Survival benefit of adjuvant therapy completion with early initiation for patients with pancreatic ductal adenocarcinoma
Abstract
Aim
To evaluate the prognostic effect of initiation timing and completion of adjuvant therapy in patients with pancreatic ductal adenocarcinoma.
Methods
The medical records of patients with pancreatic ductal adenocarcinoma who underwent radical pancreatectomy between 2006 and 2022 at Hiroshima University were retrospectively reviewed. Patient characteristics, perioperative outcomes, clinicopathological factors, and survival rates were analyzed. Adjuvant indications were for all patients who had a good postoperative status as early as possible. Early initiation was defined as adjuvant initiation within 4 weeks after surgery, and completion was defined as a total of 6 months of administration.
Results
In total, 444 (294, resectable; 150, borderline resectable or locally advanced) patients who received adjuvant therapy were enrolled in this study. The median time to adjuvant therapy initiation was 20 days. In total, 328 patients with early initiation had better overall survival than those with delayed initiation, and 409 patients with adjuvant completion had better survival rates than those with incompletion. Multivariate overall survival analysis demonstrated that early adjuvant therapy initiation and completion were independent prognostic factors for prolonged survival. In total, 310 adjuvant completions with early initiation resulted in a median survival period of 81.8 months. Multivariate analysis identified severe postoperative complication as an independent risk factor preventing adjuvant completion with early initiation.
Conclusion
Adjuvant completion with early initiation may contribute to the improved survival of patients with pancreatic ductal adenocarcinoma. Preventing severe postoperative complications may facilitate adjuvant completion with early initiation.
1 INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies and the fourth most common cause of cancer-related deaths in Western countries, with a 5-year overall survival rate of 13%.1 Surgical resection is the only potentially curative treatment for PDAC, but survival rates after resection remain extremely poor. To improve the survival of patients with PDAC, some randomized studies have shown the survival benefits of adjuvant therapy after surgery. Adjuvant therapy is a strong prognostic factor for patients with resected PDAC and is a standard treatment following curative surgery.2, 3
However, the optimal time to initiate adjuvant therapy in patients with PDAC remains controversial. Some studies4-9 evaluated patient prognosis stratified by various cut-off timings of adjuvant initiation (8–12 weeks), and the results differed among the studies. Most of these reports focused on the timing of adjuvant initiation; however, it was unclear whether the patients completed adjuvant therapy. Adjuvant completion improves the survival of patients with PDAC, and it is necessary to verify which adjuvant initiation timing and completion contribute to improved survival. Therefore, this study aimed to evaluate the prognostic effect of adjuvant therapy initiation timing and completion in patients with PDAC.
2 METHODS
2.1 Study design
This retrospective cohort study was based on a prospectively maintained institutional database of all patients with PDAC who underwent potentially curative resection at the Department of Surgery, Hiroshima University Hospital, Hiroshima, Japan, between 2006 and 2022. In this study, we initiated adjuvant therapy as early as possible for patients with pathologically confirmed PDAC who recovered sufficiently after surgery. Adjuvant regimens were combined with gemcitabine plus S-1 (GS) therapy10-12 or S-1 monotherapy,13 and radiation therapy was not performed. The exclusion criteria were as follows: (1) patients who did not receive adjuvant therapy, (2) patients who had early recurrence or death within 6 months after surgery, and (3) patients lost to follow-up within 6 months after surgery. We evaluated preoperative patient status, perioperative outcomes, adjuvant status, and survival. This study was approved by the Institutional Review Board of Hiroshima University and conducted in accordance with the principles of the Declaration of Helsinki (approval number: E-2151).
