Brain Organoids: A New, Transformative Investigational Tool for Neuroscience Research
Abstract
Brain organoids are self-assembled, three-dimensionally structured tissues that are typically derived from pluripotent stem cells. They are multicellular aggregates that more accurately recapitulate the tissue microenvironment compared to the other cell culture systems and can also reproduce organ function. They are promising models for evaluating drug leads, particularly those that target neurodegeneration, since they are genetically and phenotypically stable over prolonged durations of culturing and they reasonably reproduce critical physiological phenomena such as biochemical gradients and responses by the native tissue to stimuli. Beyond drug discovery, the use of brain organoids could also be extended to investigating early brain development and identifying the mechanisms that elicit neurodegeneration. Herein, the current state of the fabrication and use of brain organoids in drug development and medical research is summarized. Although the use of brain organoids represents a quantum leap over existing investigational tools used by the pharmaceutical industry, they are nonetheless imperfect systems that could be greatly improved through bioengineering. To this end, some key scientific challenges that would need to be addressed in order to enhance the relevance of brain organoids as model tissue are listed. Potential solutions to these challenges, including the use of bioprinting, are highlighted thereafter.
1 Introduction
The remarkable sight of a 29 year old paraplegic man kicking a football to inaugurate the 2014 FIFA World Cup in Brazil prompted Michio Kaku, the venerable theoretical physicist, to proclaim that the golden age of neuroscience had dawned.1 Observing a man precisely controlling a robotic exoskeleton with his brain would prompt most to arrive at the same conclusion. However, several veterans of the pharmaceutical industry, most notably Paul Marangos, quickly labeled Kaku's comments as deceptive. How could society be entering the golden age of neuroscience, they queried, if it was no nearer to treatments for Alzheimer's disease (AD), stroke, amyotrophic lateral sclerosis (ALS), and other neurological diseases than it was a few decades ago? While the reasons for the absence of effective treatments are manifold and complex, the chronic unavailability of pathophysiologically relevant, living brain tissue continues to be a significant impediment for pharmaceutical development and medical research. To this end, another discipline–tissue engineering–that may or may not be entering a golden age of its own2 may already have a solution to neuroscience's grandest challenges. Organoids are self-assembled, three-dimensionally structured tissues derived from pluripotent stem cells (PSCs).3 Clevers and co-workers at the Hubrecht Institute were the first group to engineer these tissues when, in 2009, they produced gut organoids from adult intestinal stem cells that had been cultured in Matrigel.4 Since then, the list of engineered organoids has grown to include the kidney,5 brain,6 retina,7 and liver.8 Although the suffix “oid” suggests that they resemble actual organs, they do not completely reproduce organ function. Nevertheless, as shown by the work of Fred Gage and his team at the Salk Institute on the generation of functional neural networks upon transplantation of brain organoids in mice brains9 and the observation by Arlotta and co-workers at the Harvard Stem Cell Institute that not only do human brain organoids develop dendritic spines and neural networks in vitro, but that neuronal activity within these tissues can be controlled using light stimulation,10 organoids can recapitulate some functions of the native organ, as well as mimic the microenvironment of the constituent tissue. Organoids are genetically and phenotypically stable over prolonged durations of culturing, which makes them excellent investigational models,11 particularly in drug discovery.
2 Genesis and Maturation of the Organoid Platform
Although it appears that organoids are recent inventions, their intellectual foundations date back to the 1980s. Then, several pioneering studies in developmental and cancer biology, notably those led by Mina Bissell on generating 3D tissue constructs by supporting the cell cultures with a nutrient-rich extracellular matrix (ECM) of the target tissue,12-15 paved the way for fabricating more realistic tissue samples. In due course, the incorporation of PSCs and the newfound ability to induce and control spatiotemporal chemical gradients within the cultures to guide differentiation of the stem cells provided another fillip to producing organ-like structures.16-18 These breakthroughs were stepping stones toward the eventual generation of structured neuronal tissue in 2001, when two groups–one led by Su-Chun Zhang at the University of Wisconsin19 and the other by Benjamin Reubinoff at the Hadassah University Hospital20–contemporaneously produced aggregates of neural progenitor cells (NPCs) in vitro from human embryonic stem cells (ESCs) through the addition of fibroblast growth factor 2 (FGF-2). The study reported by Zhang et al. is especially noteworthy since it was one of the first to demonstrate formation of transplantable, 3D neural tube-like structures from previously 2D, plated embryoid bodies (Figure 1).
