Prognostic significance of six autoantibodies in esophageal squamous cell carcinoma: A prospective multi-institutional study
Abstract
Aim
Serum autoantibodies have been reported to react with tumor-associated antigens in various cancers. This study evaluated the diagnostic and prognostic significance of six autoantibody panels in esophageal squamous cell carcinoma.
Methods
In this study, 193 patients with esophageal squamous cell carcinoma and 78 healthy controls were enrolled. Serum antibodies were detected using originally developed enzyme-linked immunosorbent assays to detect autoantibodies against the following tumor antigens: c-myc, p62, RalA, p53, Sui1, and NY-ESO-1. The positive rates of the six-autoantibody panel were compared with those of SCC antigen (SCC-Ag). The prognostic significance of these autoantibodies was also evaluated.
Results
The overall positive rate was significantly higher in the six-autoantibody panel than in SCC-Ag (72% vs. 28%; p < 0.01). The positive rates of the six-autoantibody panel were 71% in stage 0/I, 73% in stage II, and 71% in stage III. No clinicopathological factors were associated with autoantibodies. Although the difference was not significant, the overall survival of the autoantibody-positive group was worse than that of the autoantibody-negative group (p = 0.14).
Conclusion
The six-autoantibody panel was useful for detecting esophageal squamous cell carcinoma, particularly in stage 0/I; however, it showed limited prognostic significance.
1 INTRODUCTION
Patients with esophageal cancer continue to have a poor prognosis.1 Multidisciplinary treatments have been attempted to control esophageal cancer.2 Also, early detection of esophageal cancer is an important treatment strategy for improving patients' prognosis.
Serum tumor markers significantly help in cancer detection; however, conventional serum markers are not useful for detecting early esophageal cancer.3 Therefore, finding new serological biomarkers is essential to improve the diagnostic ability to detect early esophageal cancer.
The production of autoantibodies reflects greater immunological reactivity in patients with cancer and enhanced immune surveillance for cancer cells.4 Some serum autoantibodies have been reported to react with tumor-associated antigens (TAAs) even in the early stages of gastrointestinal cancer.5-8 Measurement of a single autoantibody may lack sufficient sensitivity required for cancer screening and diagnosis. Combining multiple autoantibodies may increase the sensitivity in detecting gastrointestinal cancer compared with that of established markers.5, 9, 10 The presence of serum p53 and/or NY-ESO-1 antibodies has been reported to be associated with tumor progression in esophageal and gastric cancers.10 The prognostic value of serum autoantibodies, including NY-ESO-1 and p53 in gastrointestinal cancer, has been investigated; however, the results were inconclusive.10, 11
Recently, we conducted a multi-institutional prospective study to develop a panel that comprises six autoantibodies to detect hepatocellular carcinoma.12 Autoantibody panels have been reported to be useful for detecting early hepatocellular carcinoma. However, the prognostic impact of autoantibody panels was limited. Therefore, a similar prospective study should be conducted to clarify the clinical significance of autoantibody panels in patients with esophageal cancer. Three studies have reported positive rates of the combinations of autoantibodies in patients with esophageal squamous cell carcinoma (ESCC).13-15 However, these studies did not compare these autoantibody combinations with conventional tumor markers, such as squamous cell carcinoma antigen (SCC-Ag); additionally, prognostic analysis was not performed.
We selected six autoantibodies with a higher positive rate from those detected by the serological identification of antigens by recombinant expression cloning method. Therefore, this prospective multi-institutional study investigated the diagnostic and prognostic significance of a six-autoantibody panel, including c-myc, p62, RalA, p53, Sui1, and NY-ESO-1 antibodies, in patients with ESCC.
