Site-specific fabrication of gold nanocluster-based fluorescence photoswitch enabled by the dual roles of albumin proteins
Wencheng Zhong and Xiaojian Yan contributed equally to this work.
Abstract
Fluorescence photoswitch is becoming increasingly desirable for many applications, but its controllable fabrication still remains challenging yet. In this paper, a new strategy is proposed to fabricate fluorescence photoswitch by harnessing dual roles of albumin proteins as both photochrome carriers and biotemplates of fluorophore. As an example, we demonstrated the successful fabrication of a fluorescence photoswitch by incorporating both the photochromic diarylethene dye (CMTE) and red-emitting fluorescent gold nanoclusters (AuNCs) into the specific domains of bovine serum albumin (BSA) in a highly controllable manner. Detailed spectral and photophysical characterisation showed that CMTE well-retains the photochromic properties within the CMTE–BSA–AuNC construct, although its photoconversion rate is slightly retarded. Different from previously reported photoswitches, the fluorescence of the present system is mainly modulated via the inner filter effect (IFE) mechanism, which exhibits high switching efficiency with an on–off ratio up to 90%, reversible fluorescence response and good antifatigue performance. This work provides a new, generable albumin protein-assisted strategy of fabricating advanced fluorescence photoswitch, which can find wide applications in biological, optical and information fields.
1 INTRODUCTION
Light-controlled photoswitches, as novel dynamic responsive materials, have attracted great interests for their widespread applications in diverse fields including chemical sensing, molecular machines, information storage and optical imaging.[1-5] Particularly, fluorescence photoswitchable materials, which exhibit highly sensitive and reversible fluorescence response under light stimulus, provide vast opportunities in developing advanced optical tools for sensing,[6] anticounterfeiting[7] and imaging.[8] While there are few photochromic materials with intrinsic fluorescence property, most fluorescence photoswitchable materials rely on the chemical conjugation of photochromic molecules with the fluorophores. In the latter case, the fluorescence intensity of photoswitches is modulated upon illuminating the photochromic dyes with selective wavelengths mainly through the Föster resonance energy transfer (FRET)[9] or photoinduced electron transfer (PET) mechanism.[10] Despite good achievements made in this area recently, several challenges still remain to be addressed. For example, in order to achieve optimal performance for either FRET-based or PET-based photoswitches, it is essential to precisely control the distance between fluorophores and photochromic molecules, which typically involves a cumbersome synthesis and laborious surface engineering. Moreover, in most reported cases, the photochromic dyes locate on the outer surface of the fluorescence photoswitch systems, which will restrict the scope of their application in complicated environmental conditions due to the potential stability concerns. Therefore, a simple and generable methodology allowing the construction of more robust fluorescence photoswitches would be highly beneficial and desirable.
In nature, proteins prove to be effective in confining photoresponsive molecules and providing stable microenvironment for their photosensitisation.[11, 12] For example, the function of rhodopsin as a visible light responsive ion transport channel depends on the conformational isomerisation between 11-cis-retinal and all-trans-retinal within the binding pocket of a specific protein.[13] Inspired by natural design, researchers have recently utilised protein-encapsulation strategy as a new means to fabricate fluorescence photoswitch systems. Indeed, upon protein encapsulation via host–guest chemistry, these photoswitch systems can exhibit enhanced stability and biocompatibility with their photoresponsive features well retained.[14-17] As a matter of fact, besides the distinct role as a host to carry guest molecules, proteins are also excellent biomineralisation templates for guiding the nucleation and growth of inorganic materials, such as fluorescent semiconductor quantum dots and metal nanoclusters.[18-20] Importantly, proteins possess well-defined structures and multiple binding sites that can impose exquisite control on the size, chemistry and localisation of the inorganic fluorescent product. Thus, these proteins hold great potential as biotemplates for generating fluorophores directly within the photoswitch systems, which will largely simplify the fabrication process of fluorescent photoswitches and enable a better control of their physicochemical properties. However, despite several reports on protein-based fluorescent photoswitches recently,[21, 22] the unique characteristics of proteins as biomineralisation templates have not been exploited for fabricating advanced fluorescence photoswitch systems yet.