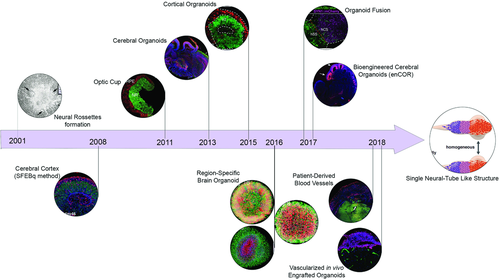
The next leap in neural tissue engineering occurred in 2008, when Yoshiki Sasai's team at the RIKEN Center for Developmental Biology reported the production of self-organized, polarized cortical tissue from serum-free cultures of floating aggregates of ESCs.21 Sasai and his team also confirmed that the regional identities of the cortical tissues could be selectively controlled by programmed addition of neural growth factors such as FGF, Wnt, and bone morphogenetic protein (BMP), and built upon these insights to generate an anatomically complete optic cup from 3D cultures of mouse ESCs 3 years later.21 A team led by Jürgen Knoblich at the Institute of Molecular Biotechnology in Austria subsequently derived complete cerebral organoids exhibiting discrete yet interdependent regions of the brain from human PSCs in 2013.22, 23 Knoblich's protocol can be summarized as a series of timely medium changes in response to specific morphological cues, and the cerebral organoids that are produced accurately reproduce all the major biochemical and physiological milestones that are observed during human cortical development. Significantly, in the same study, Knoblich and his team also created anatomically accurate models of microcephaly by altering the progenitor PSCs through RNA interference, thus becoming one of first groups to employ an engineered, 3D tissue construct derived exclusively from biotic precursors as a model for development and disease. An alternative approach utilizing low-attachment plates for generating cortical organoids from human PSCs was reported by Paşca and co-workers at Stanford University shortly afterward.24 Like Knoblich, Paşca's methodology also generates laminated, spatially organized tissue and, significantly, the organoids are electrophysiologically active and closely resemble fetal brain tissue. Finally, in 2016, a team led by Guo-li Ming and Hongjun Song at Johns Hopkins University “industrialized” the production of brain organoids by designing a miniaturized spinning bioreactor to controllably differentiate human induced PSCs into region-specific brain organoids.25 They subsequently deployed the culturing apparatus to model the exposure of forebrain organoids to the Zika virus, consequently laying the foundations for future efforts in discovery and development of suitable anti-viral agents.
Despite the extensive focus of different groups to develop region-specific brain organoids, the interregional interactions of these parts have yet to be modeled in 3D. Nevertheless, investigations into the migration of neurons from one region to another one when two or more region-specific organoids are fused together, such as a recent study by Knoblich and co-workers,26 have revealed the dynamics of this phenomenon. Likewise, Paşca and his team demonstrated the integration of interneurons between subdomain-specific forebrain spheroids and subsequently probed the fused organoids to identify transcriptional changes associated with neuronal migration.27 These investigations clearly highlight the potential of brain organoids to mimic the complex interactions involved in the development of human brain more closely, as well as their capability to illuminate molecular dynamics of development.28 We anticipate that the next generation of the brain organoids that will be developed using advanced biomaterials and through the use of controlled spatiotemporal patterning will be able to successfully demonstrate microcircuit formation and functional connectivity within a homogenous mixture of different cell types.29
Although each of the examples summarized earlier was a seminal milestone in the path toward de novo, in vitro generation of realistic brain tissue, the works of Knoblich and the team at Johns Hopkins University are particularly important since they were the first to demonstrate the suitability of utilizing organoids to model development and disease, as well as in drug discovery. The next decade in brain organoid engineering will witness focussed improvements in the protocols and tools used to develop even more realistic brain tissues (Figure 2), especially diseased ones, and the nature and scope of the refinements to the tissues will vary greatly depending on the application. For instance, brain organoids, when suitably appended with subtissues such as the blood–brain barrier (BBB),30, 31 will be a significant improvement over existing investigative tools that are used in neuroscience research.
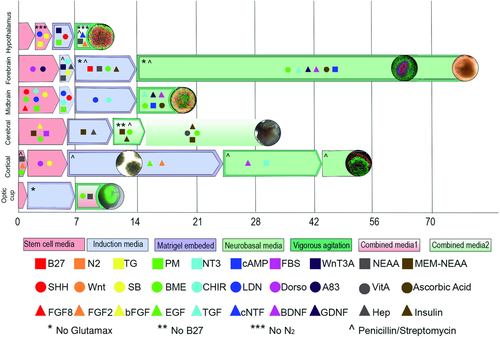
In drug discovery, for example, current 2D in vitro cultures comprising cortical neurons do not incorporate the BBB. As a result, the diffusion of the lead compound across the BBB, which has long been the Achilles' heel of therapies targeting the brain, is never actually evaluated until the molecules are tested in animal models of the disease. Here too, the tissue architecture within rodent brains that are presently utilized for in vivo testing is quite dissimilar to that of the human brain.32 Some experts have also predicted increased use of organoids in regenerative medicine, although this is a more distant prospect for brain organoids.33, 34 We outline some notable applications in the sections that follow and highlight key scientific challenges that would need to be addressed in order to enhance the relevance of brain organoids as model tissues. We will also attempt to provide potential solutions to these challenges, wherever possible, such as the incorporation of emerging biofabrication technologies such as bioprinting and microfluidics.