2 MATERIALS AND METHODS
2.1 Patients
This study analyzed 193 patients, including 163 men and 30 women, with a median age of 67 years (range, 38–88 years). Patients with histologically proven primary ESCC were eligible. Those with active coexisting cancer, that is, synchronous coexisting or metachronous cancer within 5 disease-free years, were excluded to ensure the complete absence of the influence of previous cancers on antibody concentrations and overall survival (OS). Each institution performed central monitoring to ensure data submission, patient eligibility, protocol compliance, safety, and on-schedule study progress. Patient clinicopathological variables and survival, in those positive and negative for any of the six and none of the six autoantibodies, were compared. The SCC-Ag cutoff value was 1.5 ng/mL following the instructions of the manufacturer of the assay kit.
2.2 Patient variables
The stage was assessed following the tumor–node–metastasis classification criteria of the guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus (11th Edition). Preoperative resectability and local or distant tumor extension were determined using computed tomography. Tumors associated with distant metastasis were considered unresectable. Esophagectomy was performed with radical lymphadenectomies.
2.3 Data collection
Serum samples were obtained from patients with esophageal SCC and healthy controls without previous malignant disease at the six participating institutions from 2011 to 2015. Serum samples were collected before pre-treatment. For patients who underwent neoadjuvant chemotherapy, blood samples were taken before neoadjuvant chemotherapy. The healthy controls obtained from Biobank Japan had no previous malignant disease. The average age of the control group (n = 78) was 48 years, with a male to female ratio of 49:29. The study centers included the Toho University School of Medicine, Tokyo Medical and Dental University, Showa University School of Medicine, Keio University School of Medicine, Jikei University School of Medicine, Saitama Cancer Center, Tokyo Women's Medical University, and Nihon University School of Medicine. Samples were stored at −80°C until analysis. The titers (means ± standard deviation) of autoantibodies against the six TAAs (i.e., c-myc, p62, RalA, p53, Sui1, and NY-ESO-1) were assayed in 193 patients with ESCC and 78 controls. The cutoff value for positive reactivity was an optical density greater than the mean + two standard deviations observed in healthy controls. The specificity of the assay was calculated as the percentage of controls from whom a negative result was obtained. The significance of differences in each of the six autoantibody titers to the individual antigens in the panel was assessed. Various serum markers, clinicopathological characteristics, and SCC-Ag concentrations were analyzed. Preoperative variables, pathological characteristics, and survival rates were entered in a spreadsheet and imported into a dedicated database. The prognostic value and clinical usage of the six-autoantibody panel for the diagnosis of ESCC were evaluated. OS was calculated from the time of surgery or initial date of chemoradiation until death or study conclusion.
2.4 Isolation, purification, and amplification of TAAs and enzyme-linked immunosorbent assay (ELISA) of serum antibodies
Full-length cDNA of c-myc, p62, RalA, p53, Sui1, and NY-ESO-1 were amplified via polymerase chain reaction. Briefly, the recombinant proteins were expressed in Escherichia coli BL21-CodonPlus (DE3)-RIL (Stratagene, La Jolla, California). Each TAA extract was added to Ni Sepharose 6 Fast Flow (GE Healthcare UL, Buckinghamshire, UK), and the column was washed with 50 mM imidazole in phosphate buffered saline (PBS). Purified TAA recombinant proteins were eluted with 200 mM imidazole in PBS. DNA sequencing confirmed that the correct gene was inserted into the constructed plasmid. Serum samples collected from patients and controls were analyzed using ELISA. Since it is being conducted as a corporate clinical trial, the quality of the test is PMDA approved.
2.5 Informed consent and data protection
All study participants provided their consent for future analyses of their blood samples for research purposes. The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Faculty of Medicine, Toho University (approval number: A16035_A16001_26095_25024_24038_22047), Toho University Omori Medical Center (approval number: M20219), and the Institutional Review Boards of each participating hospital. All informed consent was obtained from the subjects and/or guardians.
2.6 Statistical analysis
Fisher's exact test was used to compare differences in patients' categorical data. OS rates were estimated using the Kaplan–Meier method, and survival curve comparisons were performed using the log-rank test. Multivariate analysis was performed using a Cox regression model to identify prognostic factors associated with OS. Two-sided p-values < 0.05 were used to denote statistical significance. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan16), a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). More precisely, EZR is a modified version of R Commander (version 1.6-3) designed to facilitate the use of statistical functions frequently employed in biostatistics. Differences with p-values < 0.05 were considered statistically significant.
