Osteopenic occult vertebral fracture is associated with poor oncological outcome in patients with hepatocellular carcinoma after hepatic resection
Abstract
Background
Although osteopenia has been associated with poor outcomes in patients with hepatocellular carcinoma (HCC), the oncological impact of occult vertebral fracture (OVF) has not been investigated.
Methods
The study comprised 235 patients who had undergone primary hepatic resection for hepatocellular carcinoma between 2008 and 2019. Osteopenia was evaluated with computed tomographic measurement of pixel density in the midvertebral core of the 11th thoracic vertebra. OVF was defined if the ratios of central/anterior or central/posterior heights of the vertebrae, measured using sagittal computed tomography reconstruction between 11th thoracic vertebra to 5th lumber vertebrae, <0.8. Multivariate Cox proportional hazard models were conducted to assess disease-free and overall survival adjusting for potential confounders.
Results
Occult vertebral fracture was identified in 93 patients (40%), while osteopenia in 65 patients (28%). Osteopenic OVF was identified in 27 patients (12%). In multivariate analysis, gender (p < 0.001), serum PIVKA-II level ≥ 200 mAU/ml (p = 0.005), C-reactive protein-to-albumin ratio ≥0.04 (p = 0.03), multiple tumors (p < 0.001), type of resection (p < 0.001), low skeletal muscle index (p = 0.002), and osteopenic OVF (HR 3.07, 95% CI 1.78–5.28, p < 0.001) were independent and significant predictors of cancer recurrence, while gender (p = 0.002), Child–Pugh grade B (p = 0.009), C-reactive protein-to-albumin ratio ≥0.04 (p = 0.03), multiple tumors (p = 0.005), low skeletal muscle index (p < 0.001), and osteopenic OVF (HR 4.75, 95% CI 2.41–9.39, p < 0.001) were independent predictors of overall survival.
Conclusions
Osteopenic OVF is associated with poor oncological outcomes in patients with hepatocellular carcinoma after hepatic resection. Our findings provide a compelling rationale for the further investigation of the interplay between tumor and bone metabolism.
1 INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third cause of cancer-related death worldwide.1 Although liver resection has been the potentially curative treatment for HCC, the prognosis of patients with HCC has been far from satisfactory, due to frequent tumor recurrence.2 Therefore, in clinical decision-making, better risk prediction of tumor recurrence is critically needed.
Accumulating evidence suggests that patient-related factors representing frailty have been associated with cancer-related as well as overall survival in several kinds of tumors. Sarcopenia, characterized by loss of skeletal muscle mass, has been associated with poor prognosis in patients with various malignancies including HCC.3 Sarcopenia has been linked to low bone mineral density (BMD), known as osteopenia, which has been another poor prognostic factor in cancer patients.4, 5 Although vertebral fractures are the most common complication of osteopenia or osteoporosis,6 more than two-thirds of them cannot be clinically detected due to the lack of symptoms.7 A few studies indicated the clinical impact of vertebral fractures in patients with malignancies.8, 9 However, the oncologic impact of vertebral fracture on the prognosis of HCC patients has not been investigated. Therefore, in this study, we investigated the prognostic association of vertebral fracture with survival in patients with HCC.
2 PATIENTS AND METHODS
2.1 Patient selection
Subjects of this retrospective study were HCC patients who underwent primary hepatic resection at the Department of Surgery, The Jikei University Hospital, Tokyo, Japan between January 2008 to December 2019. We excluded patients with other malignancies, postoperative mortality, and unavailable data on preoperative computed tomography, leaving the remaining 235 patients were enrolled into this study. We collected data on clinical information, operative and pathological findings, and postoperative courses from medical records. Patients were followed until death or the end of follow-up. This study was approved by the Ethics Committee of The Jikei University School of Medicine (#27-177).
2.2 Treatment and follow-up
Generally, the extent and type of hepatic resection were determined based on preoperative tumor staging, retention rate of indocyanine green at 15 min (ICGR15) before surgery, and hepatic reserve, as previously described.10 The nomenclature of the segment and types of operations followed the Tokyo 2020 terminology.11 The type of resection was classified into two groups: anatomical resection (hemihepatectomy, sectionectomy, or segmentectomy) and non-anatomical limited partial resection.12 Tumor-Nodes-Metastasis (TNM) classification was based on tumor pathology and the General Rules for the Clinical and Pathological Study of Primary Liver Cancer by the Liver Cancer Study Group of Japan.13
The recurrence of HCC was defined as newly detected hypervascular hepatic or extrahepatic tumors by ultrasonography, computed tomography, magnetic resonance image with or without increase in serum α-fetoprotein (AFP), or protein induced by vitamin K absence or antagonist-II. For recurrent HCC in the liver, repeated hepatic resection, local ablation therapy, or transarterial chemoembolization was given based on hepatic functional reserve evaluated mainly by ICGR15. Extrahepatic recurrence was mainly treated with systemic chemotherapy.
