Evaluation value of neutrophil gelatinase-associated lipocalin for the renal dysfunction of patients with chronic kidney disease: A meta-analysis
Abstract
Objective
The role of neutrophil gelatinase-associated lipocalin (NGAL) for the evaluation of renal function in chronic kidney disease (CKD) has not yet to be determined. We aimed to perform a meta-analysis exploring the correlation between NGAL and glomerular filtration rate (GFR) in CKD patients, and to further identify factors affecting NGAL's performance.
Methods
Studies dated before November 2017 were retrieved from PubMed, Embase, Web of Science, and the Cochrane Library. A total of 28 relevant studies (involving 3082 patients from 17 countries) were included. The second version of the Quality Assessment for Studies of Diagnostic Accuracy demonstrated that no significant bias had influenced the methodological quality of the included studies.
Results
Neutrophil gelatinase-associated lipocalin showed a strong negative correlation with measured glomerular filtration rate (mGFR). The pooled correlation coefficient (r) with corresponding 95% confidence intervals for the correlation between serum NGAL (sNGAL) and GFR was −0.48, meanwhile that for urine NGAL (uNGAL) and GFR was −0.34. However, NGAL's performance is different in subgroups restricted by clinical settings, race, sex, age, and staging of renal function.
Conclusion
Neutrophil gelatinase-associated lipocalin could be a renal function evaluation marker for patients with renal dysfunction in CKD. Compared with uNGAL, there was a significant negative correlation between sNGAL and GFR. The performances of sNGAL and uNGAL were restricted by clinical factors that should be considered in regards to the sampling source selection.
1 INTRODUCTION
Chronic kidney disease (CKD) is a major public health problem and typically evolves over many years. The prevalence of all stages of CKD varies between 7% and 12% in the different regions of the world.1 CKD G3-G5 prevalence in adults varies worldwide, with values reported as 1.7% in China,2 3.1% in Canada,3 5.8% in Australia,4 and 6.7% in the United States.5 In order to screen out the severe disease earlier and more accurately, glomerular filtration rate (GFR) is still regarded as the ideal marker of kidney function. Unfortunately, measuring GFR is time-consuming and therefore GFR is usually estimated from equations that take into account endogenous filtration markers, such as serum creatinine (SCr) and cystatin C (CysC).6, 7 Another important biomarker, albuminuria,8, 9 precedes kidney function decline and has been demonstrated as having strong associations with disease progression and outcomes. Recently, more studies have focused on finding new potential biomarkers for detecting early kidney damage.
Neutrophil gelatinase-associated lipocalin (NGAL), a ubiquitous 25-kDa lipocalin iron-carrying protein,10 was originally isolated from neutrophils.11 Then people found that it also expressed in tissues such as kidney, liver, epithelial cells,12, 13 and vascular cells in atherosclerotic plaques.14 It attracted the attention of clinical scientists for it was found to be one of the earliest, most robustly induced genes and proteins in the tubular epithelium of the distal nephron and it was released from tubular epithelial cells following tissue damage, such as ischemic renal injury.15 Further studies were carried out on multiple molecular forms in urine. In contrast to the dimeric form produced by neutrophils, the monomeric form originates from kidney tubular epithelial cells.16 This difference has the potential to improve the specificity of NGAL as a renal biomarker. NGAL has been identified as an early biomarker of acute kidney injury (AKI).17, 18, 15, 19-23 AKI is increasingly recognized as a prelude to CKD. In an experimental study in rats, ongoing inflammation and immune activity were found to be involved with the pathogenesis of CKD, and NGAL was upregulated, suggesting that it may be a valuable biomarker for the development of CKD after AKI.24, 25 NGAL has recently been proven useful to quantitate CKD.26 Thus, there has been interest in NGAL as an additional measure of kidney impairment in CKD.
Baseline serum NGAL (sNGAL) and urine NGAL (uNGAL) were well correlated with residual GFR in CKD and have been shown to be excellent indicators of renal function in patients affected by autosomal dominant polycystic kidney disease.27 In a cross-sectional study performed on 80 nondiabetic patients with CKD Stages 2-4, sNGAL rose gradually, reaching a higher value in Stage 4 CKD, and was related to measured glomerular filtration rate (mGFR).28 A prospective cohort trial of 96 patients with CKD has shown that baseline sNGAL and uNGAL were predictors of mGFR decline.29 Another study of children with CKD proved the significant correlations between sNGAL and uNGAL and mGFR.30 Since then, concerns focused on NGAL for CKD prediction have been accelerating.
