AIE-based drug/gene delivery system: Evolution from fluorescence monitoring alone to augmented therapeutics
Special Issue: Emerging Investigators
Abstract
The aggregation-induced emission luminogens (AIEgens) with unique photophysical properties propel the rapid development of novel delivery systems for drugs and genes. In this review, we first presented numeral classic types of delivery systems based on light-up AIEgens for real-time monitoring of the delivery processes and evaluation of therapeutic outcomes of delivered drugs or genes. Next, general strategies were discussed for the delicately structural design of AIEgens that can self-assemble into bright therapeutic carriers through covalent or noncovalent interactions. Furthermore, given the unprecedented enhancements in therapeutic effects through the use of multimodes of therapy, AIEgens that were endowed with multi-properties and functionalities were investigated in the constructions of delivery systems for multimodality therapy. Especially, the combinational uses of AIE photosensitizers with chemo drug and small interference RNA (siRNA) were given as examples of augmenting the therapeutic performance in drug-resistant treatment and moderate immunotherapy by showing synergistic effects.
1 INTRODUCTION
With the rapid development of functional materials, versatile delivery systems had been applied as carriers to convey drugs/genes to lesion sites.[1] These delivery systems covered small molecules, polymers, peptides, supramolecular assemblies, and nanoparticles.[2] The therapeutic efficiency of the drug/gene heavily relied on their local concentrations in targeted lesions and living cells. After being taken up by disease cells, endosome/lysosome escape of therapeutic ingredients and their following release or activation rate were critical for therapy outcomes. Evaluation of the drug/gene's therapeutic effect was equally important to testify whether the treatment regimens worked well or not. Therefore, it was of great significance to develop novel delivery systems that can depict their intracellular distribution, assess the release or activation kinetics of delivered drugs/genes, and predict their therapeutic responses in malignant disease treatments. This would provide important clues for further optimization of the therapeutic regimens and advancement of disease therapy.
Till now, various tools for bioimaging have been developed for biomedical research and clinical application.[3] Fluorescence (FL) imaging showed unique advantages in real-time monitoring of dynamic biological processes with high sensitivity and rapid responsiveness.[4] So far, fluorescence probes had been widely applied for labeling drug/gene delivery systems to investigate their intracellular behaviors and distributions.[5, 6] After their accumulation in target tissues and entering into living cells, the activation process of delivered drugs could be real-time tracked, particularly for anticancer drugs linked to fluorophores through covalent bonds that can specifically respond to the tumor microenvironments. However, the performances of most fluorophores with the coplanar structure suffered shortcomings of severe photobleaching[7] and concentration-caused quenching (ACQ).[8] Unlike conventional organic fluorophores, AIEgens with rotor-like structures provided a superb option for fluorescence sensing and imaging in a turn-on manner.[9] As dispersed molecules, the AIEgens underwent intramolecular motions and dissipated exciton energy, leading to fast nonradiative decay of the excited states, and emitted out very weak fluorescence. Whereas, in aggregated/solid state, the radiative pathway was dominant for robust fluorescence emission via the mechanism of restriction of intramolecular rotation, vibration, and motion. The AIE aggregates exhibited tunable emission wavelength, high quantum yield, strong photostability, negligible random blinking, and good biocompatibility.[10, 11] They had been widely applied for cellular and tissue imaging,[12-14] physiological dynamic sensing,[15-18] noninvasive bioanalytes detection,[19-22] and imaging-guided disease therapy.[23-27] The unique features of AIEgens had propelled the development of various robust and shining drug/gene carriers,[5, 28, 29] making them promising tools for real-time tracking of the dynamic delivery processes of therapeutic ingredients and accurate evaluation of their therapeutic outcomes. The self-monitoring functionality is the most significant advantage of the AIE-based delivery system compared to the conventional lipid-based delivery system.
On the other hand, the incorporation of AIEgens into highly orchestrated architectures was a pioneering attempt to broaden AIE applications in drug and gene delivery systems. Monocomponent AIEgens could harness various approaches, including hydrophobic coprecipitations, self-assemblies of amphiphilic AIEgens, host–guest and electronic interactions, and so forth, to form well-defined structures as spherical vesicles, micelles with inner cavities, nanoparticles, and nanofibers.[30] To date, two main approaches have been employed to integrate AIEgens into the therapeutic ingredients delivery systems: (a) through a covalent bond to connect proper organic groups to the AIE unit to facilitate aggregation, or (b) harnessing noncovalent interactions to encapsulate the AIEgens in an ensemble. Among those, the strategy of molecular self-assembly was commonly used for the oriented association of AIEgens and therapeutic chemo drugs and nucleic acids, and so forth, into nano- or microscopic structures through noncovalent interactions.[31] Moreover, functional AIEgens could not only participate and even facilitate the formation of delivery systems due to their versatile structural design but also serve as perfect building frameworks for the construction of emissive nanostructural delivery systems due to their high fluorescence efficiency in the aggregated or assembly states. Herein, several molecular designing strategies for enhancing the self-assembly ability of AIEgens, promoting intermolecular interactions, and finally boosting the theranostic performances of the delivery systems would be described in detail.