3 RESULTS
3.1 Serum autoantibody titers
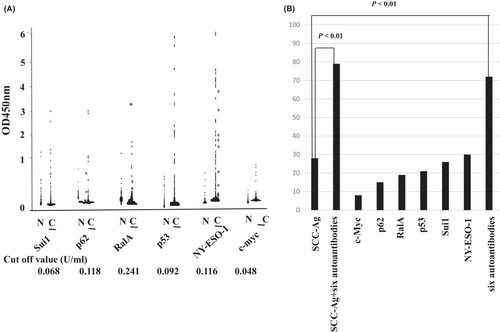
The serum titers of all six autoantibodies were significantly elevated in patients with ESCC compared with those in healthy controls (p < 0.05) (Figure 1A). The cutoff titers were 0.048 for c-myc, 0.118 for p62, 0.241 for RalA, 0.092 for p53, 0.068 for Sui1, and 0.11 for NY-ESO-1. The patients were divided into two groups (positive or negative groups) based on serum autoantibody titers using the cutoff value of each titer.
3.2 Clinicopathological characteristics and autoantibody status in patients with esophageal cancer
The clinicopathological characteristics were compared between the autoantibody-positive and autoantibody-negative groups. The results are shown in Table 1. No clinicopathological factors, including SCC-Ag status, were associated with autoantibody status.
| Panel | Total | All autoantibody negative | Six autoantibodies positive | p Valuea |
|---|---|---|---|---|
| Number | 193 | 55 | 138 | |
| Gender | ||||
| Male | 163 | 46 | 117 | 0.83 |
| Female | 30 | 9 | 21 | |
| Age | ||||
| <65 | 74 | 22 | 52 | 0.87 |
| ≧65 | 119 | 33 | 86 | |
| Tumor depth | ||||
| pT0T1 | 106 | 30 | 76 | 1 |
| pT2T3T4 | 83 | 24 | 59 | |
| Nodal status | ||||
| Negative | 110 | 30 | 80 | 0.63 |
| Positive | 83 | 25 | 58 | |
| Neoadjuvant therapy | ||||
| Absent | 105 | 28 | 77 | 0.63 |
| Present | 88 | 27 | 61 | |
| SCC-Ag | ||||
| <1.5 ng/mL | 139 | 41 | 98 | 0.72 |
| ≧1.5 ng/mL | 54 | 14 | 40 | |
- a Fisher’s exact test
3.3 Sensitivity and specificity of serum autoantibodies against tumor-associated antigens
Of the 193 patients, the sensitivity of those with autoantibodies was 7.7% (15/193) against c-myc, 15% (29/193) against p62, 19% (37/193) against RalA, 21% (40/193) against p53, 26% (50/193) against Sui1, and 30% (57/193) against NY-ESO-1 (Figure 1B). The specificity was 94% against c-myc, 96% against p62, 96% against RalA, 92% against p53, 95% against Sui1, and 96% against NY-ESO-1. The combination of all six autoantibodies increased the sensitivity to 72% (95% confidence interval (CI), 65%–78%) with a specificity of 76% (95% CI, 65%–85%) (Table 2).