However, Liu et al31 conclude that uNGAL levels do not improve risk prediction of progressive CKD. A study with type 2 diabetic patients showed that of the urinary tubular markers, NGAL was not significantly increased in the early stage of diabetic nephropathy with normoalbuminuria and microalbuminuria.32 Another cross-sectional study with type 1 diabetic patients revealed no correlation between uNGAL level and GFR.33 A matched case-control study showed that with adjustment for urinary creatinine and albumin concentration, the association between NGAL and incident CKD stage was not significant.34 Another study of 140 diabetic nephropathy patients also showed that unlike sNGAL, the uNGAL level did not change significantly throughout the varying degrees of CKD and lacked clinical value in predicting the GFR decline rate.35 Based on these controversial results, we conducted the present meta-analysis to investigate the evaluation value of sNGAL and uNGAL for GFR decline in CKD, respectively, and to further identify which factors affect its performance.
2 METHODS
2.1 Data sources and search strategy
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,36 we searched PubMed, EMBASE, Web of Science, and the Cochrane Library from inception to November 2017. The following terms were used: CKD, chronic kidney disease, chronic renal failure, chronic renal insufficiency, chronic renal dysfunction, and neutrophil gelatinase-associated lipocalin. References of the selected studies were further screened manually to identify whether additional eligible articles were available or not.
2.2 Study selection
The inclusion criteria of this study were composed of the following characteristics: (a) investigation of the relationship between NGAL and eGFR/mGFR; (b) randomized controlled trial; (c) original data of Pearson correlation coefficient; and (d) the mGFR measured by nuclear medicine techniques, such as 99Tc-diethylene triamine pentaacetic acid (99Tc-DTPA) or 51Cr-ethylenediamine tetra-acetic acid (51Cr-EDTA), or calculated by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation or Modification of Diet in Renal Disease (MDRD) formula or 24-hour creatinine clearance rate. If any disagreement existed, two investigators would check and discuss the full text. Authors were contacted when there were incomplete or missing data. Ethics approval and patient consent were not in need for this study.
2.3 Data extraction and quality assessment
Two investigators (L.L.G. and Y.Y.Z.) independently extracted information from each article using a standardized collection form. Collected parameters included the first author, publication year, clinical setting, region, age, sex, CKD diagnostic criteria, NGAL determination method, and Pearson correlation coefficient. Differences were resolved by consensus or the third researcher (W.H.Z.).
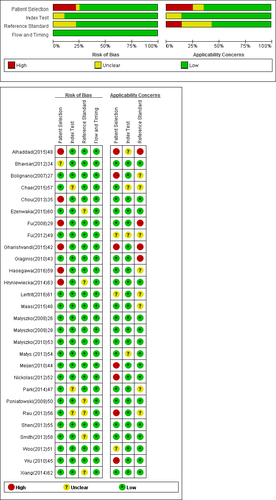
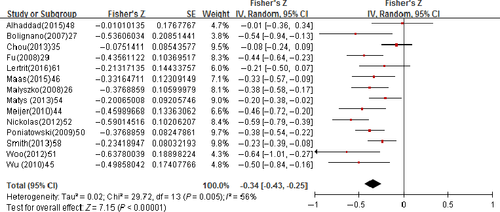
We investigated the methodological quality of the present study using the second version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2).37 The QUADAS-2 assesses the risk of bias and applicability in four domains: (a) patient selection (consecutive or random sample enrolled, case-control design, and inappropriate exclusions avoided); (b) index test (blinded interpretation of the rules); (c) reference standard (correctly excluded a fracture and blinded interpretation); and (d) flow and timing (appropriate interval between application of the rules and reference standard, all patients received the reference standard and were included in the analysis).
2.4 Statistical analysis
After appropriate conversion, data from the various studies were combined using random effects meta-analyses.38 The heterogeneity of the r values between studies was determined by calculating the Q statistic, derived from the chi-square test, and the inconsistency index (I2).39, 40 A P value < 0.05 or an I2 value >50% suggested heterogeneity.41 If notable heterogeneity was detected, a sensitivity analysis was performed for all studies to further investigate the study heterogeneity.
In a subgroup analysis, studies were stratified by the following: (a) race; (b) age; (c) sex; and (d) clinical settings. Statistical manipulation was performed with the Review Manager 5.3 (Cochrane Collaboration, Copenhagen, Denmark).