Herein, we present the first demonstration of a protein-based fluorescent photoswitch by employing proteins as both host–guest carriers and biomineralisation templates. The strategy is straightforward and easily generalised, which simply involves pre-modification of proteins with photochromic dyes via non-covalent host–guest chemistry[23] and subsequent in situ synthesis of fluorescent nanoparticles (Scheme 1). As a proof of the concept, bovine serum albumin (BSA) was chosen as the model protein owing to its widespread use in many studies. Upon incorporating a representative photochromic diarylethene dye, CMTE, into the specific hydrophobic domain of BSA, red-emitting fluorescent gold nanoclusters (AuNCs) were then synthesised within the protein cavity of BSA via a biomineralisation process. Due to the strong spectral overlap between the absorption band of CMTE and the excitation wavelength of BSA–AuNCs, the resultant CMTE–BSA–AuNCs exhibit reversible fluorescence response under UV/Vis light stimulus via a fluorescence inner filter effect (IFE). Particularly, the present strategy enables the control of localisation sites of both photochromic and fluorescent units within protein structures. The obtained fluorescence photoswitches exhibit distinct features such as easy synthesis, a high on-off ratio (∼90%) and excellent antifatigue performance, which underscore the feasibility of developing robust fluorescence photoswitches by harnessing dual roles of proteins as both host–guest carriers and biomineralisation templates.
2 RESULT AND DISCUSSION
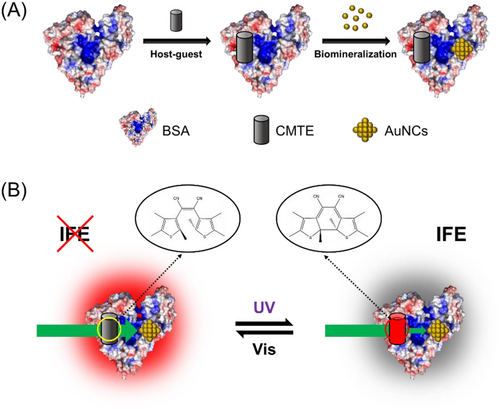
As the first step, the photochromic molecule, CMTE, was encapsulated into BSA by simply mixing in the aqueous solution. BSA is a well-known serum protein that acts as the main carrier in blood plasma owing to its abundant binding sites for either hydrophilic or hydrophobic molecules.[24] Thus, the hydrophobic CMTE with a small molecule size is expected to bind with BSA and locate within its interior hydrophobic pockets (Figure 1A). Indeed, as shown in Figure 1B, the intrinsic fluorescence intensity of BSA decreased gradually with raising the concentration of CMTE in the solution, confirming the interactions between CMTE and BSA. Meanwhile, one can observe a slight blueshift of the emission maximum, indicating that the microenvironment of the tryptophan (Trp) residue in BSA changed slightly upon CMTE binding. Note that a wavelength of 298 nm was used herein to excite the fluorescence from tryptophan rather than other luminescent residues. In good agreement with the fluorescence study, the results of circular dichroism spectroscopy also showed a mild effect of CMTE binding on the conformation of BSA (Figure S1).
The specific localisation of CMTE in BSA is important for the subsequent synthesis of fluorescent AuNCs. BSA contains two tryptophan residues in its structures: Trp213 in sub-domain IIA (site I) and Trp134 in sub-domain IB. By fitting the fluorescence quenching data with a modified Stern–Volmer equation (Experimental section in Supporting information), the fraction of the fluorophore (Trp) that is accessible to the quencher (fa) was calculated to be 0.5, indicating that only one Trp residue in BSA is quenched by CMTE (Figure 1C).[25, 26] In order to identify the exact localisation of CMTE within BSA, two well-established competitive site markers (phenylbutazone for site I and ibuprofen for site II) were then used.[27] Our results showed that the presence of phenylbutazone caused a more significant inhibit effect on the binding of CMTE to BSA than that of ibuprofen, suggesting that CMTE locates within the site I pocket of BSA that contains Trp213 (Figure S2). The site-specific binding of CMTE to BSA was further confirmed by the molecular docking study.[28] As shown in Figure 1D, CMTE was found to locate in the vicinity to Trp213 and interact with Asp450 in subdomain IIA (site I). The binding forces likely include hydrogen bonding, van der Waals force and hydrophobic interaction, which was deduced based on the more negative total energy (−27.9 kJ mol–1) of Van der Waals force, hydrogen bonding and desolvation free energy than the energy of electrostatic force (−0.1 kJ mol–1, Table S1). In addition, TMTE, the isomer of CMTE, also exhibits similar binding behaviour to BSA, and TMTE was found to form hydrogen bonds with a different residue (Ala209) due to its varied structure (Figure 1E). Altogether, both experimental and simulation results suggest the successful formation of stable CMTE-BSA complexes.