| Group | c-myc | p62 | RalA | P53 | Sui1 | NY-ESO-1 | Panel |
|---|---|---|---|---|---|---|---|
| Sensitivity | 7.8 (4.4–12) | 15 (10–21) | 19 (14–25) | 21 (15–27) | 26 (20–33) | 30 (23–37) | 72 (65–78) |
| Specificity | 94 (86–98) | 96 (89–99) | 96 (89–99) | 92 (84–97) | 95 (87–99) | 96 (89–99) | 76 (65–85) |
| PPV | 75 (51–91) | 91 (75–98) | 93 (80–98) | 87 (74–95) | 93 (82–98) | 95 (86–99) | 88 (82–93) |
| NPV | 29 (24–35) | 31 (26–38) | 33 (27–39) | 32 (26–39) | 34 (28–41) | 36 (29–42) | 52 (42–61) |
3.4 Positive rate of autoantibodies and SCC-Ag according to TNM stages
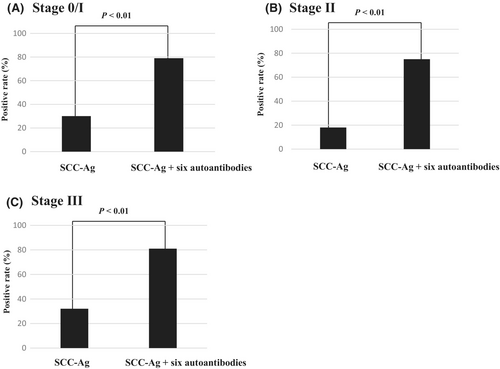
The positive rate of the combination of all six autoantibodies was higher than that of SCC-Ag (72% vs. 28%; p < 0.05) (Figure 1B). The combined positive rate of serum autoantibodies and SCC-Ag was significantly greater than that of SCC-Ag alone (p < 0.01) (Figure 1B). At each stage, the positive rate of SCC-Ag + serum autoantibodies was significantly higher than that of SCC-Ag alone (Figure 2).
3.5 Autoantibody positive rates at each TNM stage
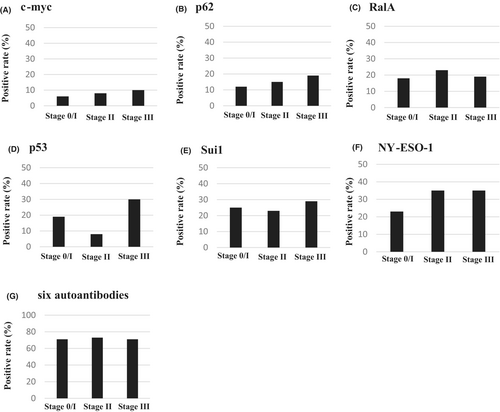
The positive rates of each autoantibody for stages0/I, II, and III are shown in Figure 3. The positive rates of the c-myc antibody were 5.9% for stage 0/I, 7.5% for stage II, and 10% for stage III. The positive rates of the p62 antibody were 12% for stage 0/I, 15% for stage II, and 19% for stage III. The positive rates of the RalA antibody were 18% for stage 0/I, 23% for stage II, and 19% for stage III. The positive rates of the p53 antibody 19% for stage 0/I, 8% for stage II, and 30% for stage III. The positive rates of the Sui1 antibody were 25% for stage 0/I, 23% for stage II, and 29% for stage III. The positive rates of the NY-ESO-1 antibody were 23% for stage 0/I, 35% for stage II, and 35% for stage III (Figure 3).
3.6 Overall survival and serum autoantibody status in each stage
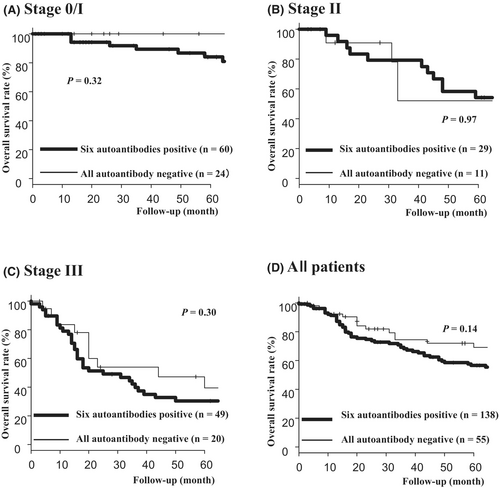
The overall survivals were compared between the autoantibody-positive and autoantibody-negative groups at each stage (Figure 4). Although no significant difference in OS rate was observed between the two groups, the autoantibody-positive group showed a relatively poor prognosis (p = 0.14) (Figure 4D).