3 An Attractive Investigational Model
The brain is the most complex organ in the human body, and its development and function is an even more complex cascade of interacting biochemical, physical and physiological processes.35 The biochemical and genetic mechanisms that underpin brain structure and function have been systematically decoded over the past half-century by neuroscientists largely owing to staggering advances in cellular and molecular biology during the same period,36, 37 and the recent mainstreaming of paradigm-altering technologies such as CRISPR/Cas-based genome engineering,38, 39 optogenetics,40 massively parallel single-cell RNA sequencing,41 and metabolomics and proteomics42 have raised very real visions of mapping the brain up from the level of individual cells in unprecedented chemical and genetic detail. Additionally, imaging techniques such as computerized tomography and magnetic resonance imaging have been invaluable tools for visualizing the structure of the brain and identifying anatomical connections, whereas positron emission tomography has permitted monitoring neural activity and overlaying functional and chemical information upon anatomical data.43 Likewise, microscopy has greatly aided assessments of structure and function at the cellular level, and encephalography has contributed to understanding about the response of brain cells and tissues to stimuli as well as measuring mental activity.
While the impact of these diverse technologies and methodologies on advancing understanding about the brain's processes is indisputable, it is clear that they are only as informative as the investigational model on which they are applied. This is especially true for cellular and molecular biology investigations, of which over 90% are conducted using rodent models.44 The rodent and human brains are quite dissimilar, and insights from rodent models do not typically extrapolate well to the human brain.32 The use of brain organoids directly addresses this limitation since they faithfully replicate the biochemical and genetic mechanisms that occur within the human brain. In many respects, brain organoids are the need of the hour for integrative neuroscience.45 Arguably, brain organoids provide their greatest utility in the study of biophysical processes such as neuronal differentiation, migration and connection; tissue morphology, shape and patterning; cortical folding; and response to physicochemical stimuli (Figure 3). These processes greatly influence development of the human brain, and, despite their importance, they remain open questions in the field since animal models cannot accurately replicate them.35
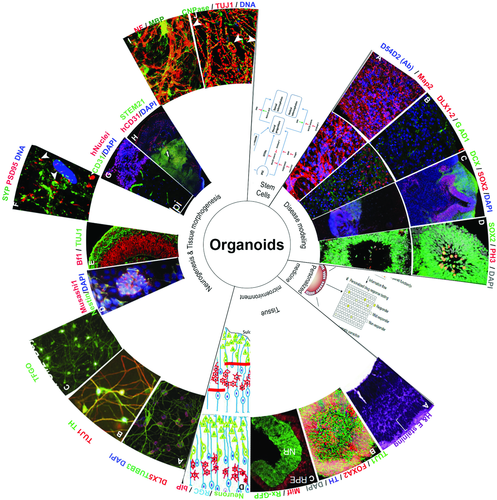
4 Modeling Organogenesis and Early Development
Organogenesis of the brain commences with the recruitment of the neural ectoderm through the receipt of BMP-inhibiting signals from proteins such as noggin.46 The neural ectoderm subsequently transforms into a flat sheet-like structure called the neural plate, which then folds to form the neural groove and, later, the neural tube. The latter is a cylindrical structure composed of apico-basally polarized neuroepithelial cells that are arranged radially around the lumen. While symmetric, proliferative division of the neuroepithelial cells increases the cellular population within the developing neural tube, neurogenesis (or the formation of specialized neural cell types such as neurons, astrocytes and glial cells) is initiated through the transformation of neuroepithelial cells into radial glial cells and basal progenitor cells by the activity of morphogens such as FGF and retinoic acid.47 Morphogens are signalling molecules that, among other roles, activate and control intra- and intercellular signals in tissues, and induce gene expression and regulate transcription and translation within cells.