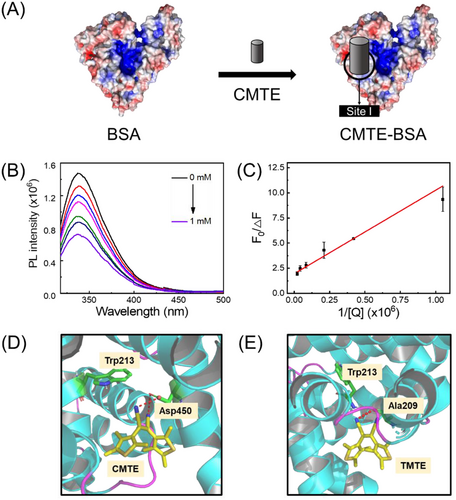
Similar to the natural photoswitch such as retinal, certain degrees of conformational flexibility is an important prerequisite for the synthetic photoswitch to achieve effective isomerisation.[29] Thus, it is interesting to assess whether CMTE still retains its photochromic property upon encapsulation into the binding pocket of BSA. As a photochromic dye of diarylethene family, CMTE has the advantage of small conformational change between two isomers, thermal stability and fatigue resistance.[30, 31] Under the irradiation of UV light at 405 nm, CMTE will undergo photocyclisation and convert into its isomer, TMTE (Figure 2A). The photoconversion process of CMTE to TMTE was monitored by absorption spectroscopy upon successive pulses of UV (405 nm, 1.0 mW·cm–2) or VIS (546 nm, 1.0 mW·cm–2) irradiation. As seen in Figure 2B, an intense absorption band centred at 520 nm will appear after irradiating CMTE at 405 nm, confirming the formation of TMTE. Upon further irradiation with a visible light at 546 nm, the absorption band will then gradually diminish until completely vanish owing to the conversion of TMTE into CMTE (Figure 2C). This photochromic feature of CMTE was still clearly observed for the CMTE–BSA complex, as seen in Figure 2D,E, which showed similar absorption spectral changes under the light irradiation. Apparently, CMTE well retains its photochromic behaviour upon forming complex with BSA, which is not surprising considering that diarylethenes require only a small degree of freedom for efficient isomerisation.[32] Further kinetic analysis showed that the photoconversion rate of CMTE into TMTE decreased slightly from 0.24 min–1 to 0.20 min–1 upon binding to BSA (Figure 2F). Similar behaviour was also observed for the reverse conversion process. Previous studies showed that photoswitching rate of photochromic molecules is closely related to the crowdedness of their surrounding microenvironment.[33, 34] Herein, the slightly retarded photoconversion of CMTE–BSA compared with that of free CMTE is likely due to the restriction effect from its interactions with the surrounding residues. Nevertheless, the CMTE–BSA complexes that are formed via host–guest chemistry inherit good photochromic features from CMTE, which is important for the further use of the CMTE–BSA complex as a biotemplate to synthesise fluorescent photoswitch.
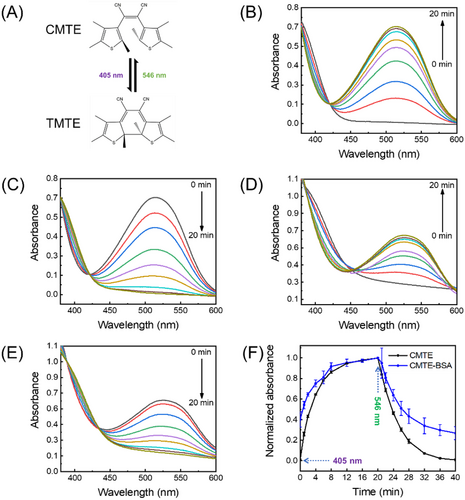
We next explored the use of above formed CMTE–BSA as biomineralisation templates for further synthesising fluorescent AuNCs. The multiple advantages of AuNCs, such as well-defined structures, excellent stability and good biocompatibility, make them attractive in widespread applications as novel fluorophores.[35-42] Proteins such as BSA prove to be capable of in situ synthesising fluorescent AuNCs, whereas BSA acts as both reductant and stabilizer.[43, 44] Based on a modified strategy to previous reports, AuNCs could be generated by simply mixing CMTE–BSA with HAuCl4 and subjected to reaction in alkaline condition for 1 h (Figure 3A). The resultant product, CMTE–BSA–AuNCs, exhibits red fluorescence with an emission maximum at 665 nm (Figure 3B). The quantum yield was measured to be 2.2%, which is smaller than that of BSA–AuNCs (5.6%) likely due to the influence of CMTE in proteins. Meanwhile, the absorption spectrum of CMTE–BSA–AuNCs exhibits featureless characteristic in the visible region as expected (Figure S3). XPS analysis also showed that the binding energy (BE) of Au 4f7/2 (84.3 eV) falls between the BE of Au(0) (84.0 eV) and Au(I) (86.0 eV), which is in good agreement with previous reports of AuNCs (Figure S4).[45-49] Apparently, the pre-incorporation of CMTE into BSA does not affect its capability of generating luminescent AuNCs despite the slight decrease of the quantum yield.