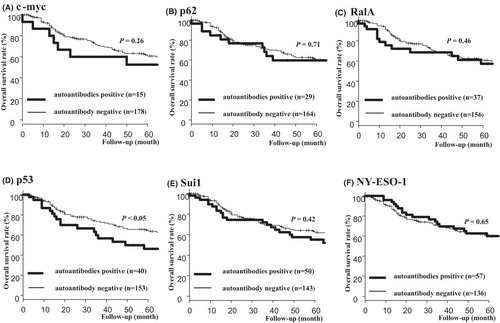
3.7 Comparison of overall survival rates between patients positive for each autoantibody and those negative for each autoantibody
The OS rates were compared between patients positive for each autoantibody and those negative for each autoantibody (Figure 5). The p53 antibody-positive group had a significantly worse prognosis than the p53 antibody-negative group (Figure 5D). However, no significant difference in prognosis was observed between the two groups in other autoantibodies.
3.8 Univariate and multivariate analyses of overall survival and relapse-free survival
Univariate analysis revealed that tumor depth (T2T3T4), positive nodal status (pN1), and positive distant metastasis were significantly associated with poor prognosis (Table 3 left panel). Multivariate analysis revealed that tumor depth (T2T3T4) and positive nodal status (pN1) were independent risk factors for poor prognosis (Table 3 right panel). Although autoantibody positivity was not an independent risk factor, it may be a risk factor for poor prognosis (hazard ratio = 1.58; p = 0.11).
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p Valuea | H.R (95% CI) | p Valueb | ||
| Age | ≧65/<65 | 0.62 | ||
| Gender | Male/Female | 0.57 | ||
| Tumor depth | pT2T3T4/pT0T1 | <0.01 | 2.75 (1.49–5.06) | <0.01 |
| Nodal status | Positive/Negative | <0.01 | 3.03 (1.66–5.53) | <0.01 |
| Neoadjuvant therapy | Present/Absent | 0.11 | 0.68 (0.40–1.14) | 0.14 |
| Serum autoantibody | Positive/All Negative | 0.14 | 1.58 (0.90–2.80) | 0.11 |
| SCC-Ag (ng/mL) | ≧1.5/<1.5 | 0.96 | ||
- a Log-rank analysis
- b Cox proportional hazards regression analysis
Also, there was also no significant difference in relapse-free survival between the six autoantibodies positive group and all autoantibody negative groups (p = 0.37) (Figure S1).
4 DISCUSSION
In this study, we prospectively analyzed the diagnostic and prognostic impact of six autoantibodies, including c-myc, p62, RalA, p53, Sui1, and NY-ESO-1 antibodies, in ESCC. The positive rate of the combination of these six autoantibodies was higher than that of SCC-Ag, even in the early stages. We analyzed the significance of these autoantibodies as prognostic factors in a prospective multi-institutional study.
Each of the six autoantibodies showed a lower positive rate than SCC-Ag. However, the positive rate of the combination of these six autoantibodies was 79%, which is significantly higher than that of SCC-Ag. Autoantibodies had no correlation with clinicopathological factors. Because autoantibody status was not associated with SCC-Ag, the combination of the six autoantibodies and SCC-Ag significantly increased the positive rate.
This is the first study to report the clinical significance of the combination of autoantibodies in esophageal SCC in a Japanese population and to simultaneously measure six types of autoantibodies and analyze their clinicopathological factors and prognostic factors significance in a prospective multi-institutional study.
There was a significant age difference between the ESCC group and the healthy control group in our present study. However, we consider that there is little correlation between age and autoantibodies. Because autoantibodies appear in the blood as cancer cells grow in the body. Our research group has previously reported on various cancers and autoantibodies.9, 10, 12, 17, 18 None of the reports found a correlation between autoantibodies and age. Also, in this study, there was not a correlation between autoantibodies and age in Table 1. Therefore, even if there was an age difference between the healthy subject group and the ESCC group, we consider that this is not a problem in this study.