2.2 Definition
This study focused on two factors of adjuvant therapy for patients with PDAC: (1) the initiation timing (early or delayed) and (2) completion status. Early initiation was defined as initiation within 4 weeks after surgery, whereas delayed initiation was defined as initiation beyond 4 weeks after surgery. The number of patients who received adjuvant therapy each week after surgery was also assessed. Completion of adjuvant therapy was defined as a total of 6 months administration after surgery. Tumor stage, lymph node metastasis, and final stage were classified based on the 8th edition of the International Union Against Cancer tumor-node-metastasis classification.14 All eligible patients had their resectability status assessed according to the National Comprehensive Cancer Network Pancreatic Cancer guidelines, version 2.2023.15 The incidence and severity of postoperative complications were graded according to the Clavien–Dindo classification system,16 and grade III or higher complications were considered severe complications. Overall survival was defined as the interval between treatment initiation and the date of death or last follow-up visit.
2.3 Statistical analyses
Analyzed data were described using the medians and interquartile ranges (IQRs) for continuous variables and numbers for categorical variables. Categorical variables were compared using chi-squared or Fisher's exact tests. Deaths from any cause were included in the survival analysis, and survival curves were plotted using the Kaplan–Meier method. Multivariate survival analyses were performed using the Cox proportional hazards model, and hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. Risk factors were evaluated using univariate and multivariate logistic regression models, and odds ratios (ORs) and 95% CIs were reported. All tests were two-sided, and p < 0.05 was considered significant. Statistical analyses were performed using JMP software (version 17.0; SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Patients
The patient flow chart is shown in Figure 1. During the study period, 614 patients received curative resection for PDAC, including 379 resectable (R) and 235 borderline resectable (BR) or locally advanced (LA) cases. Of the 614 patients, 93 without adjuvant initiation after surgery were excluded. In the remaining 521 patients, 73 patients with recurrence or death within 6 months after surgery and four patients who were lost to follow-up within 6 months after surgery were also excluded. The remaining 444 patients, including 294 R-PDAC and 150 BR/LA-PDAC cases, were included in this study; 311 patients were initially administrated combined GS therapy and 133 were administered S-1 monotherapy.
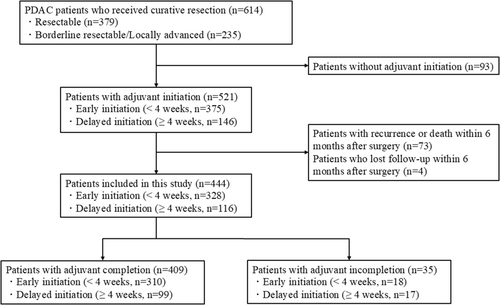
The median time to initiation was 20 days (IQR; 15–29). The eligible 444 patients were classified into two groups: 328 patients (74%) with early initiation within 4 weeks after surgery and 116 (26%) with delayed initiation beyond 4 weeks after surgery. Of the 444 patients, 409 patients (92%), including 310 with early initiation and 99 with delayed initiation, achieved completion of adjuvant therapy, while 35 patients (8%) did not achieve completion. Patient demographics, surgical outcomes, and clinicopathological factors were shown in Table 1 and Table S1. Upon comparison of the patient characteristics and outcomes between early and delayed initiation groups, neoadjuvant therapy, American Society of Anesthesiologists Physical Status (ASA-PS), cardiovascular disease, operation time, and postoperative complications were significantly associated with the initiation timing of adjuvant therapy.
