Impact of hepatospleno volume ratio on postoperative chronic liver failure after major hepatectomy for perihilar cholangiocarcinoma
Abstract
Aims
The incidence of postoperative chronic liver failure (PCLF) after major hepatectomy for perihilar cholangiocarcinoma is relatively low, but it warrants careful attention. This study aimed to analyze the risk factors for PCLF, with a specific focus on the correlation with postoperative changes in liver and spleen volumes.
Methods
A total of 172 patients who underwent major hepatectomy for perihilar cholangiocarcinoma between 2006 and 2021 were included in the study. PCLF is defined as the presence of liver failure, such as ascites, esophageal varices, encephalopathy, and jaundice at 3 mo postoperatively. Risk factors, including chronological changes in liver volume, spleen volume, and hepatospleno volume ratio for PCLF, were evaluated by univariate and multivariate analyses.
Results
PCLF occurred in 8 of the 172 patients. On univariate analysis, multiple factors including preoperative prealbumin levels, indocyanine green retention test, and future remnant liver volume were identified as risk factors for PCLF. On multivariate analysis, the hepatospleno volume ratio (p = 0.033) and prealbumin level (p = 0.015) 3 mo after surgery were significantly associated with PCLF. The cutoff value for the hepatospleno volume ratio was 3.0 (area under the curve [AUC]: 0.881, sensitivity: 91.7%, specificity: 66.7%) and that for prealbumin level was 10 mg/dL (AUC: 0.894, sensitivity: 83.3%, specificity: 88.9%).
Conclusion
Hepatospleno volume ratio <3.0 and prealbumin level <10 mg/dL 3 mo after surgery were identified as risk factors for PCLF, implying the importance of postoperative nutritional guidance to preserve the remnant liver function for patients with these risk factors.
1 INTRODUCTION
Postoperative liver failure is a significant concern following surgery for perihilar cholangiocarcinoma, and numerous studies have reported the risk factors for early liver failure, including preoperative liver function, remnant liver volume, intraoperative blood loss, and infections.1-4 In contrast, postoperative late (chronic) liver failure, which occurs over 3 mo after surgery, has received less attention and has not been extensively studied. With recent advancements in perioperative management and surgical techniques, short-term postoperative outcomes have improved, leading to an increase in the number of cases with late postoperative complications. In our facility as well, we have encountered patients exhibiting symptoms of liver failure more than 3 mo after surgery.
Chronic liver failure gradually develops through liver fibrosis, which is usually induced by factors such as alcohol, hepatitis viruses, and fatty liver. Liver fibrosis then induces portal hypertension, splenomegaly, and an increase in the hepatospleno volume ratio, finally leading to liver cirrhosis.5 Compared to this gradual change, liver and splenic volumes rapidly and dramatically change after extensive hepatectomy for perihilar cholangiocarcinoma,6, 7 and postoperative chronic liver failure (PCLF) occurs relatively rapidly during the patient's postdischarge life despite an initial stabilization of liver function in the early postoperative period. Although previous reports indicate that splenomegaly occurs in correlation with liver hypertrophy after extensive hepatectomy for hilar cholangiocarcinoma,7 the impact of liver hypertrophy or splenomegaly on long-term postoperative liver function has not been thoroughly investigated.
In this study, we hypothesized that postoperative liver hypertrophy and splenomegaly may be associated with the risk of PCLF and investigated this hypothesis.
2 METHODS
2.1 Patient selection
This single-center retrospective study included consecutive patients who underwent major hepatectomy for perihilar cholangiocarcinoma at the Cancer Institute Hospital between February 2006 and December 2021. We used the available data to analyze baseline characteristics and patient outcomes. The study population was divided into two groups based on the presence or absence of chronic liver failure. Patients with early recurrence or death within 6 mo and insufficient data were excluded from the analysis. This study was approved by the Institutional Review Board of the Cancer Institute Hospital (registration number: 2023-GB-023). The requirement for written informed consent was waived due to the retrospective nature of the study.