Further, the heterogeneity and complexity of malignant diseases (such as tumors) drove the current trend in clinical treatment gradually shift from monotherapy to combination therapy for improving treatment efficacy.[32] What is more, the synergistic interactions between several individual therapies contributed to the renaissance of multimodal therapy, which resulted in noteworthy superadditive outcomes, stronger than any individual therapy modality or their simple quantitative addition. For instance, in the case of ineffectiveness of chemotherapy that was usually caused by multidrug resistance of cancer because of long-term use of anticancer drugs,[33] the co-delivery of two or more therapeutic ingredients would play a remarkable role in enhancing the therapeutic efficiency. Beneficially, by taking advantage of AIE technology in the flexible design of multifunctional nanocarriers for the integration of chemo drug and siRNA that can regulate the expression level of the multidrug resistance-related proteins within a single delivery system, synergistic therapy could be straightforwardly operated.[34] Apart from these facile combinations, AIE fluorophores could also be delicately designed to show extra therapeutic functions. For example, numeral AIE aggregates had been demonstrated to possess superior photosensitizer character because of the efficient intersystem crossing that happens between the singlet and the triplet states. The excited triplet state could further transfer energy to nearby oxygen or react with surrounding substrates, leading to the ROS (singlet oxygen or radicals) generation for photodynamic therapy (PDT).[35] In particular, PDT-triggered enhanced immunotherapy had been demonstrated to be a promising strategy to prevent recurrence and metastasis of malignant cancers with remarkable synergistic therapeutic outcomes.[36]
In this contribution, the promising AIE-based drug and gene delivery systems will be discussed in the following three aspects: (Ⅰ) AIEgens that were associated with chemo drugs/genes for real-time fluorescence monitoring of their release and activation behaviors in living cells; (Ⅱ) AIEgens that self-assembled to generate shining therapeutic nanocarriers through covalent or noncovalent interactions, including electrostatic, host–guest, and hydrophobic–hydrophobic interactions; and (III) AIEgens that displayed versatile properties and functionalities for photo-controlled release, ROS triggered release, and enhanced therapeutics with synergistic effects (Figure 1). Clinical drug/gene delivery was focused in each system, and the close relationships between AIEgens and clinical drug/gene were highlighted in this review.
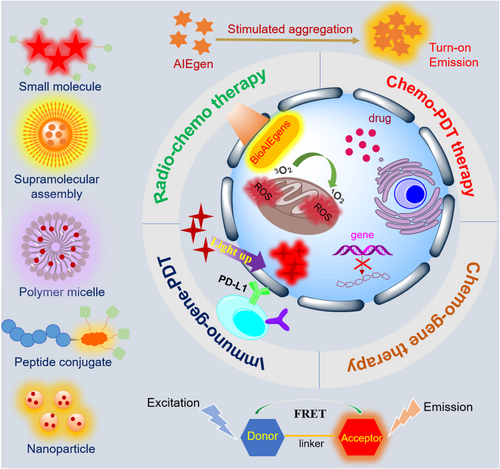
2 STARTING FROM FLUORESCENCE IMAGING
Aggregation-induced turn-on fluorescence signal provided a useful tool for real-time monitoring of the dynamic drug/gene delivery process with high sensitivity, high signal-to-noise/background ratio, and fast responsiveness.[29] Herein, water-soluble AIE-prodrug conjugate, AIEgen-drug composited energy-transfer system, and chemo drug with intrinsic AIE character were given as canonical examples to illustrate the chemo drug delivery, activation, and monitoring.
Yuan and coworkers developed an AIE conjugated Pt(IV) prodrug via a hydrophilic peptide linker for monitoring cell apoptosis in situ.[37] As shown in Figure 2A, the chemotherapeutic Pt(IV) prodrug was enriched dominantly in cancer cells by cyclic(RGD) peptide targeting the overexpressed αvβ3 integrin on the cell membrane. The active drug Pt(II) and apoptosis sensor AIEgen(TPS)-DEVD were then separated upon the reduction of the Pt(IV) prodrug in the cytoplasm. Consequently, cell apoptosis can be induced by the released Pt(II), and the DEVD peptide was cleaved by the activated caspase-3, leading to the aggregation of the hydrophobic AIEgens in the cytoplasm. The resulted turn-on fluorescence signals allowed for real-time tracking of the dynamic therapeutic responses of the delivered Pt(II) drug.