| Variables | Initiation timing of adjuvant therapy | p-Value | |
|---|---|---|---|
| Early, n = 328 (%) | Delayed, n = 116 (%) | ||
| Patient characteristics | |||
| Age, ≥80 years | 34 (10) | 17 (15) | 0.223 |
| Sex, female | 155 (47) | 48 (41) | 0.274 |
| Resectability status, BR/LA | 110 (34) | 40 (34) | 0.853 |
| Neoadjuvant therapy | 115 (35) | 54 (47) | 0.030 |
| ASA-PS, ≥3 | 5 (2) | 8 (7) | 0.006 |
| BMI, ≥25 | 48 (15) | 22 (19) | 0.279 |
| Cardiovascular disease | 24 (7) | 17 (15) | 0.025 |
| Pulmonary disease | 18 (5) | 11 (9) | 0.149 |
| Cancer history | 57 (17) | 18 (16) | 0.643 |
| Diabetes | 112 (34) | 51 (44) | 0.061 |
| Biliary drainage | 111 (34) | 41 (35) | 0.770 |
| PNI, <40 | 131 (40) | 46 (40) | 0.957 |
| Preoperative CA19-9, ≥37 U/mL | 167 (51) | 65 (56) | 0.342 |
| Operative factors | |||
| Procedure, PD | 211 (64) | 76 (66) | 0.818 |
| Portal vein resection | 92 (28) | 37 (32) | 0.435 |
| Arterial resection | 39 (12) | 19 (16) | 0.227 |
| Operation time, ≥400 min | 71 (22) | 37 (32) | 0.034 |
| Estimated Blood loss, ≥1000 mL | 81 (25) | 31 (27) | 0.703 |
| Transfusion | 43 (13) | 20 (17) | 0.281 |
| Postoperative complication, ≥III | 18 (5) | 39 (34) | <0.001 |
| Pathological findings | |||
| Pathological tumor size, ≥30 mm | 177 (54) | 66 (57) | 0.585 |
| Tumor differentiation, grade2/3 | 218 (66) | 71 (61) | 0.310 |
| Lymphovascular invasion, positive | 190 (58) | 76 (66) | 0.149 |
| Perineural invasion, positive | 261 (80) | 99 (85) | 0.164 |
| pT, T3/4 | 64 (20) | 29 (25) | 0.218 |
| pN, positive | 197 (60) | 76 (66) | 0.297 |
| Surgical margin, positive | 58 (18) | 20 (17) | 0.914 |
- Abbreviations: ASA-PS, American Society of Anesthesiologists Physical Status Classification; BMI, body mass index; BR, borderline resectable; CA19-9, Carbohydrate antigen 19–9; LA, locally advanced; PD, pancreatoduodenectomy; PNI, prognostic nutritional index.
3.2 Survival analysis
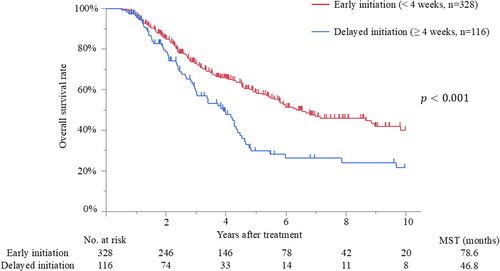
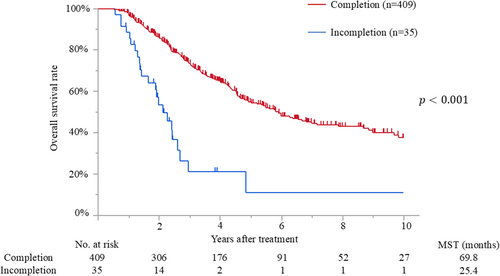
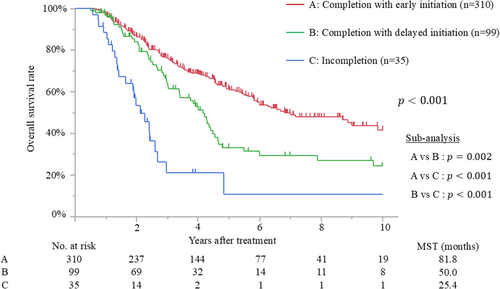
The median follow-up time after surgery for the censored patients was 47.4 months (IQR, 25.2–81.7 months). Median survival and 5-year overall survival rate were 65.0 months and 52%, respectively. Patients with early initiation of adjuvant therapy had better overall survival than those with delayed initiation (median survival time [MST], 78.6 months vs. 46.8 months; p < 0.001) (Figure 2). The MSTs of patients who initiated adjuvant treatment <2, 2–4, 4–6, 6–8, and >8 weeks after surgery were 140.7, 76.4, 46.8, 40.4, and 52.7 months, respectively (p = 0.009). Moreover, patients who completed adjuvant therapy had better survival than those who did not (MST, 69.8 months vs. 25.4 months; p < 0.001) (Figure 3). Recurrence was detected in 228 patients (51%), and the most frequent recurrence site was local (n = 76), followed by lung (n = 62), liver (n = 53), peritoneum (n = 28), and others (n = 9). Multivariate analysis demonstrated that biliary drainage, elevated preoperative CA19-9, pT3/4 status, nodal