2.2 Preoperative and postoperative evaluation
In this study, in addition to the basic patient data, we also assessed patients' nutritional status using the Glasgow Prognostic Score (GPS), Neutrophil-to-Lymphocyte Ratio (NLR), and Prognostic Nutritional Index (PNI), which were selected for their ability to evaluate systemic inflammation and nutritional reserves. Liver functional reserve was evaluated through the Child–Pugh score and the modified albumin-bilirubin (ALBI) grade. Additionally, we analyzed comorbid conditions, such as diabetes, hypertension, and hyperlipidemia, to account for their potential impact on postoperative outcomes and liver function. Furthermore, we assessed the rate of liver hypertrophy following portal vein embolization (PVE) by calculating the percentage increase in liver volume using pre- and post-PVE computed tomography (CT) scans. The Clavien–Dindo classification was used for postoperative complications. Postoperative liver failure (POLF), and bile leakage were defined as grade B or C according to the International Study Group of Liver Surgery (ISGLS) criteria.8, 9
2.3 Liver and spleen volume evaluation
The patients underwent CT before surgery, 1 wk after surgery, and at 3, 6, and 12 mo after surgery. Using CT data, the liver and spleen volumes were calculated using the 3D volume analyzer SYNAPSE VINCENT software (Fujifilm Medical, Tokyo, Japan). Volume changes in the liver and spleen at each timepoint were measured by comparing the original liver and spleen volumes. The hepatospleno volume ratio was also calculated at each timepoint.
2.4 The definition of PCLF
Postoperative chronic liver failure is defined as the presence of liver failure requiring treatment, such as ascites, esophageal varices, encephalopathy, and jaundice, occurring more than 3 mo after surgery, without recurrence or portal thrombosis at that time.10 Even in cases of postoperative liver failure, the acute phase of liver failure improves and liver failure recurs later; the subsequent episodes of liver failure are defined as chronic liver failure.
2.5 Statistical analysis
Categorical variables were compared using Fisher's exact test or the chi-squared test. Continuous variables were expressed as medians and compared using the Wilcoxon rank-sum test. Factors with p < 0.050 in univariate analyses were entered into the logistic regression model for multivariate analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for each factor. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS v. 26.0 (IBM, Armonk, NY, USA).
3 RESULTS
3.1 Study participants
During the study period, 221 patients underwent major hepatectomy for perihilar cholangiocarcinoma. Postoperative liver failure was observed in 59 patients, with 33 patients classified as grade A, 17 as grade B, and 9 as grade C. Among them, 27 patients with early recurrence or death within 6 mo after surgery and 22 patients with data deficits were excluded from the study. A total of 172 patients were evaluated, and eight patients experienced PCLF. Details of these eight patients are summarized in Table 1. PCLF was observed in six patients who underwent right hepatectomy, one patient who underwent left hepatectomy, and one patient who underwent left trisectionectomy. The initial diagnostic findings of PCLF included varices in four patients, refractory ascites in three, and encephalopathy in one. The median onset of PCLF syndrome was 8 mo (3–51 mo) after surgery.
| No. | Age (yy) | Sex | Operation type | POLF | Initial syndrome | Initial onset after surgery (mo) | Observation period (mo) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 63 | M | RH | B | Encephalopathy | 3.4 | 91 | DOLF |
| 2 | 71 | M | RH + PVR | 0 | Varix | 51.8 | 103 | DOLF |
| 3 | 67 | M | LT + PVR | A | Varix | 27.7 | 99 | Alive |
| 4 | 70 | M | RH + PD | A | Varix | 8.3 | 42 | Alive |
| 5 | 68 | M | RH + PD | B | Varix | 24.1 | 48 | DOLF |
| 6 | 71 | M | RH + PD | A | Ascites | 10.1 | 30 | DOD |
| 7 | 81 | M | LH | 0 | Ascites | 5.8 | 13 | DOLF |
| 8 | 68 | M | RH + PVR | 0 | Ascites | 4.8 | 15 | DOD |
- Abbreviations: DOD, dead of disease; DOLF, dead of liver failure; LH, left hepatectomy with S1 segmentectomy; LT, left trisectionectomy with S1 segmentectomy; M, male; PD, pancreatoduodenectomy; POPF, postoperative liver failure; PVR, portal vein resection; RH, right hepatectomy with S1 segmentectomy.