2.3 Assessment of skeletal muscle area
We used the psoas muscle to measure the skeletal muscle index (SMI) based on a previously described method.3 We simply measured the lengths of the major and minor axes of the psoas muscle at the caudal end of the third lumbar vertebra and calculated the area of the psoas muscle on preoperative CT. The SMI was calculated as the area of the psoas muscle/the height squared (cm2/m2). Given skeletal muscle mass differed by sex, we classified the SMI into two groups (high vs. low) based on the receiver operating characteristic (ROC) curves in strata of sex. The cut-off values of SMI for each sex were 10.9 for male and 7.4 for female.
2.4 Assessment of osteopenia and occult vertebral fracture
Osteopenia was defined as actual bone mineral density (BMD) below the calculated standard BMD, which was calculated as previously described (308.82–2.49 × age in men and 311.84–2.41 × age in women).4 BMD was measured in trabecular bone by calculating average pixel density within a circle in the midvertebral core at the bottom of the 11th thoracic vertebra on preoperative computed tomography.4
As previously described,8 using quantitative measurement and preoperative sagittal CT image reconstruction of vertebrae from the 11th thoracic vertebra to the 5th lumber vertebra, the anterior (A), central (C), and posterior (P) heights were measured and then the ratios of C/A and C/P were calculated. Occult vertebral fracture (OVF) were defined as C/A < 0.8 or C/p < 0.8 regardless of fracture history (Figure 1).
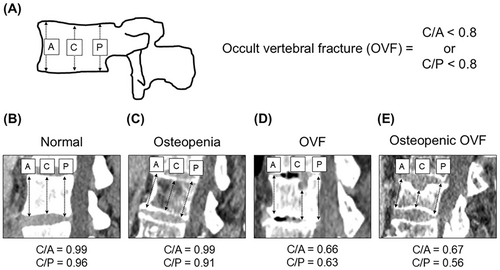
2.5 Assessment of systemic inflammatory response
Hemogram and chemistry profile were routinely measured for each patient preoperatively. Systemic inflammatory response was assessed by neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein-to-albumin ratio (CAR) within 7 days before surgery. As previously described,14 NLR was calculated as absolute neutrophil count divided by absolute lymphocyte count, while CAR was calculated as serum C-reactive protein (CRP) level (mg/dl) divided by serum albumin (g/dl). The cut-off value of each marker was defined according to the previous study as 2.6 for NLR and 0.04 for CAR.14
2.6 Statistical analysis
All statistical analyses were conducted using IBM® SPSS statistics version 25.0 (IBM Japan), and all p-values were two-sided. We used the two-sided α level of 0.05. Our primary analyses were an assessment of the survival association of vertebral bone status with disease-free and overall survival. All other tests, including assessment of risk estimates, represented secondary analyses. Data are expressed as a median, interquartile range, or ratio. Continuous and categorical variables were compared using the Mann–Whitney U-test, chi-square test, or Fisher's exact test as appropriate.
We assessed the prognostic significance of vertebral bone status in patients with HCC. Univariable and multivariable Cox proportional hazards regression models were used to estimate hazard ratio (HR) for disease-free and overall survival. The multivariable Cox regression model initially included age (≥65 vs. <65 years), gender (female vs. male), HBsAg status (positive vs. negative), HCVAb status (positive vs. negative), Child–Pugh grade (B vs. A), ICGR15 (≥15 vs. <15%), serum AFP level (≥20 vs. <20 ng/ml), serum PIVKA-II level (≥200 vs. <200 mAU/ml), NLR (≥2.6 vs. <2.6), CAR (≥0.04 vs. <0.04), tumor differentiation (well or moderate vs. poor), tumor size (>5 vs. ≤5 cm), number of tumors (multiple vs. solitary), microvascular invasion (yes vs. no), type of resection (anatomical vs. partial), duration of operation (≥360 vs. <360 min), intraoperative blood loss (≥1000 vs. <1000 g), intraoperative blood transfusion (yes vs. no), Skeletal muscle index (low vs. high), and vertebral bone status (normal vs. either OVF or osteopenia vs. osteopenic OVF). A backward elimination was conducted with a threshold p of 0.05 to select variables for the final models.