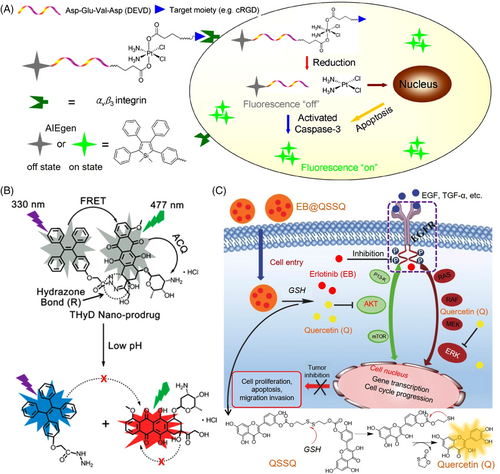
On the other hand, a self-reporting prodrug in which a switchable energy-transfer process[38] occurs between the drug and the carrier is highly desired in developing delivery systems. For instance, Liang's group constructed a self-indicating nanoprodrug via Förster resonance energy transfer (FRET) between an AIE moiety (tetraphenylethene, TPE) and the luminescent drug, DOX, to image its intracellular release behaviors (Figure 2B).[39] A pH-responsive linkage, hydrazone bond, was chosen as the spacer group between TPE and DOX. The hydrophilic DOX and hydrophobic TPE units could spontaneously self-assemble into stable and compressed nanoparticles. As the ACQ property of DOX molecules and the FRET between TPE and DOX, both of their fluorescence signals were initially inactivated. Once the link was broken in the relative acidic intracellular environment, the silenced emission of TPE and DOX was activated and evoked a dual-channel fluorescence signal, making it feasible to observe the drug-release behaviors in living cells.
Many drugs with aromatic and conjugation structures, such as quercetin, berberine chloride, myricetin, and dehydroabietic acid derivatives, had been found as natural BioAIEgen with inherent luminescence properties.[40-42] As a well-known class of natural flavonoid products, quercetin (QSSQ) exhibited excellent anticancer effects. By self-assembly of disulfide-linked QSSQ and another type of chemo drug, erlotinib (EB), an AIE-featured antitumor nanoprodrug (EB@QSSQ) was constructed.[43] As shown in Figure 2C, the high-level glutathione (GSH) in the tumor site can cleave the disulfide bond and trigger a cascade reaction, thereby breaking the nanoprodrugs, and leading to the release of both activated quercetin and EB drugs. Both released EB and quercetin served as active drugs to interfere with the EGFR signaling pathways and impeded the phosphorylation of crucial proteins, resulting in inhibition of the proliferation, growth, and survival of the cancer cells in a synergistic mode. Moreover, the enhanced fluorescence of the AIE-active quercetin was employed to self-monitor its activation process in the tumor tissue. In comparison, the last system possesses the most significant advantages in terms of biocompatibility and preparation simplicity, while the first two systems have more options in terms of properties and functions as their structures could be easy tuned and modified via chemical synthesis.
3 BEYOND FLUORESCENCE IMAGING, GOING DEEP INTO THE DELICATE STRUCTURE DESIGN
Integration of AIE moieties into well-defined structures, such as spherical vesicles, micelles, and nanoparticles, offered extra advantages in the constructed luminescent drug/gene carriers. In this section, several typical examples were given to show the delicate structural design of AIEgens that can facilitate drug or gene encapsulation and ferrying. Among them, AIE-based amphiphilic small molecules, oligomers or polymers, host–guest structures, and self-assembled nanoarchitectures were specialized in the building of efficient delivery systems.
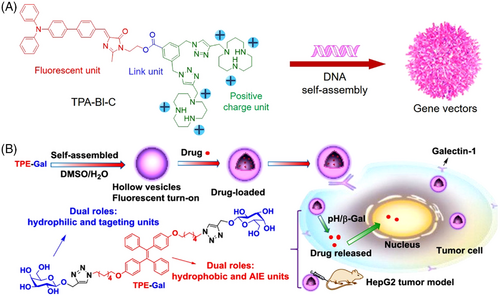
Through a combination of hydrophobic AIE unit and hydrophilic charged moiety in a small molecule, Lu and coworkers designed and synthesized an amphiphile gene vector (Figure 3A).[44] It was composited by a fluorescent triphenylamine-benzylideneimidazolone (TPA-BI) that was used as a hydrophobic moiety with two-photon AIE fluorescence and two hydrophilic heads, macrocyclic polyamine [12]aneN3 unit, with the capability of DNA uploading. Bio-reducible ester bond was used to link the TAP-BI and [12]aneN3, to realize selective responsiveness to the intracellular microenvironment and controlled gene release in both cytoplasm and nucleus. The optimal TPA-BI-C/DOPE-mediated luciferase and GFP activities were nearly 1.5-fold and 3.0-fold higher than those of lipid-based commercial agents, respectively. Through two-photon fluorescence imaging signals, the transfection process of DNA could be real-time monitored with high spatial resolution, showing great potential in developing theranostic gene vectors. Zhang et al. developed another type of small amphiphilic molecule, a conjugate of AIE-active unit (TPE) with β-D-galactose (TPE−Gal), which can straightforwardly self-assemble into pH/enzyme dual-responsive nanovesicles for responsive cell imaging, targeted drug delivery, and enhanced cancer chemotherapy (Figure 3B).[45] The hydrophobic moiety, TPE, served for fluorescence imaging and the hydrophilic Gal unit acted as the targeting ligand for galactosidase response. Both hydrophobic and hydrophilic anticancer drugs could be efficiently uploaded into the fluorescent vesicle. Much higher antitumor efficacy of the dual-responsive DOX-loaded vesicles than free DOX was achieved in the in vitro and in vivo examinations, indicating a promising therapeutic strategy.