3 RESULTS
3.1 Literature search
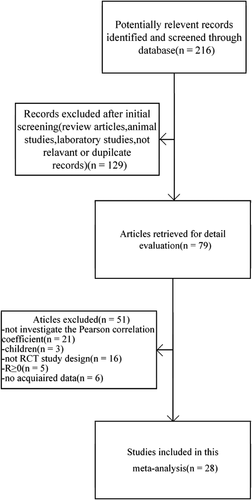
Our research initially identified 216 citations, 87 of which were excluded as they were review articles, animal studies, laboratory reports, pediatric studies, not relevant, or duplicate records. A total of 28 studies26-28, 42-49, 29, 50, 35, 51-58, 34, 59-63 finally met the inclusion criteria via full-text evaluation from 79 potentially eligible citations. A flow chart of the identification and selection process is shown in Figure 1.
3.2 Subject characteristics and quality assessment
The main characteristics of the included studies are summarized in Table 1. A total of 3082 patients (mean age 54.1 years, male 60.2%) from 17 countries were enrolled in the 28 studies. The mean GFR was 54.66 mL/min/1.73 m2. The mGFRs were measured by nuclear medicine techniques, such as 99Tc-DTPA or 51Cr-EDTA, or calculated by CKD-EPI equation, MDRD formula, or 24-hour creatinine clearance rate.
| Study | Country | Age | Setting or study population | Study design | Mean age (y) | M/F ratio | Sample size | mGFR | NGAL assay | Companies |
|---|---|---|---|---|---|---|---|---|---|---|
| Malyszko (2008)26 | Poland | Adult | Non-diabetic patients with CKD Stages 2-4 | Prospectiveobservational cohort | 46.22 | NA | 92 | MDRD | ELISA | ANTIBODYSHOP(Gentofte, Denmark) |
| Bolignano (2007)27 | Italy | Adult | Patients with autosomal-dominant polycystic kidney disease | Prospective observational ohort | 43 | 14/12 | 26 | Cockcroft-Gault formula | ELISA | Antibody Shop, Gentofte, Denmark |
| Malyszko (2009)28 | Poland | Adult | Non-diabetic patients with CKD | Prospective observational cohort | 56.9 | NA | 80 | MDRD | ELISA | ANTIBODYSHOP (Gentofte, Denmark) |
| Gharishvandi (2015)42 | Iran | Adult | Early stages of CKD in high blood pressure | Prospective observational cohort | 54.33 | 10/32 | 42 | Cockcroft-Gault formula | ELISA | Biovender,Norway |
| Giaginis (2010)43 | Greece | Adult | Patients with advanced carotid atherosclerosis | Prospective observational cohort | NA | 114/27 | 141 | MDRD | ELISA | R&D SystemsEurope, Ltd, Abingdon, UK |
| Meijer (2010)44 | Netherlands | Adult | Patients With autosomal dominant polycystic kidney disease | Prospective observational cohort | 40 | 58/1 | 59 | Clearance of iothalamate | ELISA | R&D Systems, |
| Wu (2010)45 | China | Adult | Patients with drug-induced chronic tubulointerstitial nephritis | Prospective observational cohort | 54.3 | 9/27 | 36 | MDRD | ELISA | Antibody Shop, Gentofte, Denmark |
| Maas (2015)46 | Netherlands | Adult | Patients with idiopathic membranous nephropathy | Prospective observational cohort | 51 | 41/28 | 69 | MDRD | ELISA | R&D systems (Minneapolis, MN) |
| Park (2014)47 | Korea | Adult | Patients with immunoglobulin a nephropathy | Retrospective | 35 | 48/43 | 91 | MDRD | ELISA | R&D systems, Minneapolis, MN, USA |
| Alhaddad (2015)48 | Egypt | Adult | Patients with C-related end stage liver disease | Prospective observational cohort | 51.17 | 27/8 | 35 | Tc-99mDTPA | ELISA | Wkea Med Supplies Corp. |
| Fu (2012)49 | China | Adult | Diabetic nephropathy with glomerular hyperfiltration | Prospective observational cohort | 43.1 | 18/12 | 30 | Macisaac's formulae | ELISA | Quantikine R&D SystemsInc., Abingdon, UK |
| Fu (2008)29 | Italy | Adult | Patients with CKD (22% Diabetic Patients) | Prospective observational cohort | 57 | 48/48 | 96 | MDRD | ELISA | Antibody Shop, Gentofte, Denmark |