The Kaplan–Meier method was used to estimate cumulative survival probabilities, and the differences between groups were compared using the log-rank test for trend.
3 RESULTS
3.1 Patient characteristics
Patient characteristics are outlined in Table 1 as a median, interquartile range (IQR), or ratio. The median follow-up of the entire study population was 5.0 years (IQR 2.9–7.2 years). During follow-up, 136 of 235 patients experienced tumor recurrence (57.8%) and the median time to recurrence following hepatic resection was 2.5 years (IQR 1.0–5.4 years). In the current study, the 5-year disease-free survival and overall survival rates after hepatic resection for HCC were 43.6% and 77.6%, respectively. Osteopenia was identified in 65 patients (28%), while OVF was identified in 93 patients (40%). Concomitant occurrence of osteopenia and OVF was identified in 27 patients (12%).
| Variables | Median (IQR) or ratio (%) |
|---|---|
| Age (years) | 69 (61–75) |
| Gender | |
| Female | 49 (21%) |
| Male | 186 (79%) |
| HBsAg, positive | 47 (20%) |
| HCVAb, positive | 68 (29%) |
| ICGR15 (%) | 14 (9–21) |
| Child–Pugh grade | |
| Grade B | 19 (8.1%) |
| Grade A | 216 (92%) |
| Serum AFP (ng/ml) | 8 (4–46) |
| Serum PIVKA-II level (mAU/ml) | 54 (22–811) |
| NLR | 2.1 (1.5–3.0) |
| CAR | 0.02 (0.01–0.08) |
| Tumor differentiation | |
| Poor | 35 (15%) |
| Well/moderate | 196 (85%) |
| Tumor size (cm) | 3.4 (2.2–5.5) |
| Tumor number | |
| Multiple | 45 (19%) |
| Solitary | 190 (81%) |
| Microvascular invasion, yes | 41 (18%) |
| Type of resection | |
| Anatomical resection | 146 (62%) |
| Partial resection | 89 (38%) |
| Duration of operation (min) | 378 (298–509) |
| Intraoperative blood loss (g) | 475 (202–1033) |
| Skeletal muscle index (cm2/m2) | 10.7 (8.6–13.4) |
| Bone mineral density (HU) | 160 (136–185) |
| Osteopenia, yes | 65 (28%) |
| Occult vertebral fracture | 93 (40%) |
- Abbreviations: AFP, alpha-fetoprotein; CAR, C-reactive protein to albumin ratio; HBsAg, hepatitis B surface antigen; HCVAb, hepatitis C virus antibody; HU, Hounsfield units; ICGR15, retention rate of indocyanine green at 15 min; IQR, interquartile range; NLR, neutrophil to lymphocyte ratio; PIVKA-II, protein induced by vitamin K absence or antagonist-II.