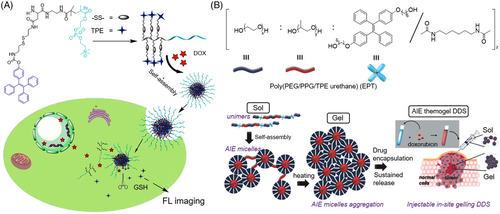
Introducing AIE units into the functional polymer and then self-assemble into nanostructure was another efficient strategy for designing therapeutic delivery systems. Wang and coworkers prepared a type of redox-sensitive polymeric micelles as an excellent drug delivery system for simultaneous fluorescence imaging and chemotherapy.[46] As shown in Figure 4A, the AIE-active TPE unit was conjugated to the side chain of an amphiphilic copolymer (TPE-SS-PLAsp-b-PMPC) by a disulfide linkage. It could further self-assemble into core–shell structured micelles with DOX embedded in the core part via hydrophobic–hydrophobic interaction with TPE. Upon the high levels of intracellular GSH, these nanomicelles can swiftly disassemble to release the encapsulated DOX. And also, the intracellular behavior of DOX release could be fluorescently traced. Compared to free DOX, these DOX-encapsulated nanomicelles showed much better performance in antitumor growth and higher compatibilities, very promising for image-guided chemotherapy. Loh et al. reported an injectable thermogel-based delivery system constructed via the self-assembly of amphiphilic copolymers (EPT) (Figure 4B).[47] The main chain consisted of hydrophilic polyethylene glycol (PEG), hydrophobic poly(propylene glycol) (PPG), and AIE-active unit (TPE). Whether EPT self-assembled into micelles or hydrogel was dictated by the hydrophobic–hydrophobic interactions between PPG and TPE segments. In dilute solution, unimers self-assembled to form micelles at critical micelle concentration, with hydrophobic PPG and TPE embedded in the core and hydrophilic PEG surrounded at the corona. Above the temperature of sol–gel transition, a high degree of aggregation occurred and the EPT micelles were transformed into a close-packed 3D network. The localized drug was slowly released from EPT thermogel, resulting in highly efficient antitumor activity in vivo. This AIE thermogel delivery system outperformed typical AIE micelle, with exceptionally higher brightness because of the enhanced restriction of the intramolecular motion process of TPE in the close-packed gel. Thus, the drug release manner and the conformation change of the gel matrix could be well illuminated by AIE emission, which was detectable for up to 18 days.[47]
The supramolecular assembly has received increasing attentions in drug delivery due to its intrinsic merits in strong encapsulation potency and convenient modification of the parental structure.[48] Jin and coworkers developed a novel type of supramolecular AIE nanocarrier via host−guest interaction-mediated coassembly of the matrix metalloproteinase-2 (MMP-2) sensitive PEG-peptide (EK6) that covalently linked to the anticancer drug gemcitabine (GEM) and functional α-cyclodextrins (α-CD) derivatives that were linked with a near-infrared AIEgen (Figure 5A).[49] After passively accumulating in tumor tissues, the supramolecular nanocarrier can respond to the overexpressed MMP-2 of cancer and intracellular reductive circumstance, leading to selective GEM release and excellent antitumor activity. In another example, Stang et al. reported a powerful theranostic supramolecular nanoparticle based on highly emissive metallacage via coordination-driven assembly of TPE and platin-composited chemo drug.[50] After coprecipitation with amphiphilic 1,2-distearoyl-phosphatidylethanolamine (DSPE)-PEG and biotin-PEG-DSPE, the resultant metallacage-loaded nanoparticles can specifically deliver the drug to the cancer cells with overexpression of biotin receptors, showing excellent stability and targeting ability. The coordination-triggered fluorescence enhancements and excellent performance in chemotherapy were achieved from this supramolecular metallacage nanoparticle in cancer therapy, indicating a promising platform for efficient chemo drug delivery. Multifunctional peptide-conjugated vector was another good choice in designing delivery systems, considering their excellent biocompatibility and bio/chem-interfaceable components. Lou's group developed a versatile AIEgen-conjugated peptide carrier, TDNCP, for the sequentially targeted delivery and tracking of an antisense single-stranded DNA oligonucleotide (ASO) into the nucleus.[51] As shown in Figure 5B, the peptide carrier contained four parts: (a) a peptide (RGD) targeting integrin αvβ3 receptor; (b) a nuclear localization peptide (KRRRR); (c) a cell-penetrating peptide (RRRR) with positive charges for therapeutic genes uploading and intracellular delivery; and (d) the AIEgen, serving not only for fluorescence imaging but also enhancing the hydrophobicity of TDNCP to initiate self-assembly. Compared to siRNA delivery, TDNCP/ASO-NPs that functioned in the nucleus afforded a much higher interference efficiency in vivo.
