDs are sensitive luminophores, there are two possible pathways that may be mutually existed and imperative to the chiral emissions, as shown in Figure 5B–E[35]: (a) when the organogels are activated, the absorption to right-handed circularly polarized light (RCP) and left-handed circularly polarized light (LCP) would be different depending on the CD responses of the chiral co-assembly to the incident light (Figure 5B,C). Then, the excited electrons, generated by both RCP and LCP, would undergo a donor–acceptor-like interfacial energy transfer (IET) process[13, 34] to arrive at the excited state of CPDs, and release corresponding CPL signals depending on the chirality of the co-assembly. (b) On the other hand, the helically assembled achiral CPDs, which possess strong chiral dipole–dipole interactions (DDI) originated from their chiral morphologies would inevitably express CPL responses as well when they were excited by the incident light, and obviously such chirality is overwhelmingly related to the helicity of the CPDs (Figure 5D,E). Therefore, the overall output will be decided by the synergistic-competition between the two phenomena. In brief, when the incident light possesses high energy (short wavelength, namely 254–310 nm), the CD signal of the co-assembly, L-CA for instance, is negative (Figure 3D), indicating the absorption of RCP is higher than LCP, the chiral emissions generated via IET effect would mainly be right-handed (RCP > LCP), suggesting negative CPL signals (Figure 5B). Then, in case for 330–375 nm irradiations, the CD signal of the co-assembly is positive and the measured CPL signals therefore show positive signs (LCP > RCP) (Figure 5C). Afterwards, when relatively low energy light source (long wavelength, such as higher than ∼400 nm) is applied, the IET-induced CPL signals should be right-handed again and then change to left-handed when incident light is higher than ∼500 nm. However, the observed CPL signals remain invariably positive for which we assert the chiral DDI effect starts to be dominated in these cases because the absorption increases rapidly for the CPDs (Figure 2B) while decreases sharply for the gels (Figure 2O), indicating less electrons are transferred from the chiral gels to the achiral CPDs via IET process, but more DDI-related chiral responses are achieved in parallel. The synergistic-competition between IET and DDI effects eventually turn out positive CPL signals, as suggested in Figure 5D. Vice versa, such synergistic-competition phenomenon is also applicable for D-CA system, which can be explained by the pathways illustrated in Figure 5C–E with just opposite CPL behaviors in return (Figure 4D,E). Notably, although the forementioned chiral light-matter interactions provide critical insights for the chiral emissions, parameters such as energy conversion efficiency during IET, radiative transition efficiency, degree of chiral DDI, and environmental variations would affect as well the overall CPL behaviors of the sample, which might be further investigated soon with more advanced experimental and theoretical support.
2.4 Theoretical understandings on CD/CPL performances of CPD helix
To further investigate the interfacial energy transfer, we employ DFT simulations to take a closer look at the interface between the CPDs and chiral gels. According to previous publication,[36] the self-assembly is formed by linking two Fmoc glutamic acid molecules to one lysine with peptide bonds on its two ends. The large molecules then accumulate by stacking the lysine parts with the rings in the Fmoc parts exposed on the gel surface. In this case, the CPD is attached to the benzene rings via H-bond interactions with –SO3H groups on it. With further calculation of the molecular orbitals via Gaussian 09[37] using the B3LYP/6-31G[38] basis set, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) and LUMO+1 can be obtained, as exhibited in Figure 6. It can be observed that the excitation is from the –SO3H group on the CPD to the hydrogel molecule, indicating that the CPL activities are relevant to this surface state of the CPD. This observation can be further confirmed from the red-edge effect[39] observed from the sample as indicated by the fluorescence lifetime scale of the co-assemblies (Figure S10). With solvents of higher polarizability, the CPL intensity increases, which indicates that the CPL is highly relevant with polar interactions with the solvent molecules that favors the longer wavelength excitations, that is, the H-bond linkage between the gel molecule and the CPD-SO3H here. Because of this H-bond relevant fluorescence mechanism, as the excitation wavelength becomes longer, the excited states are more relevant near the H-bond as the other requires higher energy. This preference of the H-bond states under longer wavelength excitation favors here the surface states of the CPD-SO3H, while for shorter wavelength excitations, the CPL still prefers the gel states. Thus, as the wavelength increases, the emission states gradually shift from the gel to the CPD-SO3H, resulting in a CPL signal inversion.
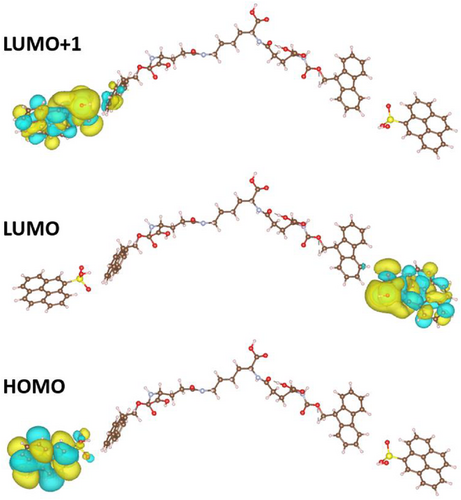
Further analysis on the excitations is demonstrated to illustrate the inversion of the chirality of the CPL. After the calculation, the orbitals were visualized according to the indices of the states involved in the calculated excitations. For the excitations with energy lower than 330 nm, the excitations are mostly from the hydrogel to carbon, while for energy higher than 330 nm, the excitations involve more of the hydrogels and end up mostly with orbitals located on the gel. Two relevant excitations are shown in Figure 8. Therefore, through simulations-based TDDFT method, an excitation energy-dependent CPL behavior is witnessed in our chiral CA system with a switch around 330 nm, demonstrating a synergistic-competition phenomenon existing between configurational chirality and intermolecular energy-transfer dynamics as proposed by IET and DDI effects.