positive, and surgical margin positive were independent poor prognostic factors, while adjuvant early initiation (HR, 0.66; p = 0.009) and completion (HR, 0.35; p < 0.001) were independent prognostic factors for prolonged survival (Table 2). When the 444 patients were divided into three groups according to adjuvant completion and initiation status: (A) adjuvant completion with early initiation (n = 310), (B) adjuvant completion with delayed initiation (n = 99), and (C) adjuvant incompletion (n = 35), patients who experienced adjuvant completion with early initiation had better survival than those who experienced adjuvant completion with delayed initiation (p = 0.002) and those with adjuvant incompletion (p < 0.001), whereas those who experienced adjuvant completion with delayed initiation had better survival than those with adjuvant incompletion (p < 0.001). These survival curves are shown in Figure 4.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| No. of patients (%) | MST (months) | p-Value | HR | 95% CI | p-Value | |
| Age, ≥80 years | 51 (11) | 86.0 | 0.868 | |||
| Sex, female | 203 (46) | 59.0 | 0.450 | |||
| Resectability status, BR/LA | 150 (34) | 46.8 | 0.005 | 1.18 | 0.83–1.67 | 0.362 |
| Neoadjuvant therapy | 169 (38) | 58.0 | 0.838 | |||
| ASA-PS, ≥3 | 13 (3) | 40.3 | 0.047 | 1.37 | 0.62–3.03 | 0.435 |
| BMI, ≥25 | 70 (16) | 64.1 | 0.807 | |||
| Cardiovascular disease | 41 (9) | 58.0 | 0.375 | |||
| Pulmonary disease | 29 (7) | 46.1 | 0.050 | |||
| Cancer history | 75 (17) | 71.8 | 0.739 | |||
| Diabetes | 163 (37) | 54.1 | 0.029 | 1.25 | 0.94–1.68 | 0.129 |
| Biliary drainage | 152 (34) | 49.1 | <0.001 | 1.60 | 1.17–2.18 | 0.003 |
| PNI, <40 | 177 (40) | 57.7 | 0.120 | |||
| Preoperative CA19-9, ≥37 U/mL | 232 (52) | 54.4 | <0.001 | 1.43 | 1.06–1.92 | 0.019 |
| Procedure, PD | 287 (65) | 57.7 | 0.142 | |||
| Portal vein resection | 129 (29) | 41.5 | <0.001 | 1.23 | 0.84–1.80 | 0.282 |
| Arterial resection | 58 (13) | 48.1 | 0.267 | |||
| Operation time, ≥400 min | 108 (24) | 38.6 | 0.004 | 1.13 | 0.79–1.61 | 0.514 |
| Estimated blood loss, ≥1000 mL | 112 (25) | 42.1 | <0.001 | 1.07 | 0.75–1.55 | 0.699 |
| Transfusion | 63 (14) | 32.6 | <0.001 | 1.35 | 0.89–2.04 | 0.151 |
| Postoperative complication, ≥III | 57 (13) | 44.9 | 0.315 | |||
| pT3/4 | 93 (21) | 35.0 | <0.001 | 1.72 | 1.22–2.42 | 0.002 |
| pN, positive | 273 (61) | 44.9 | <0.001 | 2.34 | 1.64–3.35 | <0.001 |
| Surgical margin, positive | 78 (18) | 32.6 | <0.001 | 1.79 | 1.28–2.51 | <0.001 |
| Adjuvant initiation, early (<4 weeks) | 328 (74) | 78.6 | <0.001 | 0.66 | 0.48–0.90 | 0.009 |
| Adjuvant completion, complete | 409 (92) | 69.8 | <0.001 | 0.35 | 0.21–0.57 | <0.001 |
- Abbreviations: 95% CI, 95% confidence interval; ASA-PS, American Society of Anesthesiologists Physical Status Classification; BMI, body mass index; BR, borderline resectable; CA19-9, carbohydrate antigen 19–9; HR, hazard ratio; LA, locally advanced; MST, median survival time; PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; PNI, prognostic nutritional index.