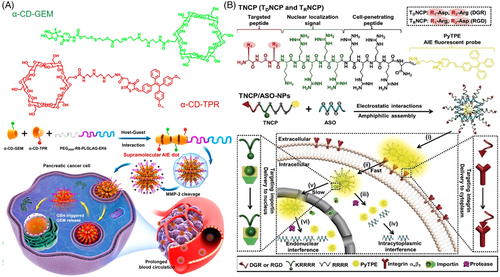
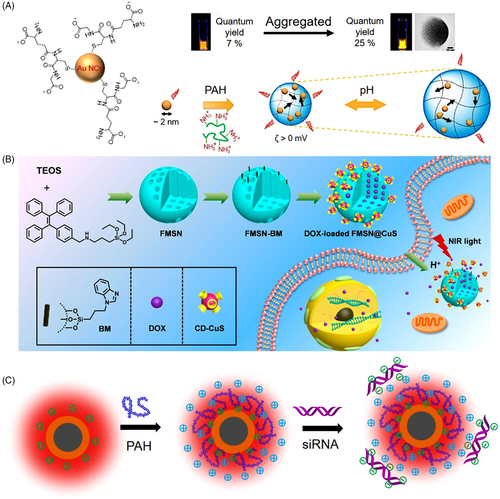
As a type of non-canonic AIEgen, metal nanocluster has also been widely applied in biomedical research with advantageous luminescent properties.[52-54] For example, Guével's group developed an excellent drug delivery vehicle based on gold nanoclusters (Figure 6A).[55] Mediated by cationic polymers, the self-assembled gold nanoclusters showed unique properties of reversible pH-dependent swelling and shrinking and volume-dependent variable fluorescence intensities and lifetime. Significant enhancements in delivery efficiency were demonstrated compared to the free drug, accompanied by a strong self-tracking function because of red-NIR fluorescence and long luminescence lifetime. Inorganic–organic hybrid nanoparticles that shared the advantages of both components were also successfully applied in efficient drug/gene delivery. Yu and coworkers reported a type of fluorescent mesoporous silica nanoparticles (FMSN) synthesized by AIEgen-containing silica precursors.[56] Figure 6B shows DOX and per-6-thio-β-cyclodextrin-decorated ultrasmall CuS nanoparticles (CD-CuS) were encapsulated in the inner cavities or attached to the surface of benzimidazole-modified FMSN, respectively. Desired drug release in the intracellular low pH circumstance was realized attributing to the pH-gated close or open of CD-CuS. Simultaneously, the fluorescence signal of DOX-loaded FMSN@CuS intensified along with the release of ACQ-active DOX. Thus, the intracellular dynamic process of drug release can be real-time traced. Upon exposure to an 808-nm laser, the attached CuS nanoparticles can generate heat to accelerate the DOX release acting, resulting in an excellent synergistic effect from a combination of chemotherapy and photothermal therapy. In another example, Tang et al. developed another type of novel inorganic–organic hybrid nanocarrier, Ag@AIE core@shell nanoparticles with tunable shell thickness for efficient siRNA delivery, and real-time monitoring of the intracellular behavior (Figure 6C).[23] Survivin siRNA was uploaded via layer-by-layer coating with positively charged PAH polymers, which acted as the intermediary between the nanoparticle and siRNA. The tunable shell thicknesses made it feasible to screen out the vehicle with optimal delivery efficiency. Attributing to the ultrahigh stability of AIE fluorescence signal, real-time tracking of siRNA endocytosis and escape from the vehicle as well as long-lasting tumor tissue observation were successfully realized. What is more, significant improvements in siRNA delivery efficiency and reproducibility were achieved compared to the commercial lipofectamine agent, indicating great potential in RNA interference-related cancer therapeutics.
4 PERFORMANCE LEAP: ENDOWING MULTI-PROPERTIES AND FUNCTIONALITIES FOR AUGMENTED THERAPEUTICS
AIEgens with a particular molecular design could afford multitherapeutic functionalities for drug/gene delivery and augmented therapy. Herein, we focused on photo- and ROS-controlled drug/gene delivery, combined therapy for overcoming the resistance of traditional chemotherapy, and enhanced immunotherapy with synergistic effects from multimodality treatments.