| Poniatowski (2009)50 | Poland | Adult | Patients with CKD in chronic heart failure and coronary artery disease | Prospective observational cohort | 64.55 | NA | 150 | MDRD | ELISA | Antibody Shop, Gentofte, Denmark |
| Chou (2013)35 | Taiwan | Adult | Patients with type 2 diabetes mellitus | Prospective observational cohort | 58.5 | 72/68 | 140 | MDRD | ELISA | Human NGAL ELISA kit, Abnova Co., CA, US |
| Woo (2012)51 | Korea | Adult | Patients with diabetic nephropathy | Prospective observational cohort | 62.4 | 11/20 | 31 | MDRD | ELISA | ARCHITECT NGAL assay (Abbott Laboratories, Abbott Park, IL, USA) |
| Nickolas (2012)52 | USA | Adult | Patients with CKD (10% diabetic patients) | Prospective observational cohort | 52.2 | 70/29 | 99 | MDRD | ELISA | Alpco, Salem, NH |
| Malyszko (2010)53 | Poland | Elderly | Patients with CKD | Prospective observational cohort | 77.74 | NA | 412 | CKD-EPI | ELISA | BIOPORTO (Gentofte, Denmark) |
| Matys (2013)54 | Poland | Adult | Patients with diabetic nephropathy | Prospective observational cohort | 66.5 | 41/80 | 121 | MDRD | ELISA | AntibodyShop, Gentofte, Denmark |
| Shen (2013)55 | China | Adult | Patients with CKD | Prospective observational cohort | NA | 45/47 | 92 | MDRD | NA | NA |
| Rau (2013)56 | Germany | Adult | Patients with BK virus-associated nephropathy | Retrospective | 50.7 | 5/12 | 17 | MDRD | ELISA | NGAL Rapid ELISA Kit036, BioPorto Diagnostics, Gentofte, Denmark |
| Chae (2015)57 | Korea | Adult | Patients with multiple myeloma | Prospective observational cohort | 60.6 | NA | 199 | MDRD | ELISA | TriageMeterPro (Alere; San Diego, CA) |
| Smith (2013)58 | UK | Adult | Patients with CKD Stages 3 and 4 (25% diabetic patients) | Prospective observational cohort | 69 | 119/39 | 158 | CKD-EPI | ELISA | BioPorto Diagnostics (Gentofte, Denmark) |
| Bhavsar (2012)34 | Germany | Adult | Patients with CKD (18.2% diabetic patients) | Prospective observational cohort | 64.7 | 66/77 | 143 | MDRD | ELISA | Rules BasedMedicine |
| Hasegawa (2016)59 | Japan | Adult | Patients with CKD (35.7% diabetic patients) | Prospective observational cohort | NA | 149/103 | 252 | MDRD | ELISA | BioPorto Diagnostics, Gentofte, Denmark |
| Ezenwaka (2015)60 | Trinidad and Tobago | Adult | Patients with CKD (10% diabetic patients) | Prospective observational cohort | 60.5 | 50/23 | 73 | CKD-EPI | ELISA | Biovendor Laborotoni medicina a.s. Karasek 176/1 621 00Brno Czech Republic |
| Lertrit (2016)61 | Thailand | Adult | Patients with interstitial fibrosis and tubular atrophy in primary glomerulonephritis | Prospective observational cohort | 39 | 18/33 | 51 | CKD-EPI | ELISA | Abbott Laboratories |
| Xiang (2014)62 | China | Adult | Patients with CKD (10% diabetic patients) | Prospective observational cohort | 43.15 | 142/98 | 240 | CKD-EPI | ELISA | Roche, Mannheim, Germany |
| Hryniewiecka (2014)63 | Poland | Adult | Patients with CKD (32.7% diabetic patients) | Prospective observational cohort | 52 | 63/44 | 107 | CKD-EPI | ELISA | R&D Systems, Minneapolis, Minnesota |
- CKD, chronic kidney disease; CKD-EPI, GFR estimated by Chronic Kidney Disease Epidemiology Collaboration formula; GFR, glomerular filtration rate; mGFR, measured GFR; MDRD, GFR estimated by Modification of Diet in Renal Disease formula; NA, not applicable; NGAL, neutrophil gelatinase-associated lipocalin.
The QUADAS-2 plot demonstrated that no significant bias had influenced the methodological quality of the included studies (Figure 2).