3.2 Chronological changes in liver, spleen volume, and hepatospleno volume ratio
Among 172 patients, the estimated remnant liver volume, expressed as a percentage of the preoperative volume, was 50.5% at the time of hepatectomy (Figure 1). Liver volume increased to 69.6% 1 wk after surgery, followed by a gradual and subsequent increase over time. Finally, the liver volume reached 82.1% at the time of 1 y after surgery.

In contrast, the spleen volume increased to 134.6% of the original spleen volume at 1 wk postoperatively, then stabilized for a while, and finally decreased slightly to 125.4% at 1 y postoperatively. The hepatospleno volume ratio was 9.4 before surgery, 5.3 at 1 wk after surgery, and increased gradually to 6.1 at 1 y after surgery.
3.3 Patients' characteristics and perioperative outcomes
The patient characteristics and preoperative factors are shown in Table 2. The analyses, including nutritional status, comorbidities, liver function assessment, and the rate of liver hypertrophy following PVE revealed no significant differences between the two groups. Body mass index (BMI) and indocyanine green (ICG) R15 levels were significantly higher in the PCLF group than in the non-PCLF group. In the PCLF group, prealbumin level, platelet count, future remnant liver volume, and hepatospleno volume ratio before surgery were lower than those in the non-PCLF group.
| Variables | PCLF+ (n = 8) | PCLF− (n = 164) | p Value |
|---|---|---|---|
| Age, yy | 69 (63–81) | 69 (38–85) | 0.768 |
| Sex, female/male | 0 (0%)/8 (100%) | 46 (28%)/118 (72%) | 0.080 |
| BMI, kg/m2 | 23.9 (20.5–27.5) | 21.8 (15.0–29.7) | 0.038 |
| Diabetes, −/+ | 7 (87%)/1 (13%) | 146 (89%)/18 (11%) | 0.893 |
| Hypertension, −/+ | 4 (50%)/4 (50%) | 121 (74%)/43 (26%) | 0.141 |
| Hyperlipidemia, −/+ | 8 (100%)/0 (0%) | 150 (91%)/14 (9%) | 0.389 |
| GPS, 0/1/2 | 4 (50%)/2 (25%)/2 (25%) | 102 (62%)/39 (23%)/23 (15%) | 0.663 |
| NLR | 2.5 (1.4–3.9) | 2.0 (0.4–10.6) | 0.579 |
| PNI | 43 (29–46) | 44 (28–56) | 0.130 |
| Child–Pugh, A/B/C | 7 (87%)/1 (13%)/0 (0%) | 157 (96%)/7 (4%) | 0.280 |
| mALBI grade, 1/2a/2b/3 | 1 (13%)/2 (26%)/5 (61%)/0 (0%) | 41 (25%)/58 (35%)/65 (40%)/ 0 (0%) | 0.427 |
| Neoadjuvant chemotherapy, −/+ | 8 (100%)/0 (0%) | 155 (94%)/9 (6%) | 0.496 |
| Cholangitis, −/+ | 4 (50%)/4 (50%) | 130 (8%)/34 (92%) | 0.051 |
| Biliary drainage, −/+ | 2 (25%)/6 (75%) | 34 (21%)/130 (79%) | 0.772 |
| Alb, g/dL | 3.5 (2.7–3.8) | 3.6 (2.6–4.7) | 0.119 |
| Prealbumin | 13.3 (8.1–24.1) | 21.4 (6.1–37.2) | 0.017 |
| Platelet, ×104/μL | 175 (77–277) | 234 (97–576) | 0.028 |
| T. Bil, μmol/L | 0.8 (0.5–1.7) | 0.8 (0.3–3.2) | 0.870 |
| PT, % | 93 (72–100) | 97 (69–130) | 0.093 |
| ICGR 15, % | 14.7 (4.1–34.0) | 10.6 (2.8–30.8) | 0.038 |
| FRLV, % | 40.4 (27.1–56.1) | 50.9 (28.3–84.3) | 0.034 |
| Hepatospleno volume ratio (preoperative) | 6.0 (3.8–9.5) | 9.6 (3.6–30.2) | 0.003 |
| PVE, −/+ | 1 (13%)/7 (87%) | 77 (47%)/87 (53%) | 0.056 |
| Rate of hypertrophy after PVE, % | 13.4 (1.3–19.0) | 9.4 (1.6–21.5) | 0.512 |
| Hepatectomy | |||
| S1, 5, 6, 7, 8 | 6 (75%) | 75 (46%) | 0.481 |
| S1, 2, 3, 4 | 1 (13%) | 65 (40%) | |
| S1, 4, 5, 6, 7, 8 | 0 (0%) | 4 (2%) | |
| S1,2,3,4,5,8 | 1 (13%) | 14 (9%) | |
| Others | 0 (0%) | 6 (4%) | |
- Note: Continuous data are presented as medians and ranges.