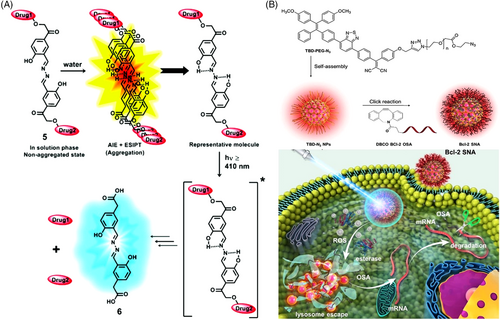
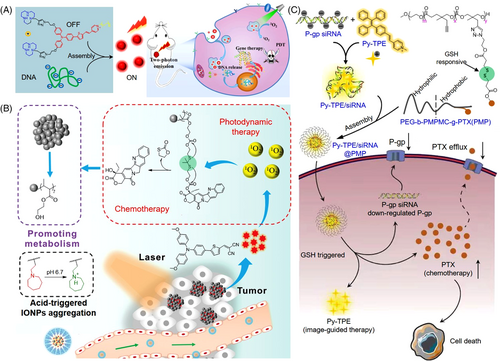
By facile incorporation of a salicylaldazine unit to a pHP photo trigger, Jana and coworkers designed a photoresponsive drug delivery system showing both AIE and excited-state intramolecular proton transfer (ESIPT) properties.[57] As shown in Figure 7A, upon irradiation, the aggregated-state carrier went to the singlet excited state, where it experienced a rapid ESIPT process from the pHP group to the imine moiety with the generation of the deprotonated species. Via an intersystem crossing, this zwitterionic intermedia could then go to its triplet excited state and liberate either of the drugs by producing the putative spirodiketone. Another drug was expulsed following the opening of a hydrolytic ring of the spirodiketone under light excitation. The distinct change in fluorescent color provided distinguished signals to monitor the drug release behavior in a real-time manner. In another example, by harnessing an amphiphilic AIE photosensitizer, Liu's group designed a non-cationic gene delivery system with self endo/lysosome escape capacity (Figure 7B).[58] A spherical nucleic acid (SNA) vector was constructed after Bcl-2 antisense oligonucleotides (OSAs) were covalently conjugated onto the surface of the nanoparticles formed via the self-assembly of AIE photosensitizers. Once the SNAs entered tumor cells, the accumulative 1O2 generated by the AIE photosensitizers broke the endo/lysosome structure to propel OSA escape. Efficient downregulation of the anti-apoptosis protein (Bcl-2) in tumor cells was realized, which in turn can reverse inherent oxidative resistance and immensely enhance the efficiency of PDT treatment. Thus, a synergistic cancer therapeutic outcome could be achieved via mutual facilitation between the AIE photosensitizer and the uploaded gene.
Integration of extra functionalities into gene vectors holds great promise for the augmented therapy of gene-related diseases. Lu et al. reported a novel gene vector with conjugated triphenylamine derivatives bearing two polar [12]aneN3 heads and a lipophilic n-hexadecane tail for PDT and gene combinatorial therapy.[59] As shown in Figure 8A, excellent DNA condensation abilities via electrostatic interaction were demonstrated with 6.7-fold efficiency in gene transfection compared to that of the commercial lipo-based agent. And What is more, the vector possessed bright AIE fluorescence and efficient 1O2 generation capability, leading to synergetic gene/PDT therapeutics. By introducing a powerful AIE photosensitizer, Wang and coworkers reported another nanotheranostic agent (denoted as AtkCPTNPs) for chemo/PDT combination therapy (Figure 8B).[60] It was prepared by the self-assembly of hydrophobic AIE photosensitizer, ROS-responsive poly camptothecin (CPT) prodrug-decorated iron oxide nanoparticles, and pH-responsive amphiphilic polymer (PEG-PC7A). Under irradiation, the AIE photosensitizer started to generate ROS, which can be used for both PDT and triggering CPT release, resulting in controlled drug release and efficient chemo/PDT combination therapy. To relieve resistance during tumor chemotherapy, Mao et al. reported an AIE featured metallacycle constructed by perylene bisimide AIEgen (PPy) and TPE-based di-Pt(II) organometallic precursor (TPE-Pt) for imaging-guided cancer radio-chemotherapy.[61] After encapsulation by a GSH-sensitive amphiphilic copolymer, metallacycle-loaded nanoparticles formed and the TPE-Pt acted as both an anticancer drug and also a radiosensitizer, thereby incorporating radiotherapy to enhance the therapeutic efficacy and conquer chemo drug resistance. Guided by the NIR-emissive PPy, radio-chemotherapy was precisely conducted and excellent performances in inhibition of the tumor growth and extension of life of cisplatin-resistant mice were successfully achieved. In another example, Lou's group proposed a novel RNA-interference-assisted chemotherapy strategy.[34] As shown in Figure 8C, a reduction-sensitive prodrug was designed via conjugation of PTX into an amphiphilic polymer skeleton, poly(ethylene glycol)-b-poly(5-methyl-5-propargyl 1,3-dioxane-2-one)-g-paclitaxel (PMP) using a disulfide linker. The negative-charge siRNA and positive-charge AIE unit (Py-TPE) were incorporated via electrostatic interactions and self-assembled with PMP to form Py-TPE/siRNA@PMP nanoformulations. After being intravenously injected into PTX-resistant ovarian cancer models, the high-level GSH cleaved the disulfide bond and released the activated PTX, Py-TPE, and P-gp siRNA. Apart from the native chemotherapy activity of PTX, the effluxion of PTX driven by the drug resistance channel was also effectively prohibited due to the RNA interference of P-gp siRNA. The AIE moiety enabled it to be an image-guided siRNA-chemo drug synergistic therapeutics.