In order to evaluate the photoswitching performance of luminescent CMTE–BSA–AuNCs, we first studied their photochromic behaviour by absorption spectroscopy. As shown in Figure S5, CMTE–BSA–AuNCs exhibit obvious spectral changes as that of CMTE upon irradiation with 405 nm light, as also visualised by the obvious colour change of the aqueous solution from light yellow to dark red (Figure 3C). Upon further irradiation with 546 nm light, colour of the solution slowly faded as expected, which is in good agreement with the absorption spectral evolution (Figure S5). The well-retained photochromic properties of CMTE–BSA–AuNCs suggest that further generation of AuNCs inside BSA does not significantly affect the isomerisation of encapsulated CMTE. However, the photoswitching rate of CMTE in CMTE–BSA–AuNCs (0.15 min–1) is slightly slower than either CMTE–BSA or free CMTE, as shown in Figure 3D. Although the precise structure of protein-protected AuNCs remains unknown yet due to the difficulty of crystallisation, several recent studies proposed that the nucleation site of AuNCs in serum albumin is close to cysteine residues, especially in domain IIB.[50, 51] Despite localising in a different pocket of BSA from that of CMTE (site I), the presence of these AuNCs will inevitably pose additional effect to squeeze their surrounding peptide motifs. Consequently, the microenvironment around CMTE will become more crowded, and the isomerisation process of CMTE is further hindered, as evidenced by a slower photoswitching rate in Figure 3E. Meanwhile, we note that the hydrodynamic size of BSA keeps essentially unchanged upon incorporating either CMTE or both CMTE and AuNCs (Figure S6), suggesting a good colloidal stability of protein-templated nanosystems.
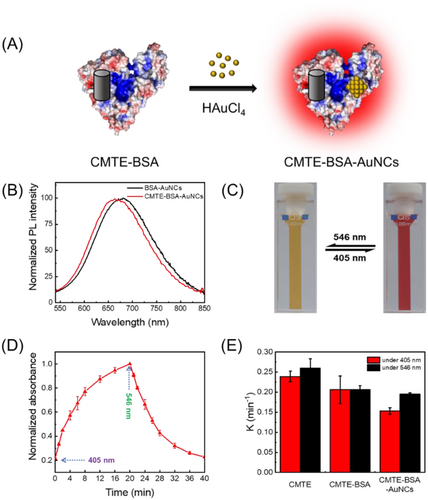
The fluorescence response of our photoswitching system was then investigated by monitoring their fluorescence intensity at 665 nm. As shown in Figure 4A, under the irradiation of 405/546 nm light, the fluorescence intensity of CMTE–BSA–AuNCs is decreased/increased, confirming the effective fluorescence photoswitching of the present system. Different from most reported fluorescence photoswitches, the present system is designed to work through an IFE mechanism. As seen in Figure 4B, when CMTE is photoconverted to TMTE, the absorption band of TMTE overlaps significantly with the excitation spectrum of fluorescent BSA–AuNCs. When exciting CMTE–BSA–AuNCs at 520 nm, the photons of excitation light will be competitively absorbed by TMTE via the IFE process. Consequently, the amounts of photons that can finally excite the fluorophores (AuNCs) will be decreased, and the fluorescence of CMTE–BSA–AuNCs will be switched off (Figure 4C). However, when TMTE is photoswitched back to CMTE, the influence of IFE on the fluorescence of AuNCs will be vanished, and their fluorescence will be recovered. Typically, the efficiency of IFE-caused fluorescence quenching is strongly affected by the excitation wavelength.[52, 53] Adopting an excitation wavelength near to the absorption maximum will result in a stronger IFE effect and higher fluorescence modulation efficiency. Indeed, as seen in Figure 4D, the fluorescence switching efficiency of CMTE–BSA–AuNCs exhibits obvious excitation wavelength dependence. The maximum switching efficiency was obtained at 520 nm (Figure 4B), which is near the absorption peak of TMTE. Fluorescence lifetime of CMTE–BSA–AuNCs in both “on” and “off” states were then measured, suggesting a slight decrease of the lifetime from 6.3 to 5.6 μs upon the isomerisation of CMTE to TMTE (Figure 4E). While the fluorescence lifetime of fluorophores is expected to keep unchanged upon quenching by the IFE process, the slight decrease herein can be attributed to the existence of weak energy transfer between AuNCs and TMTE.[54, 55] Note that the distance between AuNCs and CMTE in our system is within the FRET range (<10 nm), and there is a slight spectral overlap between the absorption band of TMTE and the emission band of AuNCs (Figure S7). Therefore, weak energy transfer from AuNCs to TMTE is likely to occur, resulting in the quenched fluorescence of AuNCs. Nevertheless, considering the weak FRET efficiency of the present system due to the poor spectral overlap, IFE is believed to be the dominating mechanism in our fluorescence photoswitching system.