- Abbreviations: BMI, body mass index; FRLV, future remnant liver volume; GPS, Glasgow prognostic score; ICG, indocyanine green; NLR, neutrophile-lymphocyte ratio; PCLF, postoperative chronic liver failure; PNI, prognostic nutrition index; PVE, portal vein embolization.
The perioperative and postoperative characteristics of the patients are presented in Table 3. Postoperative complications showed no significant differences between the two groups. In the PCLF group, the prealbumin levels at 3 mo after surgery were significantly lower than those in the non-PCLF group.
| Variables | PCLF+ (n = 8) | PCLF− (n = 164) | p Value |
|---|---|---|---|
| Operation time, min | 657 (571–751) | 593 (392–966) | 0.087 |
| Bleeding, ml | 935 (400–2630) | 895 (80–5750) | 0.621 |
| Vascular resection, −/+ | 6 (75%)/2 (25%) | 120 (73%)/44 (27%) | 0.909 |
| HPD, −/+ | 5 (63%)/3 (37%) | 137 (84%)/27 (16%) | 0.126 |
| POLF (Grade ≧B), −/+ | 6 (75%)/2 (25%) | 152 (93%)/12 (7%) | 0.074 |
| Biliary fistula, −/+ | 8 (100%)/0 (0%) | 136 (83%)/28 (17%) | 0.202 |
| Clavien–Dindo classification≧3a, −/+ | 4 (50%)/4 (50%) | 127 (77%)/37 (23%) | 0.075 |
| Adjuvant chemotherapy, −/+ | 7 (86%)/1 (14%) | 128 (78%)/36 | 0.525 |
| Prealbumin (3 mo) | 6.3 (3.0–9.4) | 14.7 (4.4–26.4) | 0.001 |
| Liver volume (3 mo), % | 61.5 (57.8–98.1) | 76.8 (42.7–129.6) | 0.107 |
| Spleen volume (3 mo), % | 162.7 (98.7–206.1) | 131.5 (72.9–301.6) | 0.149 |
| Hepatospleno volume ratio (3 mo), % | 3.1 (2.4–4.5) | 5.6 (2.0–17.5) | 0.002 |
- Note: Continuous data are presented as medians and ranges.
- Abbreviations: HPD, hepatopancreatoduodenectomy; PCLF, postoperative chronic liver failure; POLF, postoperative liver failure.
The hepatospleno volume ratio at 3 mo after surgery was 3.1 in the PCLF group and 5.6 in the non-PCLF group. The PCLF group had a significantly larger spleen compared to the liver than the non-PCLF group (p = 0.002).
3.4 Multivariable analysis to identify predictive risk factors for PCLF
On univariable analysis, BMI (p = 0.038), prealbumin (p = 0.017), platelet (p = 0.028), ICG R15 (p = 0.038), FRLV (p = 0.034), hepatospleno volume ratio before surgery (p = 0.003), prealbumin at 3 mo after surgery (p = 0.001), and hepatospleno volume ratio at 3 mo after surgery (p = 0.002) were significantly associated with the incidence of PCLF (Tables 2, 3). On multivariate analysis, prealbumin at 3 mo after surgery (HR: 0.606, 95% CI 0.404–0.908, p = 0.015) and hepatospleno volume ratio at 3 mo after surgery (HR: 0.095, 95% CI 0.011–0.823, p = 0.033) were independent risk factors for PCLF (Table 4).