With respect to the relative dose intensity (RDI) of adjuvant therapy, when patients were divided into high (≥50%, n = 383) and low (<50%, n = 61) RDI groups, patients who received high RDI had significantly better survival than those who received low RDI (MST, 69.7 months vs. 38.7 months; p < 0.001). In comparisons of survival outcomes between adjuvant GS and S-1 therapies, the MSTs of patients with GS and S-1 therapies were 65.9 and 71.8 months, respectively (p = 0.291).
Evaluation of the optimal cut-off timing of adjuvant initiation is demonstrated in Table 3. The MSTs between early and delayed adjuvant initiation were compared, with cut-off timings of adjuvant therapy set from 2 to 10 weeks after surgery. Early groups had significantly better survival than delayed groups when the cut-off timing was 3, 4, 5, and 7 weeks, whereas no significant differences were found between these groups when the cut-off timing was 2, 6, 8, 9, and 10 weeks.
| Cut-off timing | MST (months) | p-Value | |
|---|---|---|---|
| Early | Delayed | ||
| 2 weeks (Early, n = 43/Delayed, n = 401) | 140.7 | 62.5 | 0.131 |
| 3 weeks (Early, n = 245/Delayed, n = 199) | 78.6 | 51.4 | 0.006 |
| 4 weeks (Early, n = 328/Delayed, n = 116) | 78.6 | 46.8 | <0.001 |
| 5 weeks (Early, n = 382/Delayed, n = 62) | 69.7 | 40.7 | 0.022 |
| 6 weeks (Early, n = 412/Delayed, n = 32) | 69.1 | 44.9 | 0.073 |
| 7 weeks (Early, n = 418/Delayed, n = 26) | 69.1 | 44.9 | 0.015 |
| 8 weeks (Early, n = 424/Delayed, n = 20) | 65.9 | 52.7 | 0.166 |
| 9 weeks (Early, n = 429/Delayed, n = 15) | 65.9 | 52.7 | 0.404 |
| 10 weeks (Early, n = 432/Delayed, n = 12) | 65.8 | 53.7 | 0.323 |
- Abbreviation: MST, median survival time.
The analysis of risk factors preventing adjuvant completion with early initiation is shown in Table 4. The multivariate analysis revealed that ASA-PS ≥3 (OR, 4.64; p = 0.024), cardiovascular disease (OR, 2.18; p = 0.036), postoperative complications ≥III (OR, 5.88; p < 0.001), and pT3/4 (OR, 1.77; p = 0.036) were independent risk factors of adjuvant incompletion with early initiation.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| No. of patients (%) | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, ≥80 years | 51 (11) | 1.73 (0.95–3.16) | 0.076 | ||
| Sex, female | 203 (46) | 1.15 (0.77–1.73) | 0.498 | ||
| Resectability status, BR/LA | 150 (34) | 1.31 (0.86–2.00) | 0.213 | ||
| Neoadjuvant therapy | 169 (38) | 1.71 (1.13–2.58) | 0.011 | 1.40 (0.84–2.32) | 0.194 |
| ASA-PS, ≥3 | 13 (3) | 5.51 (1.67–18.2) | 0.003 | 4.64 (1.23–17.5) | 0.024 |
| BMI, ≥25 | 70 (16) | 1.35 (0.79–2.31) | 0.278 | ||
| Cardiovascular disease | 41 (9) | 2.69 (1.41–5.16) | 0.003 | 2.18 (1.05–4.51) | 0.036 |
| Pulmonary disease | 29 (7) | 2.29 (1.07–4.90) | 0.035 | 1.64 (0.70–3.85) | 0.252 |
| Cancer history | 75 (17) | 1.03 (0.60–1.76) | 0.920 | ||
| Diabetes | 163 (37) | 1.36 (0.90–2.06) | 0.146 | ||
| Biliary drainage | 152 (34) | 0.96 (0.63–1.47) | 0.849 | ||
| PNI, <40 | 177 (40) | 1.11 (0.74–1.69) | 0.609 | ||
| Preoperative CA19-9, ≥37 U/mL | 232 (52) | 1.29 (0.86–1.94) | 0.215 | ||
| Procedure, PD | 287 (65) | 1.01 (0.67–1.56) | 0.934 | ||
| Portal vein resection | 129 (29) | 1.23 (0.79–1.91) | 0.357 | ||