3.2 Univariate and multivariate analyses of clinicopathological variables associated with disease-free survival after hepatic resection for HCC
Table 2 lists the association of the clinical variables with disease-free survival after hepatic resection for HCC. In univariate analysis, the disease-free survival was significantly associated with female (p = 0.005), serum PIVKA-II level ≥ 200 mAU/ml (p = 0.01), CAR ≥0.04 (p = 0.008), poorly differentiated tumor (p = 0.02), tumor size >5 cm (p = 0.002), multiple tumors (p < 0.001), microvascular invasion (p = 0.03), intraoperative blood loss ≥1000 g, SMI low (p = 0.02), and vertebral bone status (p < 0.001, Figure 2A). In multivariate analysis, female (p < 0.001), serum PIVKA-II level ≥ 200 mAU/ml (p = 0.005), CAR ≥0.04 (p = 0.03), multiple tumors (p < 0.001), type of resection (p < 0.001), SMI low (p = 0.002), and vertebral bone status (p < 0.001) were independent predictors of disease-free survival. HRs [95% confidence intervals (CIs)] for either OVF or osteopenia, and osteopenic OVF were 2.01 (1.36–2.96) and 3.07 (1.78–5.28).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| N = 235 | HR (95% CI) | p-Value | HR (95% CI) | p-Valuea |
| Age ≥ 65 years | 1.12 (0.79–1.61) | 0.52 | NS | |
| Gender, female | 0.48 (0.29–0.80) | 0.005 | 0.34 (0.19–0.59) | <0.001 |
| HBsAg, positive | 0.65 (0.41–1.02) | 0.06 | NS | |
| HCVAb, positive | 1.06 (0.88–1.27) | 0.57 | NS | |
| ICGR15 ≥ 15% | 1.25 (0.89–1.75) | 0.19 | NS | |
| Child–Pugh grade, grade B | 0.97 (0.52–1.80) | 0.93 | NS | |
| Serum AFP ≥20 ng/mL | 1.25 (0.88–1.78) | 0.21 | NS | |
| Serum PIVKA-II 200 mAU/ml | 1.55 (1.09–2.19) | 0.01 | 1.72 (1.15–2.57) | 0.005 |
| NLR ≥2.6 | 0.66 (1.08–1.54) | 0.66 | NS | |
| CAR ≥0.04 | 1.61 (1.14–2.29) | 0.008 | 1.62 (1.09–2.43) | 0.03 |
| Tumor differentiation, poor | 1.70 (1.10–2.64) | 0.02 | NS | |
| Tumor size, >5 cm | 1.75 (1.23–2.51) | 0.002 | NS | |
| Tumor number, multiple | 2.47 (1.70–3.60) | <0.001 | 3.52 (2.33–5.34) | <0.001 |
| Microvascular invasion, yes | 1.60 (1.04–2.46) | 0.03 | NS | |
| Type of resection, anatomical | 0.80 (0.57–1.12) | 0.19 | 0.42 (0.29–0.62) | <0.001 |
| Duration of operation ≥360 min | 1.16 (0.82–1.63) | 0.40 | NS | |
| Intraoperative blood loss ≥1000 g | 1.51 (1.06–2.17) | 0.02 | NS | |
| Skeletal muscle index, low | 1.52 (1.08–2.13) | 0.02 | 1.85 (1.27–2.68) | 0.002 |
| Vertebral bone status | NS | |||
| Normal | 1 (referent) | 1 (referent) | ||
| Either OVF or osteopenia | 1.64 (1.13–2.38) | 2.01 (1.36–2.96) | ||
| Osteopenic OVF | 2.89 (1.74–4.81) | <0.001b | 3.07 (1.78–5.28) | <0.001b |
- Abbreviations: AFP, alpha-fetoprotein; CAR, C-reactive protein to albumin ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen; HCV-Ab, hepatitis C virus antibody; HR, hazard ratio; ICGR15, retention rate of indocyanine green at 15 min; NLR, neutrophil to lymphocyte ratio; OVF, occult vertebral fracture; PIVKA-II, protein induced by vitamin K absence or antagonist-II.
- a The multivariable Cox regression model initially included age (≥65 vs. <65 years), gender (female vs. male), HBsAg status (positive vs. negative), HCVAb status (positive vs. negative), Child–Pugh grade (B vs. A), ICGR15 (≥15 vs. <15%), serum AFP level (≥20 vs. <20 ng/mL), serum PIVKA-II level (≥200 vs. <200 mAU/ml), NLR (≥2.6 vs. <2.6), CAR (≥0.04 vs. <0.04), tumor differentiation (well or moderate vs. poor), tumor size (>5 vs. ≤5 cm), number of tumors (multiple vs. solitary), microvascular invasion (yes vs. no), type of resection (anatomical vs. partial), duration of operation (≥360 vs. <360 min), intraoperative blood loss (≥1000 vs. <1000 g), intraoperative blood transfusion (yes vs. no), skeletal muscle index (low vs. high), and vertebral bone status (normal vs. either OVF or osteopenia vs. osteopenic OVF). A backward elimination was conducted with a threshold p of 0.05 to select variables for the final models.
- b p for trend was calculated across the categories of vertebral bone status (normal, either OVF or osteopenia, and osteopenic OVF, as an ordinal predictor variable).