3.3 Evaluation value of NGAL for GFR in CKD
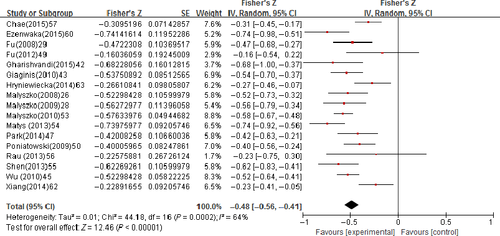
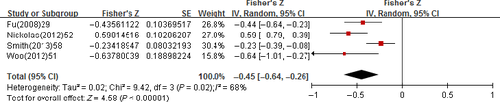
The pooled correlation coefficient (r) with corresponding 95% confidence intervals (CIs) for the correlation between the uNGAL and GFR was −0.34 (95% CI: −0.43 to −0.25); however, an apparent heterogeneity (P = 0.005, I2 = 56%) has to be declared (Figure 3). The pooled r for the correlation between the sNGAL and GFR was −0.48 (95% CI: −0.56 to −0.41); also, an apparent heterogeneity (P = 0.0002, I2 = 64%) has to be declared (Figure 4). The pooled r for uNGAL and GFR in CKD Stage 3-5 was −0.45 (95% CI: −0.64 to −0.26) with notable heterogeneity (P = 0.02, I2 = 68%; Figure 5); meanwhile, the r was −0.60 (95% CI: −0.76 to −0.44) for sNGAL and GFR, and exhibited notable heterogeneity (P = 0.14, I2 = 49%; Figure 6).

3.4 Influence factors affecting NGAL of CKD
The pooled r values estimated for the different subgroups are presented in Tables 2 and 3, based on race, age, sex, and clinical settings. Regarding subgroup analysis, there are sufficient data to support a strong negative correlation between the uNGAL and GFR in Asian patients, aged ≤60 years, and male rate >70%, with no significant heterogeneity. The pooled r value was −0.71 (95% CI: −0.82 to −0.60, P = 0.38, I2 = 0%), −0.51 (95% CI: −0.63 to −0.38, P = 0.2, I2 = 48%), and −0.40 (95% CI: −0.52 to −0.27, P = 0.1, I2 = 49%), respectively. The serum NGAL performed a strong negative correlation with GFR in the non-Asians, elderly, male patients, with no significant heterogeneity. The pooled r value was −0.54 (95% CI: −0.61 to −0.46, P = 0.3, I2 = 40%), −0.57 (95% CI: −0.63 to −0.47, P = 0.2, I2 = 44%), and −0.51 (95% CI: −0.61 to −0.41, P = 0.2, I2 = 41%), respectively. There is a strong negative correlation between uNGAL and mGFR in Asians (r = −0.71, 95% CI: −0.82 to −0.60, I2 = 0%) that is apparently higher than in non-Asia (r = −0.38, 95% CI: −0.47 to −0.3, I2 = 29%). However, the data are opposite in sNGAL, where the r is higher in non-Asians (r = −0.54, 95% CI: −0.61 to −0.46, I2 = 40%) than in Asia (r = −0.33, 95% CI: −0.45 to −0.20, I2 = 56%). Furthermore, we observed that the correlation between uNGAL and GFR is higher in patients aged ≤60 years (r = −0.51, 95% CI: −0.63 to −0.38, I2 = 48%) than in patients aged >60 years (r = −0.23, 95% CI: −0.33 to −0.12, I2 = 59%). However, the correlation between sNGAL and GFR was the opposite: higher in patients aged >60 years (r = −0.57, 95% CI: −0.63 to −0.47, I2 = 44%) than in patients aged ≤60 (r = −0.44, 95% CI: −0.53 to −0.35, I2 = 66%).