| Arterial resection | 58 (13) | 1.93 (1.09–3.39) | 0.025 | 1.32 (0.66–2.64) | 0.428 |
| Operation time, ≥400 min | 108 (24) | 1.67 (1.06–2.63) | 0.029 | 1.38 (0.83–2.29) | 0.209 |
| Estimated blood loss, ≥1000 mL | 112 (25) | 1.25 (0.79–1.97) | 0.348 | ||
| Transfusion | 63 (14) | 1.52 (0.87–2.64) | 0.147 | ||
| Postoperative complication, ≥III | 57 (13) | 6.66 (3.64–12.2) | <0.001 | 5.88 (3.12–11.0) | <0.001 |
| pT, T3/4 | 93 (21) | 1.73 (1.07–2.79) | 0.026 | 1.77 (1.04–3.01) | 0.036 |
| pN, positive | 273 (61) | 1.23 (0.81–1.88) | 0.326 | ||
| Surgical margin, positive | 78 (18) | 1.03 (0.61–1.76) | 0.901 | ||
- Abbreviations: ASA-PS, American Society of Anesthesiologists Physical Status Classification; BMI, body mass index; BR, borderline resectable; CA19-9, carbohydrate antigen 19–9; LA, locally advanced; PD, pancreatoduodenectomy; PNI, prognostic nutritional index.
4 DISCUSSION
Adjuvant therapy is the most important treatment strategy for improving the survival of patients with curatively resected PDAC. Several survival analyses2, 3 of patients with PDAC have revealed that adjuvant therapy is strongly associated with prolonged survival, as well as tumor markers.17, 18 However, the optimal timing of adjuvant initiation remains unclear. During the study period, the treatment policy in which recovered patients received adjuvant therapy as early as possible was consistent. Consequently, the median time to initiation of adjuvant therapy was 20 days, and 74% of eligible patients received adjuvant therapy within 4 weeks after surgery. Moreover, the adjuvant completion rate in this study was relatively high (92%), probably because patients in good condition intended to complete adjuvant therapy. In this study, patients with early recurrence or without adjuvant initiation were excluded, and the results of this study reflect the survival effects of adjuvant therapy in patients with resected PDAC.
Survival analysis revealed that both early initiation and completion of adjuvant therapy were independent prognostic factors. The MST of the adjuvant completion with early initiation group was 81.8 months, a relatively better prognosis than that noted in previous studies, which had MSTs of 22–57 months.4-8 Valle et al.9 demonstrated that six cycles of adjuvant completion rather than early initiation contributed to prolonged survival. Other studies have focused not on adjuvant completion but on adjuvant timing.4-8 Early initiation was better than delayed initiation in some studies,4, 5 whereas it was equivalent in other studies.6-8 This study set the cut-off timing of adjuvant initiation at 4 weeks, relatively earlier than those of previous studies, and found that the early initiation group had better survival than the delayed initiation group. In addition, when the cut-off timings of adjuvant initiation were set at 3, 4, 5, and 7 weeks, the early groups had prolonged survival, whereas there were no significant differences when the cut-off timings were set at 2, 6, 8, 9, and 10 weeks. Some studies6-8 without significant differences between the early and delayed groups set the cut-off timing at 9–12 weeks. These results suggest that the results may differ depending on the cut-off timing between early and delayed initiation.