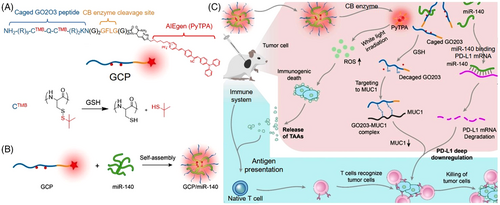
The current immunotherapy mainly utilized tumor-associated antigens (TAAs) to enhance the antitumor immune response or activate the immune cells by infusing or promoting the production of immune factors.[62, 63] However, the moderate anticancer performance showed it was inadequate to adopt immunotherapy alone. Inspired by the synergistic effect of drugs, Lou et al. proposed a combined strategy aiming at deep regulation of immunotherapy. A peptide-conjugated probe (GCP) consisting of caged GO203 peptide, cathepsin B enzyme cleavage peptide, and PDT-active AIE moiety (PyTPA), was delicately designed and prepared (shown in Figure 9).[36] Due to the hydrophobic PyTPA and positive-charge peptide, GCP can self-assemble with negative-charge miRNA-140 to form GCP/miRNA-140 nanoformulations. Upon intracellular cathepsin B enzyme, GCP/miR-140 disassembled and the three components were released. The caged GO203 was first activated by the reductive environment in tumor cells to remove the tert-butyl mercaptan. The exposed sequence can effectively inhibit the homodimerization process of MUC1 and downregulate PD-L1 expression. Meanwhile, miR-140 effectively inhibited the translation of PD-L1. Further, PyTPA-mediated PDT killed the tumor cells and release TAAs to enhance antigen presentation. As a result, the immature dendritic cells (DCs) were transformed into mature DCs, triggering an immune attack on tumor cells. Thus, the hydrophobic AIE moiety served not only in structure to build the delivery system but also in functionality to augment immunotherapy, representing a promising strategy for synergistic cancer therapeutics.
Collectively, through self-assembly with drug/gene, AIE small molecules could form nanoprodrug delivery systems and showed advantages of high drug loading efficiency, high assembly stability, and high capability in avoiding the toxic side effects caused by the carrier material. In the AIE polymer micelle delivery system, drugs are stored and delivered in a polymer-encapsulated form. The hydrophilic outer surface enables them to avoid the phagocytosis of macrophages. In addition, pH, redox, enzyme, and light-sensitive block copolymers allow controlled micelle dissociation and trigger drug release. However, the polymer micelle particles normally lack the “tolerance” of water-soluble drugs. The AIE supramolecular prodrugs are stimuli-responsive recognition-based drug delivery systems with the advantages of easy construction, molecular-level protection, quantitative loading, controllable release, good controllability, multidrug applicability, and so forth. The AIE nano-drug/gene delivery systems can improve drug/gene stability, thereby reducing drug/gene dosage and improving drug efficacy. After functional modification, the nano-drug carrier can achieve precise treatment through active/passive targeting. It can improve the blood circulation time of the drug in the body. It is expected to realize multifunctionalization and integration of diagnosis and therapy in a single structure (Table 1).