3 CONCLUSION
In conclusion, red-emissive CPD-based superstructures with tunable and enhanced chiroptical properties were synthesized via biomolecule-tailored organic–inorganic co-assembly strategy. Further experimental measurements and theoretical simulations provided: (a) by tuning parameters such as concentrations and gelation time, surface states-related CD/CPL behaviors can be stimulated and manipulated with anisotropic factors as high as gCD,max= 5.4 × 10−3/glum,max = 1.5 × 10−2, respectively, which is the first time observation of enhanced CD/CPL signals from intrinsically achiral CPDs stemmed from surface states transitions. (b) L-/D-CA could exhibit excitation-dependent CPL activities, for which a synergistic-competition phenomenon between IET and DDI effects is proposed as the very possible explanation for the CPL inversion/modulation. (c) Theoretical simulations based on TDDFT method consistent with red-edge effect were applied to investigate the chirogenesis and origins of IET and DDI, suggesting the chemical environments and geometrical morphologies of the co-assemblies would affect greatly the IET and DDI effects, providing unique insights to the overall chiroptical behaviors of L-/D-CA systems. These findings, in one way, prominently extended enantioselective synthesis of CPD-based nanocomposites with enhanced chiroptical activities and on the other hand, spotlighted research community to re-look at the interfacial emission properties of host–guest nano-luminophores for chirality-related biological applications, 3D display devices, and next-generation chiral optoelectronics’ design.
4 EXPERIMENTAL SECTION
4.1 Synthesis of the carbonized polymer dots
The CPDs were prepared from o-phenylenediamine in a one-pot solvothermal process. 0.16 g of o-phenylenediamine was added to 25 mL dilute sulfuric acid solution with a concentration of 4 mol/L, and then transferred to Teflon-lined stainless-steel autoclave after ultrasonic dissolution and dispersion, and heated at 180°C for 12 h. Then, it was cooled to room temperature in the annealing furnace, and the black powder obtained after centrifugation of the stock solution was washed five times with a mixture of deionized water and ethanol (6:1), finally dried in vacuum 40°C.
4.2 Synthesis of chiral gels
The chiral gels were prepared according to the previous approach. Typically, an equimolar mixture of (0.1, 0.2, 0.3, and 0.4 mol) Fmoc and L- or D-lysine were added to 1 mL DI water, undergoing heating and shaking treatments until all hybrids dissolved in the aqueous solution. Different concentrations of chiral gels would be produced after cooling down to room temperature naturally, following with subsequent morphology analysis and optical measurements. The vial inversion method could verify the successful preparation of gels.
4.3 Synthesis of carbon dots co-assembled gel
A certain mass of CPDs powders (0.1, 0.3, 0.5, 0.7, and 1.0 mg), 0.4 mol Fmoc, and L- or D-lysine were added to 1 mL of water, all assembled complexes would be dissolved in solution after heating treatment, following cooling to room temperature. The carbon dots co-assembled gel is thus formed (the best temperature is above 90°C).
4.4 Simulation methods
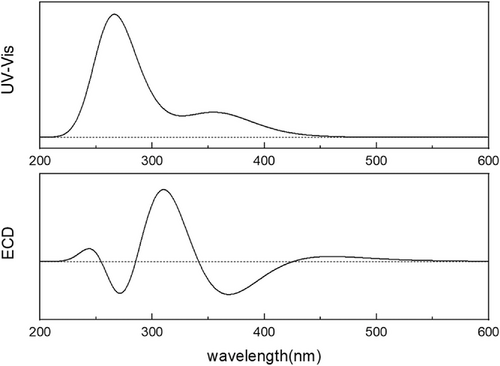
The geometric optimization, excitation states, and ECD spectrum are calculated using Gaussian 09 software under B3LYP/6-31G basis set. Ground state geometries were optimized at the DFT. Time-dependent-DFT (TD-DFT)[41] was utilized for UV and ECD spectrum. The structures combining with carbon dots, sulfonic acid group, and gel molecules are modeled by C78H68N4O18S2.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 22075240), Shenzhen Fundamental Research Foundation (JCYJ20180508162801893), National Natural Science Foundation of Hubei Province (No. 2020CFB200 and No. 2021CFB018), the Guangdong Basic and Applied Basic Research Foundation (2019A1515012094), the Project of Department of Education of Guangdong Province (2018KTSCX198), and the Shenzhen Basic Research Project of Science and Technology (JCYJ20210324094414039). And we sincerely thank Dr. Xuesong Li from Northwestern University for the language editing of the manuscript.
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.