| Subgroup | No. of experiments | r (95% CI) | I2 (%) | P value |
|---|---|---|---|---|
| Region | ||||
| Asia | 3 | −0.71 (−0.82 to −0.60) | 0 | 0.38 |
| Non-Asia | 11 | −0.38 (−0.47 to −0.30) | 29 | 0.17 |
| Participant mean age | ||||
| ≤60 y | 7 | −0.45 (−0.55 to −0.36) | 0 | 0.74 |
| >60 y | 7 | −0.23 (−0.33 to −0.12) | 59 | 0.02 |
| Male rate | ||||
| ≤70% | 8 | −0.32 (−0.46 to −0.19) | 60 | 0.01 |
| >70% | 6 | −0.40 (−0.52 to −0.27) | 49 | 0.1 |
| Setting or study population | ||||
| Patients with diabetes mellitus | 6 | −0.49 (−0.56 to −0.32) | 60 | 0.01 |
| Non-diabetic patients | 8 | −0.32 (−0.49 to −0.22) | 0 | 0.92 |
| Subgroup | No. of experiments | r (95% CI) | I2 (%) | P value |
|---|---|---|---|---|
| Region | ||||
| Asia | 6 | −0.36 (−0.49 to −0.23) | 50 | 0.07 |
| Non-Asia | 11 | −0.54 (−0.61 to −0.46) | 40 | 0.3 |
| Participant mean age | ||||
| ≤60 y | 14 | −0.46 (−0.54 to −0.37) | 58 | 0.003 |
| >60 y | 3 | −0.57 (−0.63 to −0.47) | 44 | 0.2 |
| Male rate | ||||
| ≤70% | 10 | −0.45 (−0.57 to −0.34) | 65 | 0.02 |
| >70% | 7 | −0.51 (−0.61 to −0.41) | 41 | 0.2 |
| Setting or study population | ||||
| Patients with diabetes mellitus | 7 | −0.52 (−0.65 to −0.38) | 69 | 0.003 |
| Non-diabetic patients | 10 | −0.46 (−0.55 to −0.37) | 61 | 0.006 |
3.5 Publication bias
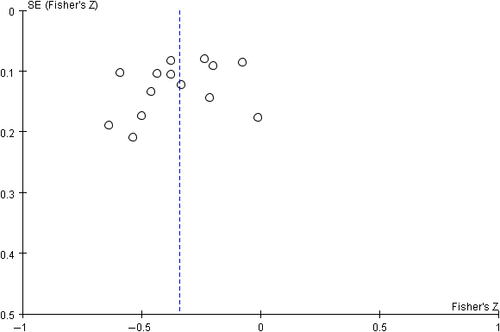
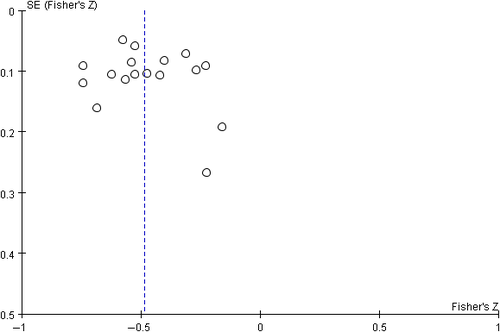
Begg's funnel plot proved that there was no evidence of notable publication bias of the included studies (Figures 7 and 8).
4 DISCUSSION
We performed a systematic review and meta-analysis to clarify the correlation between NGAL and GFR in CKD patients and to investigate whether NGAL could be identified as a maker for kidney function in CKD. As tubular epithelial cells play a crucial role in the pathogenesis of CKD progression, the tubular injury marker NGAL is expected to be useful in reflecting disease activity and kidney function. Many studies showed that urinary and serum NGAL could be biological makers for disease activity and kidney function: immunoglobulin A nephropathy,64 glomerulonephritis,65 pediatric lupus nephritis,66 children with CKD,67 diabetic nephropathy,68 and drug-induced chronic tubulointerstitial nephritis.45 However, discrepancies between several recent prospective studies have resulted in controversy regarding the potential clinical value of uNGAL for CKD.29-35 So far, there have been no specific assays assessing the discriminative value of the two markers in CKD patients. Whether both sNGAL and uNGAL could be satisfactory markers of CKD is still in debate. Therefore, in our systematic review, we sought to provide a more persuasive argument to this debate.
This meta-analysis set rigorous inclusion and exclusion criteria at the very start. One of the essential selected conditions should be randomized controlled trials. After a literature search, 28 studies were finally included. The pooled correlation coefficient with corresponding 95% CIs for the correlation between NGAL and GFR was −0.34 (uNGAL) and −0.48 (sNGAL), respectively. Our findings provide evidence that compared with uNGAL, there was a significant negative correlation between sNGAL and renal function, especially in CKD Stages 3-5. These findings efficiently demonstrated that sNGAL would be better than uNGAL for renal function evaluation in CKD.