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| BMI | 2.462 | 0.400–15.11 | 0.331 |
| Prealbumin | 0.444 | 0.122–1.622 | 0.220 |
| Platelet | 1.021 | 0.960–1.085 | 0.508 |
| ICGR 15 | 0.515 | 0.132–2.006 | 0.339 |
| FRLV | 0.829 | 0.491–1.401 | 0.484 |
| Prealbumin (3 mo) | 0.606 | 0.404–0.908 | 0.015 |
| Hepatospleno volume ratio (preoperative) | 1.675 | 0.867–3.235 | 0.124 |
| Hepatospleno volume ratio (3 mo) | 0.095 | 0.011–0.823 | 0.033 |
- Abbreviations: BMI, body mass index; FRLV, future remnant liver volume; ICG, indocyanine green; PCLF, postoperative chronic liver failure.
3.5 ROC analysis to predict PCLF
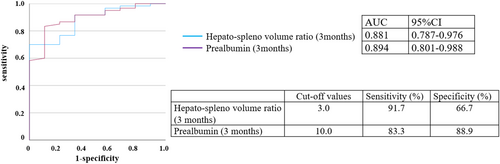
Receiver operating characteristic (ROC) analysis of the two variables that were independent factors for PCLF revealed that prealbumin level and hepatospleno volume ratio at 3 mo after surgery were predictors of PCLF (AUC, 0.894; 95% CI, 0.801–0.988, AUC, 0.881: 95% CI, 0.787–0.976, respectively) (Figure 2). Based on the results of the ROC analysis, the best cutoff values for prealbumin and hepatospleno volume ratio at 3 mo after surgery to predict PCLF were 10 mg/dL and 3, respectively. Hepatospleno volume ratio <3 at 3 mo after surgery showed an accuracy with a sensitivity of 91.7% and specificity of 66.7%, as for prealbumin <10 mg/dL at 3 mo after surgery, with a sensitivity of 83.3% and specificity of 88.9%. When both cutoff values were met, PCLF occurred in five of six patients (83.3%).
3.6 Analysis of patients with low liver regeneration
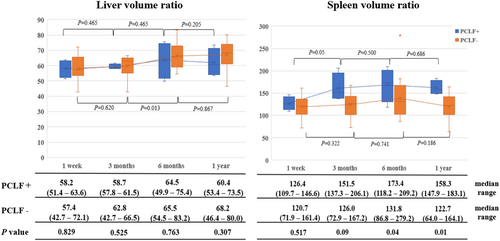
Although impaired liver regeneration was not a risk factor for PCLF in the multivariate analysis, the decrease in liver regeneration at 3 mo postoperatively may reflect a decline in liver function. Therefore, we compared cases with low liver regeneration below the 25th percentile (≤66.6%) at 3 mo postoperatively, comparing those who developed PCLF to those who did not. In cases without PCLF, there were significant preoperative decreases in platelet count (149 × 104/μL vs. 233 × 104/μL, p = 0.013) and a trend toward a poorer indocyanine green clearance rate (13.9 vs. 10.4, p = 0.081) compared to cases that developed PCLF (Table 5). Additionally, examining the postoperative chronological changes in the spleen and liver volumes revealed that patients without PCLF exhibited favorable liver hypertrophy at 1 y postoperatively compared to patients with PCLF (68.2 vs. 60.4, p = 0.307), with a significantly lower spleen volume (122.7 vs. 158.3, p = 0.01) (Figure 3).