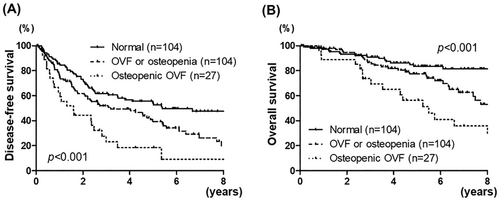
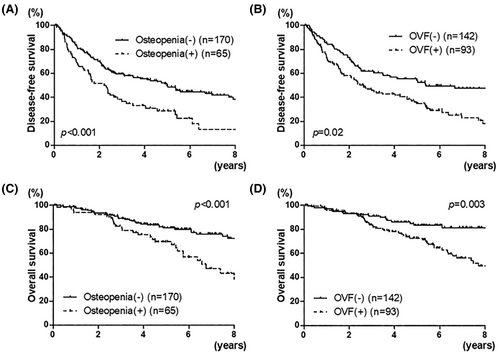
Both osteopenia and OVF were associated with worse disease-free survival (p < 0.001 and p = 0.02, respectively, Figures 1B and 3A).
3.3 Univariate and multivariate analyses of clinicopathological variables associated with overall survival after hepatic resection for HCC
Table 3 lists the association of the clinical variables with overall survival after hepatic resection for HCC. In univariate analysis, the overall survival was significantly associated with HBsAg positivity (p = 0.04), CAR ≥0.04 (p = 0.04), poorly differentiated tumor (p = 0.02), tumor size >5 cm (p = 0.007), multiple tumors (p = 0.01), microvascular invasion (p = 0.04), SMI low (p < 0.001), and vertebral bone status (p < 0.001, Figure 2B). In multivariate analysis, female (p = 0.002), Child–Pugh grade B (p = 0.009), CAR ≥0.04 (p = 0.03), multiple tumors (p = 0.005), SMI low (p < 0.001), and vertebral bone status (p < 0.001) were independent predictors of overall survival. HRs (95% CIs) for either OVF or osteopenia, and osteopenic OVF were 2.68 (1.45–4.98) and 4.75 (2.41–9.39).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| N = 235 | HR (95% CI) | p-Value | HR (95% CI) | p-Valuea |
| Age ≥ 65 years | 1.08 (0.65–1.79) | 0.76 | NS | |
| Gender, female | 0.49 (0.22–1.07) | 0.07 | 0.24 (0.10–0.55) | 0.002 |
| HBsAg, positive | 0.47 (0.23–0.95) | 0.04 | NS | |
| HCVAb, positive | 1.19 (0.93–1.54) | 0.17 | NS | |
| ICGR15 ≥ 15% | 1.58 (0.97–2.59) | 0.07 | NS | |
| Child–Pugh grade, grade B | 1.88 (0.93–3.81) | 0.08 | 3.21 (1.54–6.70) | 0.009 |
| Serum AFP ≥20 ng/mL | 1.62 (0.98–2.65) | 0.06 | NS | |
| Serum PIVKA-II 200 mAU/ml | 1.19 (0.72–1.97) | 0.51 | NS | |
| NLR ≥2.6 | 0.93 (0.56–1.56) | 0.79 | NS | |
| CAR ≥0.04 | 1.70 (1.03–2.80) | 0.04 | 1.79 (1.06–3.02) | 0.03 |
| Tumor differentiation, poor | 2.00 (1.11–3.63) | 0.02 | NS | |
| Tumor size, >5 cm | 2.00 (1.21–3.31) | 0.007 | NS | |
| Tumor number, multiple | 2.00 (1.18–3.39) | 0.01 | 2.08 (1.20–3.60) | 0.005 |
| Microvascular invasion, yes | 1.82 (1.02–3.25) | 0.04 | NS | |
| Type of resection, anatomical | 0.77 (0.47–1.25) | 0.29 | NS | |
| Duration of operation ≥360 min | 1.13 (0.69–1.86) | 0.62 | NS | |
| Intraoperative blood loss ≥1000 g | 1.59 (0.96–2.63) | 0.07 | NS | |
| Skeletal muscle index, low | 4.64 (2.52–8.53) | <0.001 | 6.36 (3.33–12.18) | <0.001 |
| Vertebral bone status | NS | |||
| Normal | 1 (referent) | 1 (referent) | ||
| Either OVF or osteopenia | 1.91 (1.06–3.44) | 2.68 (1.45–4.98) | ||
| Osteopenic OVF | 4.46 (2.32–8.57) | <0.001b | 4.75 (2.41–9.39) | <0.001b |
- Abbreviations: AFP, alpha-fetoprotein; CAR, C-reactive protein to albumin ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen; HCV-Ab, hepatitis C virus antibody; HR, hazard ratio; ICGR15, retention rate of indocyanine green at 15 min; NLR, neutrophil to lymphocyte ratio; OVF, occult vertebral fracture; PIVKA-II, protein induced by vitamin K absence or antagonist-II.