Further subgroup analysis indicated several influential factors that should be noted as follows: (a) both sNGAL and uNGAL perform stronger negative correlation with GFR in patients with CKD Stages 3-5 than in the CKD general population; (b) uNGAL shows better renal function evaluation value in Asia and male patients than in non-Asians and female patients; (c) in contrast to uNGAL, the analysis proved significant correlations between sNGAL and GFR in non-Asian, elderly, male patients; and (d) both sNGAL and uNGAL showed a higher correlation with GFR in diabetic nephropathy than in non-diabetic nephropathy.
Otherwise, factors potentially influencing NGAL could be explained by the following factors:
- Different ethnicities and lifestyles, such as diet, which lead to different physical states. The included studies used GFR based on formulas that are different in the West and Asia, and the existing formulas to assess renal function are all just include the black as an influencing factor to adjust the calculated result.
- Kidney aging presents as global glomerulosclerosis and subsequent interstitial fibrosis. Decreasing podocyte density and total numbers are also associated with aging.69 Aging has been reported to be an independent risk of acute on chronic renal failure, and recovery from AKI decreases with aging. Older individuals show a lower rate of full recovery than younger counterparts after acute on chronic renal failure.79
- Sex is an important influencing factor for susceptibility to AKI and young females exhibit the lowest incidence.71 Many studies report that differences in sex may influence the susceptibility, progression, and response to AKI and/or to treatment.72 Evidence shows male sex to be an important risk factor73 and proves higher susceptibility to cisplatin-induced nephrotoxicity than that in females.74 In contrast, the incidence of AKI in women is lower than that in men.75
- Diabetic nephropathy is still demonstrated as the leading cause of end-stage renal disease. Tubular injury plays a critical role in the progression of diabetic nephropathy. High NGAL expression in diabetic nephropathy tubular histology and increased NGAL expressions are independent factors for subsequent rapid GFR decline.76
- The difference of the incidence of other basic diseases, for instance multiple myeloma, immunoglobulin A nephropathy, high blood pressure, and autosomal dominant polycystic kidney disease, may be the nonrenal factors influencing NGAL level.
Why do the two NGAL markers present discriminative values in CKD? It is likely due to the following factors:
- NGAL exists in three main forms: monomer, homodimer, and NGAL/MMP-9 complex.77 The monomer form of NGAL is predominantly released by tubular cells, whereas the homodimer form is mainly released from neutrophils.78, 79 Therefore, different forms may be specific for the different function and causes of CKD.
- The uNGAL consists of more complex forms, originally secreted from neutrophils and excreted by the renal epithelium of distal tubules respectively.11-13, 16
- Theoretically, a serum sample is more stable than urine, as the latter has various influencing factors, such as urinary output, timing of sampling collection, and storage temperature.
For all meta-analyses, heterogeneity is a potential problem when interpreting the results. Heterogeneity is one limitation of this research across all included studies of uNGAL and sNGAL (I2 = 88% and 83%, respectively). However, heterogeneity was inevitable because these studies were based on different institutions and settings worldwide. In these included studies, the NGAL was not assessed by the same assay of NGAL concentration examination. Some different kits for measuring urinary NGAL from multiple companies, such as Abbott or Bioport, were used in the cited reports. However, standard substance of NGAL is not determined between the kits.
Another limitation of this study is the assays used to evaluate GFR. Inulin clearance and nuclear medicine techniques, such as 99Tc-DTPA or 51Cr-EDTA, are considered as the gold standards to define CKD. However, these methods cannot be used routinely in clinical practice because they are invasive and time-consuming.
In conclusion, despite the limitations of our meta-analysis, all currently available evidence supports a strong negative correlation between NGAL and GFR in CKD patients, particularly in Stages 3-5. Several factors should be considered on the sampling choice, from blood or urine. More multicenter randomized controlled trials are needed to further investigate the accuracy of NGAL.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (H0511-81670677), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801), Jiangsu Provincial Key Discipline of Medicine (ZDXKA2016003), Jiangsu Provincial Key Laboratory of Geriatrics, Jiangsu Province's Key Medical Talents Program (ZDRCA2016021), and Jiangsu Province 333 Project (BRA2017409).
CONFLICT OF INTEREST
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
Conception and design: L.L.G. and W.H.Z. Development of methodology: L.L.G., Z.Z.Y. Acquisition of data: L.L.G. and Y.Y.Z. Analysis and interpretation of data: L.L.G., Y.Y.Z., and Z.Z.Y. Writing, review and/or revision of the manuscript: L.L.G., Y.Y.Z., and W.H.Z.