Various combinations of autoantibodies have been discovered, and a combination of 4–11 antibodies has been reported.13-15, 19 Zhang19 reported that a combination of four serum antibodies from nine autoantibodies achieved a sensitivity and specificity of 60%. However, in our present study, both the sensitivity and specificity were over 70%, which is higher than previous reports. Such superiority was also observed in the early stages of cancer (stage 0/I). We consider that this combination of autoantibodies may be more useful for early diagnosis of ESCC than previous reports. Also, regarding the number of antibodies to be combined, the smaller the number, the easier it is. Even with six types of antibodies, the false positive rate tends to be high; therefore, there is concern that the more the number of antibodies, the less useful they will be in clinical practice. The positive rate of the six autoantibodies in this study to detect ESCC was higher than that of the antibodies in the previous report.14 Considering the cost tolerance evaluation, the positive rate of each autoantibody was lower than that of SCC-Ag, and the survival curve in the panel was not an independent prognostic predictor, so its use in clinical practice is questionable, remains. However, since the use of an autoantibody panel has shown a high positive rate in early-stage cancers, we consider that it may help improve diagnosis in the future.
Previous reports did not compare the usefulness of autoantibodies with that of SCC-Ag. This is also the first multi-institutional study to measure SCC-Ag and autoantibodies simultaneously and compare the positive rate. In Table 1, no relationship between autoantibodies and SCC-Ag was observed; therefore, it was confirmed that combining SCC-Ag with autoantibodies increased the positive rate. In this study, Sui1, NY-ESO-1, p-53, and RalA antibodies showed a relatively high positive rate of approximately 20% in stage 0/I. For the NY-ESO-1 antibody, a positive rate of >30% was observed in stages II and III. We considered that the Sui1, NY-ESO-1, p-53, and RalA antibodies are necessary for their combinatory use. The levels of c-myc and p62 autoantibodies are relatively low in ESCC, and optimized combinations of autoantibodies should be selected to increase the positive rate and specificity for the diagnosis of ESCC. Regarding the relationship between autoantibodies and SCC-Ag, similar to the analysis results for other cancers, there is no correlation between autoantibodies and conventional tumor markers.17, 20 Because autoantibodies have little correlation with SCC-Ag, using both autoantibodies and SCC-Ag significantly increases the positive rate.
Okada R. of our research group reported clinical impact of six autoantibodies in hepatocellular carcinoma.12 They reported that those six autoantibodies were useful for early detection, and that the positive rate increased when combined with traditional tumor markers, AFP. In our present study, we also showed that the autoantibodies were useful for early detection, and that the positive rate increased when combined with traditional tumor markers, SCC-Ag.
Comparisons of OS between individuals positive for the six autoantibodies and those negative for all autoantibodies did not show a consistent trend. The p53 antibody-positive group had a significantly worse prognosis than the p53 antibody-negative group. The c-myc, p62, RalA, and Sui1 antibody-positive group tended to have a worse prognosis than the c-myc, p62, RalA, and Sui1 antibody-negative group.
We consider that Sui1, NY-ESO-1, p-53, and RalA antibodies should be used in combination to diagnose ESCC, so we analyzed the prognostic impact of these four autoantibodies combinations (Sui1, NY-ESO-1, p-53 and RalA). However, there was no significant difference in the prognosis between the antibody-positive and antibody-negative groups (p = 0.08) (Figure S2). Furthermore, we examined whether there was a relationship between the number of positive markers among the six and overall survival and relapse-free survival. There was no statistically significant difference between the number of positive markers for the six autoantibodies and overall survival (p = 0.39) (data not shown) or relapse-free survival (p = 0.32) (data not shown). The median number of positive autoantibodies was one. Therefore, we compared the overall survival rate and relapse-free survival rate between the group with one or less positive markers for autoantibodies and the group with two or more positive markers for autoantibodies. There was no statistically significant difference in the overall survival rate (p = 0.32) (Figure S3 left panel) and relapse-free survival rate (p = 0.42) (Figure S3 right panel) between the group with one or less positive markers for autoantibodies and the group with two or more positive markers for autoantibodies.