| Variables | PCLF+ (n = 4) | PCLF− (n = 20) | p Value |
|---|---|---|---|
| Age, y | 69 (63–71) | 72 (55–80) | 0.157 |
| Sex, female/male | 0 (0%)/4 (100%) | 7 (35%)/13 (65%) | 0.160 |
| BMI, kg/m2 | 23.6 (20.5–26.9) | 21.2 (15.5–25.3) | 0.135 |
| Alb, g/dL | 3.5 (2.7–3.7) | 3.6 (3.0–4.1) | 0.273 |
| Prealbumin | 13.3 (8.1–24.1) | 20.3 (11.1–25.2) | 0.291 |
| Platelet, ×104/μL | 149 (127–199) | 233 (145–477) | 0.013 |
| T. Bil, μmol/L | 0.8 (0.6–1.7) | 0.9 (0.3–2.7) | 0.737 |
| PT, % | 94 (93–100) | 96 (81–126) | 0.911 |
| ICGR 15, % | 13.9 (11.9–34.0) | 10.4 (6.0–25.4) | 0.081 |
| FRLV, % | 33.2 (27.1–54.6) | 43.7 (33.2–62.7) | 0.115 |
| Hepatospleno volume ratio (preoperative) | 7.1 (4.9–9.5) | 8.7 (3.6–14.9) | 0.347 |
| PVE, −/+ | 0 (0%)/4 (100%) | 2 (10%)/18 (90%) | 0.509 |
| Hepatectomy | |||
| S1, 5, 6, 7, 8 | 4 (100%) | 14 (70%) | 0.659 |
| S1, 2, 3, 4 | 0 (0%) | 2 (10%) | |
| S1, 4, 5, 6, 7, 8 | 0 (0%) | 1 (5%) | |
| S1, 2, 3, 4, 5, 8 | 0 (0%) | 3 (15%) | |
| Others | 0 (0%) | 0 (0%) | |
| Operation time, min | 662 (602–751) | 588 (445–795) | 0.135 |
| Bleeding, mL | 815 (400–1450) | 875 (440–3370) | 0.525 |
| Vascular resection, −/+ | 3 (75%)/1 (25%) | 16 (80%)/4 (20%) | 0.822 |
| HPD, −/+ | 3 (75%)/1 (25%) | 16 (80%)/4 (20%) | 0.822 |
| POLF (Grade ≥ B), −/+ | 3 (75%)/1 (25%) | 17 (85%)/3 (15%) | 0.624 |
- Note: Continuous data are presented as medians and ranges.
- Abbreviations: BMI, body mass index; FRLV, future remnant liver volume; HPD, hepatopancreatoduodenectomy; ICG, indocyanine green; PCLF, postoperative chronic liver failure; POLF, postoperative liver failure; PVE, portal vein embolization.
4 DISCUSSION
This study focused on PCLF after major hepatectomy for perihilar cholangiocarcinoma and examined the associated risk factors. The findings revealed that prealbumin level and hepatospleno volume ratio at 3 mo after surgery emerged as significant predictors of PCLF. Patients exhibiting positive outcomes for these factors warrant enhanced monitoring, necessitating careful attention to nutritional interventions, alcohol abstinence, and varix screening beyond the standard follow-up protocols.
Few studies have focused on liver regeneration and spleen hypertrophy following hepatectomy for perihilar cholangiocarcinoma. In our study, the liver volume at 1 y after hepatectomy for perihilar cholangiocarcinoma was 82.1%. Nagino et al reported that the liver volume at 1 y was 76%, which was lower than that in our data.6 This may be due to the fact that the report by Nagino et al only examined right hepatectomy for perihilar cholangiocarcinoma. Similar to liver regeneration, spleen volume has been reported to predominantly increase, which is consistent with our findings. Ando et al reported a 1.74-fold increase in spleen volume 1 y after right hepatectomy for hilar cholangiocarcinoma.7 In contrast, our investigation found an overall increase of ~1.25-fold, with a 1.42-fold increase after right hepatectomy and a 1.19-fold increase after left hepatectomy. Interestingly, examination of the chronological changes in the volumes of the liver and spleen revealed a rapid increase in both organs for up to 3 mo. Subsequently, the liver continued to increase slowly, while the spleen showed a tendency to decrease after peaking at 6 mo, indicating the stabilization of liver function and the role of the spleen as a reservoir that regulates portal vein pressure. Nevertheless, patients with a high rate of spleen volume increase at 3 mo postoperatively, especially those with low liver enlargement rates, tend to maintain high spleen volume and low liver regeneration rates even 1 y after surgery. These results support the findings of our study, suggesting that PCLF cannot be predicted solely by a decrease in liver regeneration rate or an increase in spleen volume, but appears to arise from the simultaneous occurrence of both factors.