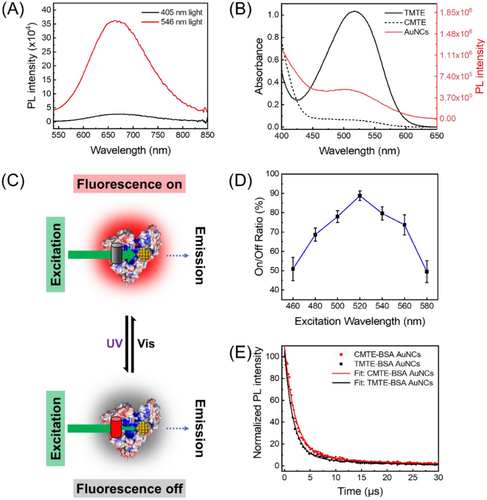
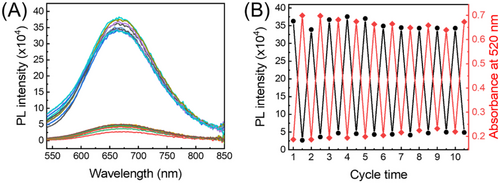
For potential application that would benefit from photocontrollable fluorescence, it is necessary to ensure that the photoswitching process is reversible and fatigue-resistant. Accordingly, we monitored the spectral response of CMTE–BSA–AuNCs upon irradiation with 405/546 nm light for 10 successive cycles. As seen in Figure 5A, the photoswitchable CMTE to TMTE isomers enable remarkable (ca. 90%) and reversible quenching of AuNCs’ fluorescence over cycled UV/VIS light irradiation. Meanwhile, the absorbance of the system at the excitation wavelength (520 nm) also exhibits reversible change that is well complementary to the fluorescence response, further confirming the IFE-based modulation principle (Figure 5B). The fluctuation of either fluorescence or absorbance response is calculated to be less than 4% within 10 repeated cycles, suggesting a good stability of our fabricated fluorescence photoswitch system. Particularly, we note that the on/off ratio of the present IFE-based fluorescence photoswitch is higher than those reported ones that work via FRET or PET mechanisms.[10, 56-60] Compared with other modulation mechanisms, IFE-based photoswitch does not demand the strict control on the distance between the photochrome and the fluorophore, thus making the design of fluorescence photoswitch more flexible and simple. Meanwhile, owing to the competitive absorption of excitation light by the photochrome, direct interaction of fluorophore with the excitation light will be much weaker in IFE-based photoswitch than that in FRET or PET-based photoswitches, which will be favourable for resisting the typical photobleaching issues of most fluorophores. As a result, the IFE-based photoswitch exhibits good antifatigue performances.
3 CONCLUSION
In summary, we have demonstrated a new strategy of fabricating fluorescence photoswitch by employing albumin protein as both photochrome carrier and biotemplate of fluorophore. The unique structural features of albumin protein endow the photoswitch with better controllability and stability without compromising their optical properties. As an example, we successfully fabricated AuNC-based photoswitch within CMTE-incorporated BSA via a simple and versatile approach. Different from most reported photoswitches, the fluorescence of the present system is mainly modulated via the IFE mechanism, which exhibits high switching efficiency, reversible fluorescence response and good antifatigue performance. Moreover, we envision that these photo-stimulus systems will be useful in anticounterfeiting, super-resolution imaging and optical memory applications. Considering the rich availability of proteins and well-established protein engineering toolbox, one can also envision numerous extension of the present strategy for developing advanced photoswitches.