Unfortunately, this study did not show that autoantibodies may affect OS or relapse-free survival. Regarding cancer diagnosis and prognosis, we consider that autoantibodies should be evaluated in combination with other biomarkers. Recently, circulating tumor DNA (ctDNA) has been extensively analyzed and used to diagnose and predict the prognosis of tumor diseases.21, 22 ctDNA can be extracted from plasma or serum. However, even in advanced cancers, the amount of ctDNA contained in cell-free DNA in the blood is very small and the half-life of ctDNA is short, about 1 h, requiring highly sensitive detection techniques. On the other hand, analysis of autoantibodies is simple and inexpensive. In addition, autoantibody samples are stable even when stored at room temperature without losing activity, making them highly versatile. We consider that combining ctDNA with autoantibodies will further increase the rate of cancer diagnosis and predict the prognosis of tumor diseases. In addition, we consider that by preventing unnecessary follow-up treatment, we can obtain beneficial results in terms of costs.
The NY-ESO-1 autoantibody did not show any prognostic significance. In all stages, the group positive for the six autoantibodies had a worse prognosis than the autoantibody-negative group; however, the difference was not statistically significant.
Recently, vaccination against cancer has attracted attention. The impact of the NY-ESO-1 antibodies in patients treated with CHP-NY-ESO-1 vaccine for esophageal cancer has been reported.23 This suggests that autoantibodies are useful for selecting good candidates for vaccine treatment. This assumption should be clarified in future clinical trials.
This study had two limitations. First, immunohistochemical analyses of tumor tissues were not performed in this study. However, studies have reported that serum autoantibodies and expression in tumor tissue may be correlated.13, 18, 24 Second, because of the lack of postoperative antibody data, the transition before and after treatment could not be confirmed. In future research, we should clarify the changes in serum antibody titers before and after treatment.
In conclusion, a panel of autoantibodies against TAAs, including Sui1, p62, RalA, p53, NY-ESO-1, and c-myc, had an additive effect with SCC-Ag in detecting esophageal SCC even in the early stage. Although the difference was not significant, the autoantibody-positive group tended to have a worse prognosis than the autoantibody-negative group. Thus, the prognostic impact of autoantibodies in ESCC is limited.
AUTHOR CONTRIBUTIONS
Fumiaki Shiratori: Data curation; writing – original draft. Naoto Fujiwara: Data curation. Yasuaki Nakajima: Conceptualization; data curation; methodology. Koji Otsuka: Data curation. Masahiko Murakami: Data curation. Satoru Matsuda: Data curation. Hirofumi Kawakubo: Data curation. Keita Takahashi: Data curation. Yuichiro Tanishima: Data curation. Daiji Oka: Data curation. Shunichi Ito: Data curation. Kosuke Narumiya: Data curation. Osamu Aramaki: Data curation. Tadatoshi Takayama: Data curation. Takashi Suzuki: Data curation. Satoshi Yajima: Data curation. Hideaki Shimada: Conceptualization; methodology; validation; writing – original draft.
ACKNOWLEDGMENTS
This work was partially supported by Grants-in-Aid for Scientific Research (C) (JSPS KAKENHI Nos. 15K10117, 16K10520). The authors would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
FUNDING INFORMATION
Hideaki Shimada has received research grants from Medical & Biological Laboratories Co., Ltd., Nagoya, Japan.
CONFLICT OF INTEREST STATEMENT
Hideaki Shimada has received research grants from Medical & Biological Laboratories Co., Ltd., Nagoya, Japan. Hideaki Shimada and Satoru Matsuda are editorial board members of Annals of Gastroenterological Surgery. The other authors have no conflict of interest associated with this present study.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Toho University, Graduate School of Medicine (approval number: M20219).
Informed consent: Participants provided paper-based informed consent at the time of admission, and their consent forms were digitized and stored in a database.
Registry and the registration No. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.