Rapid elevation of portal vein pressure occurs after liver resection, subsequently causing the spleen volume to increase.11, 12 Persistent portal hypertension is known to elevate the levels of multiple cytokines, and this association is closely linked to impaired liver regeneration and the progression of liver fibrosis.13-16 Dysregulation of cytokines can significantly contribute to the pathological changes observed in the liver, affecting its ability to regenerate, and leading to fibrosis. In the context of liver transplantation, portal vein pressure (PVP) plays a crucial role in the postoperative course. Splenectomy has been reported as a means to reduce PVP in patients with portal hypertension, leading to improved postoperative outcomes.17 Yao et al proposed a splenectomy algorithm for the PVP modulation strategy, and other positive aspects of splenectomy have also been frequently reported,18 particularly the prevention of portal hypertension and the improvement of hepatic vascular compliance.19-21 Similar to liver transplantation, case reports have documented the successful treatment of portal hypertension and postoperative liver failure after major hepatectomy through splenectomy for portal vein pressure modulation.22 Considering these reports, splenectomy has the potential to alleviate symptoms in patients with PCLF.
Eight of the 172 patients were diagnosed with PCLF in this study, of whom four ultimately died of liver failure, indicating a high mortality rate. This is similar to the pathogenesis of acute-on-chronic liver failure (ACLF), which is an acute deterioration of liver function in patients with cirrhosis that progresses to liver failure in a short period of time due to exacerbating factors such as increased systemic inflammatory cytokines, including bleeding and infection.23 ACLF requires multidisciplinary treatment for multiple organ failure, and fatality rates of 50–90% have been reported.24 Similar to patients with ACLF, patients at high risk of POLF may benefit from a multidisciplinary treatment approach. This approach may involve nutritional therapy, alcohol abstinence, and screening for gastrointestinal varices using gastrointestinal endoscopy.25 Implementing a comprehensive plan that addresses various aspects of patient care can contribute to better outcomes and management of patients at risk of postoperative liver complications.26, 27
The present study had some limitations. First, it had a small sample size and was retrospective. A larger number of cases should be considered and prospective cases should be reviewed. Second, this study did not evaluate the degree of steatosis and liver fibrosis pathologically in the background liver of the resected specimens, nor assess PVP during surgery. These factors could provide additional insights for predicting PCLF and may need to be investigated in future studies. Third, while we defined PCLF as symptoms of liver failure 3 mo postoperatively, this definition of PCLF is not universally accepted. Postoperative liver failure in the perioperative period has been defined previously9; however, the concept of chronic liver failure postoperatively is not commonly defined. It is necessary to further investigate the pathophysiology of PCLF in a larger number of cases and consider a more widely accepted definition of PCLF.
In conclusion, the present study highlights the significance of the hepatospleno volume ratio and prealbumin levels at 3 mo postoperatively in assessing the risk of PCLF. For patients at high risk of PCLF, postoperative intensive nutritional guidance is recommended to preserve the remnant liver function.
AUTHOR CONTRIBUTIONS
Atsushi Takahashi: Conceptualization; data curation; formal analysis; writing – original draft; writing – review and editing. Yoshihiro Ono: Conceptualization; data curation; writing – review and editing. Kosuke Kobayashi: Conceptualization. Atsushi Oba: Conceptualization. Takafumi Sato: Conceptualization. Hiromichi Ito: Conceptualization. Yosuke Inoue: Conceptualization. Akio Saiura: Conceptualization. Yu Takahashi: Conceptualization.
ACKNOWLEDGMENTS
We express our thanks to members of the Division of Hepatobiliary Pancreatic Surgery at Cancer Institute Hospital who gave their advice and support during this research.
FUNDING INFORMATION
No funding was available for this study. No specific grants from funding agencies in the public, commercial, or not-for-profit sectors were received to fund this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest for this article.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: This study was approved by the Institutional Review Board of the Cancer Institute Hospital (registration number: 2023-GB-023).
Informed Consent: This study was conducted in accordance with the guidelines of the Declaration of Helsinki. There was no need for consent to participate due to this being a retrospective study. The opt-out method to obtain patient consent was utilized.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.