Interestingly, this study revealed that the adjuvant completion with delayed initiation group had a significantly better prognosis than the incompletion group, but still had a significantly poorer prognosis than the early initiation group. With respect to the recurrence patterns in this study, which excluded patients without adjuvant therapy or early recurrence within 6 months after surgery, 143 (63%) patients had distant metastases in the liver, lung, and peritoneum. Delayed initiation of adjuvant therapy due to postoperative inflammation, immunosuppression, or complications may cause the progression of distant occult metastases.19, 20 From the viewpoint of basic science, Javed et al.21 demonstrated that postoperative circulating tumor cell positivity was associated with poor survival in patients who either experienced delayed initiation or lack of adjuvant therapy. They hypothesized that a delay or lack of adjuvant therapy allowed circulating tumor cells to proliferate and could potentially cause residual systemic disease, subsequently resulting in disease recurrence. In addition, this study suggested that it was insufficient to complete adjuvant therapy, even if delayed, but rather that adjuvant completion with early initiation led to prolonged survival.
The risk factors for delayed or incomplete adjuvant therapy were poor patient status, such as a higher ASA-PS or combined cardiovascular disease, and severe postoperative complications. An American nationwide survey22 revealed that postoperative complications were common following pancreatic surgery and were associated with the omission of adjuvant chemotherapy and treatment delays. Postoperative complications following pancreatic surgery mainly include surgical site infections, such as postoperative pancreatic fistula, abdominal abscess, and anastomotic leakage. Postoperative infections can cause immunodeficiency and delayed recovery. To administer cytotoxic chemotherapy agents to patients after surgery, sufficient recovery and good general condition are required. A multicenter cohort study by the Japanese Society of Surgical Infection23 reported that postoperative infection after pancreatic cancer surgery was strongly associated with adjuvant incompletion and indirectly caused a worse prognosis. In addition, this study demonstrated that postoperative complications were strongly associated with delayed initiation or incomplete adjuvant therapy. Careful perioperative management and surgical skills to prevent postoperative complications are expected to improve the survival of patients with PDAC.
This study has some limitations. First, this was a retrospective cohort study with a relatively small number of patients with PDAC at a single institution. There were several potential biases in patient selection, neoadjuvant strategies, surgical skills, and follow-up strategies over the long study period. However, early adjuvant strategies remained consistent throughout the study period. To overcome these limitations, prospective studies with a larger number of patients with resected PDAC who intend to receive early adjuvant therapy after surgery at multiple institutions are warranted. Second, to prove the survival benefit of early adjuvant initiation, comparisons of the prognostic significance between early and delayed adjuvant therapy in patients with good status and good postoperative course are required. A randomized control trial is warranted. Finally, the adjuvant therapy in this study included only chemotherapy consisting of S-1 and/or gemcitabine. The timing and duration of adjuvant radiation and chemoradiotherapy were not considered. Moreover, the survival benefits of stronger combined regimens24-26 for high-risk patients and precision medicine, including gene or immunotherapy,27-29 should be considered adjuvant strategies in the future.
In conclusion, adjuvant completion with early initiation may contribute to better survival in patients with PDAC, and the prevention of severe postoperative complications may facilitate adjuvant completion with early initiation.
AUTHOR CONTRIBUTIONS
Kenjiro Okada: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; writing – original draft. Kenichiro Uemura: Conceptualization; methodology; project administration; supervision; writing – review and editing. Tatsuaki Sumiyoshi: Conceptualization; data curation; writing – review and editing. Ryuta Shintakuya: Conceptualization; data curation; writing – review and editing. Kenta Baba: Data curation; writing – review and editing. Takumi Harada: Data curation; writing – review and editing. Yasutaka Ishii: Conceptualization; writing – review and editing. Shiro Oka: Conceptualization; writing – review and editing. Yoshiaki Murakami: Conceptualization; writing – review and editing. Shinya Takahashi: Conceptualization; project administration; writing – review and editing.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Nanami Taketomi and Dr. Yasushi Orihashi (Clinical Research Center in Hiroshima, Hiroshima University Hospital) for their expert advice on the statistical analyses and Editage (www.editage.com) for English-language editing.
FUNDING INFORMATION
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests for this article.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The protocol for this research project has been approved by the Ethics Committee of Hiroshima University (Approval No. E-2151) and it conforms to the provisions of the Declaration of Helsinki.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.