- a The multivariable Cox regression model initially included age (≥65 vs. <65 years), gender (female vs. male), HBsAg status (positive vs. negative), HCVAb status (positive vs. negative), Child–Pugh grade (B vs. A), ICGR15 (≥15 vs. <15%), serum AFP level (≥20 vs. <20 ng/mL), serum PIVKA-II level (≥200 vs. <200 mAU/ml), NLR (≥2.6 vs. <2.6), CAR (≥0.04 vs. <0.04), tumor differentiation (well or moderate vs. poor), tumor size (>5 vs. ≤5 cm), number of tumors (multiple vs. solitary), microvascular invasion (yes vs. no), type of resection (anatomical vs. partial), duration of operation (≥360 vs. <360 min), intraoperative blood loss (≥1000 vs. <1000 g), intraoperative blood transfusion (yes vs. no), skeletal muscle index (low vs. high), and vertebral bone status (normal vs. either OVF or osteopenia vs. osteopenic OVF). A backward elimination was conducted with a threshold p of 0.05 to select variables for the final models.
- b p for trend was calculated across the categories of vertebral bone status (normal, either OVF or osteopenia, and osteopenic OVF, as an ordinal predictor variable).
Both osteopenia and OVF were associated with worse disease-free survival (p < 0.001 and p = 0.003, respectively, Figures 1D and 3C).
3.4 Association between clinicopathologic variables and vertebral bone status
Table 4 lists the clinicopathologic variables according to the status of vertebral bone. In univariate analysis, HCVAb positivity (p = 0.049), serum AFP level (p = 0.003), tumor differentiation (p = 0.03), and BMD (p < 0.001) were associated with vertebral bone status. Skeletal muscle index was not associated with vertebral bone status (p > 0.05).
| Variables | Vertebral bone status | p-Valuea | ||
|---|---|---|---|---|
| Normal | OVF or osteopenia | Osteopenic OVF | ||
| (n = 104) | (n = 104) | (n = 27) | ||
| Age (years) | 67 (62–74) | 69 (61–76) | 69 (55–75) | 0.60 |
| Gender | 0.16 | |||
| Female | 19 (18%) | 27 (26%) | 3 (11%) | |
| Male | 85 (82%) | 77 (74%) | 24 (89%) | |
| HBsAg, positive | 24 (23%) | 18 (17%) | 5 (19%) | 0.57 |
| HCVAb, positive | 22 (21%) | 35 (34%) | 11 (41%) | 0.049 |
| ICGR15 (%) | 13 (9–20) | 15 (10–22) | 13 (8–21) | 0.53 |
| Child–Pugh grade | 0.30 | |||
| Grade B | 9 (8.7%) | 6 (5.8%) | 4 (15%) | |
| Grade A | 95 (91%) | 98 (94%) | 23 (85%) | |
| Serum AFP (ng/ml) | 5 (3–22) | 11 (6–65) | 30 (5–70) | 0.003 |
| Serum PIVKA-II (mAU/ml) | 63 (22–839) | 50 (20–735) | 50 (24–1017) | 0.96 |
| NLR | 2.0 (1.5–3.1) | 2.0 (1.5–2.9) | 2.5 (1.6–3.1) | 0.66 |
| CAR | 0.02 (0.01–0.07) | 0.02 (0.01–0.07) | 0.03 (0.01–0.16) | 0.30 |
| Tumor differentiation | 0.03 | |||
| Poor | 9 (8.7%) | 19 (19%) | 7 (26%) | |
| Well/moderate | 94 (91%) | 82 (81%) | 20 (74%) | |
| Tumor size (cm) | 3.5 (2.3–5.5) | 3.0 (2.2–5.5) | 3.5 (2.0–7.4) | 0.64 |
| Tumor number | 0.99 | |||
| Multiple | 20 (19%) | 20 (19%) | 5 (18%) | |
| Single | 84 (81%) | 84 (81%) | 22 (82%) | |
| Microvascular invasion, yes | 17 (17%) | 20 (19%) | 4 (15%) | 0.81 |
| Type of resection | 0.49 | |||
| Anatomical resection | 61 (59%) | 69 (34%) | 16 (59%) | |
| Partial resection | 43 (41%) | 35 (66%) | 11 (41%) | |
| Duration of operation (min) | 367 (294–480) | 393 (303–506) | 390 (260–547) | 0.80 |
| Intraoperative blood loss (g) | 475 (221–934) | 470 (200–1030) | 495 (180–1275) | 0.94 |
| Pathological liver cirrhosis, yes | 26 (26%) | 33 (32%) | 11 (41%) | 0.28 |
| Postoperative complications, yes | 29 (28%) | 31 (30%) | 10 (37%) | 0.65 |
| Skeletal muscle index (cm2/m2) | 10.9 (8.8–13.5) | 10.8 (8.5–13.5) | 9.8 (7.6–11.3) | 0.19 |
| Bone mineral density (HU) | 174 (155–203) | 150 (127–175) | 123 (105–147) | <0.001 |