4 EXPERIMENTAL
4.1 Reagents
All the materials were of analytical grade. Gold (III) chloride trihydrate (HAuCl4·3H2O) and BSA were purchased from Sigma–Aldrich.
Cis-1,2-dicyano-1,2-bis(2,4,5-trimethyl-3-thienyl)ethene (CMTE) was purchased from TCI (Tokyo, Japan). Phenylbutazone (PB) and ibuprofen (IBU) were purchased from Aladdin (Shanghai, China). Phosphate buffer saline (PBS) tablet was purchased from Sangon Biotech (Shanghai, China). 1,4-dioxane was purchased from J&K (Beijing, China).
4.2 Synthesis of CMTE–BSA
CMTE–BSA was prepared by adding 0.5 mL CMTE (8 × 10–3 M, in 1,4-dioxane) to 5 mL BSA (50 mg·mL–1, in PBS solution), and the mixture was incubated at room temperature for 1 h. As-prepared CMTE–BSA was purified by triple centrifugation filtration, using Sartorius filters (Vivaspin® Turbo 15) with a molecular weight cut-off of 30 kDa to remove free CMTE and 1,4-dioxane. The concentration of CMTE in BSA was calculated by quantifying the characteristic absorption peak of TMTE (Figure S8) after irradiation of 405 nm light.
4.3 Synthesis of CMTE–BSA–AuNCs
All glassware was cleaned in freshly prepared aqua regia (HCl:HNO3, 3:1 by volume) before rinsed thoroughly in H2O before the use. According to previous reported methods with slight modification, a typical synthesis is as follows.[61] Aqueous HAuCl4 solution (0.04 mL, 50 mM) was added to CMTE–BSA solution (1 mL, 2 mM) under vigorous stirring. 0.137 mL of NaOH solution (1 M) was added 2 min later, and further stirred at room temperature for 1 h before centrifugal purification by using Sartorius filters (Vivaspin® Turbo 4) with a molecular weight cut-off of 30 kDa. The solution after purification was stored in the dark at 4°C before further use.
4.4 Characterisation of CMTE–BSA and CMTE–BSA–AuNCs
All UV–Vis absorption spectra were measured by a U-3900H spectrophotometer (Hitachi, Tokyo, Japan). The fluorescence measurements were performed on a FLS980 Series of Fluorescence Spectrometers (Edinburgh, UK) with a 1.0 cm quartz cell. The quantum yield was measured by using fluorescein (quantum yield: 79%) as the reference. The fluorescence lifetime decay curve was recorded on a Fluorescence Spectrometer (FLS1000, Edinburgh, UK). All photoswitchable fluorescence and absorbance measurements were conducted in circular irradiation of 405 nm and 546 nm light for 20 min. X-ray photoelectron spectroscopy (XPS) measurements were performed on an Axis Ultra DLD XPS spectrometer (Kratos, Japan), with Al Kα X-ray radiation (1486.6 eV) as the excitation. All measured spectra were corrected by using the C1s peak at 285.0 eV as the reference.
4.5 Circular dichroism measurements
The circular dichroism (CD) spectral measurements were performed on a Chirascan Circular Dichroism Spectrometer (Applied Photophysics, UK) equipped with 1.0 cm quartz cells. For CD measurement, 20 μL CMTE was added into 4 mL BSA (6 mg·mL–1). The mole ratio of CMTE and BSA was set as 1:1. CD spectra were recorded from 200 to 260 nm.
4.6 Molecular modelling
The structural information of CMTE (CID: 44630141) and its trans form trans-1,2-dicyano-1,2-bis(2,4,5-trimethyl-3-thienyl)ethene TMTE (CID: 14122209) was obtained from PubChem (U.S. National Center for Biotechnology Information, http://pubchem.ncbi.nlm.nih.gov). Structural information of BSA (PDB: 3V03) was obtained from the Protein Data Bank (PDB). We used AutoDock 4.2 (The Scripps Research Institute) for the molecular docking calculation based on the method reported in the literature.[24, 28] The output from AutoDock was further analysed using PyMOL software.
ACKNOWLEDGEMENTS
Wencheng Zhong and Xiaojian Yan contributed equally to this work. This work was supported by the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0980), the Fundamental Research Fund for the Central University (3102019jcc005), the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (CX2021053) and the Research Fund of the State Key Laboratory of Solidification Processing (NPU, 2020-QZ-01).
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
ETHICS STATEMENT
There are no ethical issues in this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