| Functionality | Structure | Reference | |
|---|---|---|---|
| TPS-DEVD | Imaging and chemotherapy | Small molecule | [37] |
| THyD | Imaging and chemotherapy | Small molecule | [39] |
| EB@QSSQ | Imaging and chemotherapy | Small molecule | [43] |
| TPA-BI-C | Imaging and gene therapy | Small molecule | [44] |
| TPE-GAI | Imaging and chemotherapy | Small molecule | [45] |
| TPE-SS-PLAsp-b-PMPC | Imaging and chemotherapy | Micelles | [46] |
| Poly(PEG/PPG/TPE urethane)(EPT) | Imaging and chemotherapy | Micelles | [47] |
| α-CD-TPR/α-CD-GEM | Imaging and chemotherapy | Host–guest structures | [49] |
| TPE-cis-(PEt3)2Pt(OTf)2 | Imaging and chemotherapy | Metallacage | [50] |
| TNCP/ASO | Imaging and gene therapy | Nanoparticles | [51] |
| Au-NCs-PAH | Imaging and drug delivery | Nanocluster | [55] |
| FMSN@CuS | Imaging, chemo/photothermal therapy | Nanoparticles | [56] |
| AgNP/AIEgen | Imaging and gene therapy | Nanoparticles | [23] |
| pHP | Imaging and photo-controlled drug release | Small molecule | [57] |
| TBD-N3 NPs | Imaging, PDT/gene therapy | Nanoparticles | [58] |
| [12]aneN3-TPA derivative | Imaging, gene/PDT therapy | Supramolecular | [59] |
| AtkCPTNPs | Imaging, chemo/PDT therapy | Polymer | [60] |
| PPy-TPE-Pt | Imaging, radio-chemotherapy | Metallacycle | [61] |
| Py-TPE/siRNA@PMP | Imaging, chemo/gene therapy | Polymer | [34] |
| CPG | Imaging, gene/immuno/PDT therapy | Peptide conjugate | [36] |
5 CONCLUSION AND PERSPECTIVE
In conclusion, various types of AIE-based therapeutic agent delivery systems had been discussed in aspects from imaging alone to structural design and finally to synergistic therapeutics. The unique light-up fluorescence with ultrahigh photostability of AIE carriers displayed superior advantages in real-time monitoring of the dynamic process of drug/gene endocytosis and activation in living cells and small animals. Through delicately molecular design, a series of robust delivery systems were developed by grafting AIE-active units onto amphiphiles, peptides, polymers, supramolecular structures, and nanoplatforms, to achieve sensitive microenvironment responsiveness and targeted release of therapeutic agents in the targeted cells and lesion sites. Furthermore, by exploration of multifunctional AIEgens (like PDT-active AIEgens) and integration of several therapeutic modalities (PDT/chemotherapy, RNAi/chemotherapy, PDT/RNAi/immunotherapy, etc.) within a single delivery system, multimodal therapy that combined the advantages and offset the shortcomings of individual monotherapy showed synergistic therapeutic effects that were more robust than each monotherapy.
In addition to this progress, other scientific issues should be paid more attention to, aimed at promoting and accelerating the extensive biomedical applications and clinical translation of AIE-based therapeutic delivery systems in the future. The first issue that needs to be focused on is the biocompatibility of AIEgen. Although thousands of AIEgens have been synthesized and reported, their biocompatibilities are still waiting for systematical examinations before further clinical translations. Alternatively, the natural chemicals extracted from plants are green and bioactive compounds that have been widely examined in the advanced pharmaceutic fields owning to their unique inherent properties, including natural availability, biodegradability, biocompatibility, and so forth.[40] Therefore, with more and more BioAIEgens from natural resources being discovered, it would be promising to harness them as perfect components to construct biocompatible delivery systems.
Second, the current delivery systems mainly use the AIEgens with absorption/emission in the visible light ranges, which were not applicable for tracking the delivery process and evaluation of therapeutic outcomes because of the limited tissue penetration depth and the strong interferences of autofluorescence in vivo. Thus, designing AIEgen with NIR absorption/emission wavelength or multiphoton absorption characteristics can theoretically increase the penetration depth of the light path.[64, 65] Another option is to use AIEgen with chemiluminescence or phosphorescence properties. No external energy excitation is required in chemiluminescence,[66, 67] which will significantly reduce the interference of background fluorescence signals. Similarly, through a time-resolved imaging tool, a long-lived phosphorescence signal can also significantly reduce the interference of background in the body.[68, 69] With the advancement of both types of AIE material, we anticipate that the derived delivery systems will provide much clearer information on disease treatment and help realize the final target of curing malignant diseases (like cancer) in the future.
ACKNOWLEDGMENTS
The authors acknowledge funding from the National Science Foundation of China (22274106, 22104104), the Natural Science Foundation of Jiangsu Province (BK20210701), and startup funds from Soochow University.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Biographies

Jie Sun received her B.S. degree in 2021 from College of Chemistry and Materials Science, Anhui Normal University, China. Now she is studying for a master's degree in College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, China under the supervision of Prof. Xuewen He. Her current research interest includes fluorescence probes, molecular imaging, programmed nanoparticle assembly, and biomarker detection.

Xuewen He received bachelor's and master's degrees in 2009 and 2012, respectively, from Huazhong University of Science and Technology (HUST), and a PhD in Chemistry at the Hong Kong University of Science and Technology (HKUST) in 2019, under the supervision of Prof. Ben Zhong Tang. Between 2012 and 2016, he worked in Prof. Nan Ma's laboratory at Soochow University as a Research Assistant. From 2019 to 2021, he conducted postdoctoral research at HKUST. In 2021, He joined Soochow University as a professor. He has published over 40 scientific papers. His research interests mainly focus on luminescence probes, biosensing, biomarkers, and pathogen analysis.