| Osteopenia, yes | 0 (0%) | 38 (37%) | 27 (100%) | <0.001 |
| OVF, yes | 0 (0%) | 66 (64%) | 27 (100%) | <0.001 |
- Abbreviations: AFP, alpha-fetoprotein; CAR, C-reactive protein to albumin ratio; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HU, Hounsfield units; ICGR15, retention rate of indocyanine green at 15 min; NLR, neutrophil to lymphocyte ratio; OVF, occult vertebral fracture; PIVKA-II, protein induced by vitamin K absence or antagonist-II.
- a To compare categorical data between vertebral bone status, the chi-square test was performed. To compare continuous variables, Mann–Whitney U-test was performed.
Table S1 lists clinicopathologic variables according to the presence of osteopenia. In univariate analysis, age (p < 0.001), pathological liver cirrhosis (p = 0.04), and BMD (p < 0.001) were associated with vertebral bone status.
Table S2 lists clinicopathologic variables according to the presence of OVF. In univariate analysis, age (p = 0.003), HCVAb positivity (p = 0.002), serum AFP level (p = 0.001), and tumor differentiation (p = 0.02), and BMD (p = 0.005) were associated with OVF. Multivariate logistic regression model revealed that HCVAb positivity (p = 0.01), poor tumor differentiation (p = 0.046), and low BMD (p = 0.02) were independent risk factors for OVF (Table S3).
3.5 Cancer-specific survival and vertebral bone status
Among 65 deaths in 235 patients, seven patients died due to other causes including pneumonia (n = 2), cerebral infarction (n = 1), sepsis (n = 1), and unknown sudden death (n = 3). As a secondary analysis, we investigated the association of vertebral bone status with cancer-specific survival. Vertebral bone status was associated with cancer-specific survival (p < 0.001; Figure S1).
3.6 Survival analyses of vertebral bone status in strata of gender
As a sensitivity analysis, we investigated the association of vertebral bone status with disease-free and overall survival in strata of sex, considering the difference in bone status between gender. In males, vertebral bone status was associated with disease-free and overall survival (both p < 0.001, Figure S2A,B). In females, although the sample size was limited, osteopenic OVF showed worse disease-free and overall survival (p = 0.09, p = 0.35, respectively, Figure S2C,D).
4 DISCUSSION
In the current study, we found that osteopenic OVF was associated with poor oncologic outcomes in patients with HCC after resection. Notably, osteopenic OVF showed poorer disease-free and overall survival than those in patients with either osteopenia or OVF. Our findings suggest that impaired bone metabolism might be associated with the recurrence and progression of HCC, providing a novel insight into the further investigation of the interplay between tumor and bone metabolism.
Vertebral fracture may decrease muscle strength and activities of daily living, resulting in the negative impact on quality of life and mortality.15, 16 Decreased physical frailty has been associated with reduced survival rates, especially due to cancer-related deaths and pulmonary deaths.16 Vertebral fracture is a common consequence of osteopenia, which is characterized by low BMD.6 A growing body of evidence indicates that osteopenia has been associated with worse long-term outcomes in patients with malignancies.4, 5 However, there have been limited studies on OVF in cancer patients.8, 9 In this study, the concomitant presence of osteopenia and OVF showed worse disease-free and overall survival than either osteopenia or OVF. This result indicated that the degree of osteoporosis may have a negative impact on prognosis in patients with cancers.
The biological mechanisms of osteopenia and OVF have not been clarified. There have been direct and indirect interplay between cancer cells and bone metabolism.17 Pro-inflammatory and pro-osteolytic cytokines, such as interleukin1, 6, and tumor necrosis factor-α, secreted by cancer cells cause bone loss by activating osteoclastogenesis via the RANK/RANKL (receptor of activator of nuclear factor kappa-B ligand) signaling.18 RANKL released by osteoblasts stimulates the expression of RANK on the surface of the osteoblasts and enhances osteoclastogenesis. On the process of bone resorption, various cytokines, including transforming growth factor-β and insulin-like growth factor, are secreted and those cytokines can promote tumor growth, invasion, and metastasis.19 Thus, RANK/RANKL signaling, associated with bone metabolism, has contributed to cancer development in the tumor microenvironment.20 CD109, a multifunctional glycosylphosphatidylinositol-anchored glycoprotein expressed in various malignant tumors, has been reported as one of the regulatory factors of bone homeostasis.21 CD109 may promote tumor cell proliferation22 as well as metastasis through JAK/STAT signaling pathway,23 while CD109 can regulate the activity of osteoblasts and osteoclasts.21 Moreover, Wnt/beta-catenin signaling pathway, which has been known as a major molecular pathway in cancer metabolism including HCC,24 has played a key role in osteoblast differentiation and bone formation.25 In this study, HCVAb positivity, poor tumor differentiation, and low BMD were identified as risk factors for OVF. Although studies indicated the chronic liver diseases have been associated with reduced BMD,26 our results revealed that tumor factors may be also associated with the development of OVF in patients with HCC.
In this study, osteopenia was identified in 28%, while OVF was identified in 40%. Out results suggested that OVF can be developed in patients without osteopenia. Osteopenia represents the decrease in BMD but is not equal to osteoporosis. In clinical practice, osteoporosis is diagnosed by dual-energy X-ray (DXA) absorptiometry and the presence of a fracture. A study has shown that normal BMD by DXA was observed in 10% of patients with vertebral fracture and BMD alone does not evaluate bone quality and may not adequately capture a patient's fracture risk.27 Therefore, OVF may occur in patients without osteopenia but with abnormal bone quality. In this study, patients with either of osteopenia or OVF had worse long-term outcomes than those in normal bone status. Our results were consistent with previous studies showing that osteoporosis can be associated with poor mortality in cancer patients. Considering the association between osteoporosis and poor prognosis, early interventions such as rehabilitation and osteoporotic agents such as vitamin D, calcium, and bisphosphonate to improve osteoporosis or to prevent further bone loss may improve postoperative survival after hepatic resection for HCC.28, 29
We acknowledge the potential limitations of this study. First, considering the retrospective nature of this study, the potential bias could not be completely excluded. Second, in the present study, OVF was determined by quantitative measurements using preoperative CT image reconstruction from the 11th thoracic vertebra to the 5th lumber vertebra. However, in clinical practice, vertebral fracture is assessed using spinal radiographs with a semiquantitative approach.30 Thus, the appropriate evaluation of OVF needs to be determined in cancer patients. Third, the current study was conducted in a single institution. Our findings need to be validated in large-scale studies.
In conclusion, we have shown that osteopenic OVF was associated with poor long-term outcomes in patients with HCC after hepatic resection. Osteopenic OVF had a poorer prognosis than that of patients with either osteopenia or OVF. Our findings provide a compelling rationale for the further investigation of the interplay between bone metabolism and tumor recurrence and progression in hepatocellular carcinoma.
AUTHOR CONTRIBUTIONS
Koichiro Haruki: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; writing – original draft. Kenei Furukawa: Conceptualization; data curation; methodology; writing – review and editing. Munetoshi Akaoka: Data curation; investigation; writing – review and editing. Masashi Tsunematsu: Writing – review and editing. Michinori Matsumoto: Data curation; writing – review and editing. Tomohiko Taniai: Data curation; writing – review and editing. Yoshihiro Shirai: Writing – review and editing. Shinji Onda: Writing – review and editing. Ryoga Hamura: Writing – review and editing. Toru Ikegami: Funding acquisition; project administration; supervision; writing – original draft.
ACKNOWLEDGMENTS
The authors have nothing to report.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI Grants (24K11920 to K.H. and 24K11898 to T.I.) and research grant from the Takeda Science Foundation (to K.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study protocol was approved by the Ethics Committee of The Jikei University School of Medicine (#27-177). Patients were given an opportunity to opt-out of this study through public announcements.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.