The neuroepithelial, radial glial and basal progenitor cells subsequently undergo asymmetric and symmetric neurogenic division to produce a denser mass of neural cells, which then further specialize into distinct regions of the brain, the spinal cord and the ventricular system depending on their exposure to morphogens such as sonic hedgehog, Wnt and BMP.48, 49 Recent advances in our ability to isolate and manufacture ESCs50-52 and induce PSCs53 have facilitated the generation of a variety of brain organoids and organoid subtypes that are tailored to address specific questions while preserving biological context. For instance, Zhang and co-workers at the University of Wisconsin have successfully differentiated PSCs into plated cultures of neuroepithelial cells called neural rosettes that closely mimic the radial organization of those cells in the neural tube.19 Although neural rosettes are 2D cultures, their relevance as an investigational model could be enhanced further through the use of layer-by-layer assembly to produce the 3D equivalent of a neural tube. Layer-by-layer assembly has been successfully utilized to construct other tissues, notably cardiac tissue. For example, Ali Khademhosseini's group at Harvard Medical School employed self-assembled graphene oxide-based thin films as an adhesive to link together individual layers of cardiomyocytes and eventually form tightly connected cardiac tissue.54
Brain organoids are particularly informative models to investigate the biochemical, physical and physiological mechanisms that underpin tissue organization and patterning. The intellectual foundations of these investigations were laid by pioneering experiments conducted by H. V. Wilson, who successfully regenerated entire marine sponges from a suspension of their cells.55 This phenomenon was later investigated in greater detail by Weiss and co-workers, who were able to reconstitute entire organs from suspensions of single cells isolated from chicken embryos in advanced stages of differentiation.56 These studies revealed that tissue morphogenesis and organogenesis are driven by two complementary mechanisms that are not only reproducible in vitro, but they can be modulated to produce tissues with defined physiologies. The first mechanism is governed by the “differential adhesion hypothesis,”57 which states that cells with similar degrees of adhesiveness cluster together in order to minimize their free energies, and results in the formation of thermodynamically favorable structures. These emergent microenvironments influence gene expression within the differentiated daughter cells and form the basis of the second mechanism, which commits their phenotype. As a consequence, lineage commitment is spatially restricted58 and produces morphological segmentation within the developing tissues. For example, dissociated neural progenitor cells from early-stage chicken embryos have been shown to produce clusters of neuroepithelial cells that organize radially around a lumen that is eventually filled with fluid, much like how the neural tube forms in vivo.59 Not only do brain organoids recapitulate neural development as it occurs in vivo, but the scales and reliability with which brain organoids can be produced and the relative ease with which they can be handled also makes them ideally suited for use in high-throughput, multiplexed experimentation across time and biological scales. Among other examples, the use of single-cell sequencing-based technologies to explore brain organoids could uncover cellular lineage relationships and provide a spatiotemporal map of the functional state of the constituent cells in unprecedented detail.60
5 Understanding How the Brain Microenvironment Takes Shape
Neurons also originate from neural stem cells (NSCs) that line the ventricles of the neural tube.46 Like the neuroepithelial cells, NSCs too undergo symmetric and asymmetric division to expand and differentiate into neurons, intermediate progenitors and a special class of non-self-renewing cells called non-stem-cell progenitors. NSCs also produce radial glial cells that later specialize into epithelial and astroglial lineages.61-63 Oligodendrocytes and astrocytes, in turn, are produced from the differentiated neurons.64 Much of neurogenesis within the brain originates from division of the NSCs and specialization of radial glial cells, and differential adhesion and spatial restriction of lineage commitment results in thickening of the nascent brain tissue and its subsequent extension to the subventricular zone and cortical plate, and, finally, formation of a multi-tiered structure comprising the medulla, optic tectum and cerebral cortex.65, 66 Neurogenesis and brain development in humans commences after roughly 7 weeks of gestation, and roughly 20 billion neurons are produced and spatially organized over the next 15 weeks.67-69 The foundations of the cerebral cortex are laid during 20 to 35 weeks of gestation, and organization of the neural network commences at 24 weeks and persists throughout the individual's life. In contrast, neurogenesis in mice, which is one of the most common investigational models, lasts for only 6 days and produces tissue that are not as complex as human tissue. As a consequence, investigations into neurogenesis, particularly multi-omics studies for unraveling process kinetics and biochemical mechanisms that guide healthy development of the brain, have been exclusively performed on 2D cultures. The use of brain organoids to model early development in the manner pioneered by Madeline Lancaster48 and I. H. Smart70 could prove to be game-changers for such research.
Likewise, brain organoids have also been used to elucidate stem cell lineage selection within the context of neurodevelopment.6 These processes have been hitherto difficult to replicate in a nonembryonic context.71-73 However, brain organoids provide researchers with a unique platform wherein they can combine the characteristics of stem cells to differentiate and self-organize with the ability to manipulate cellular interactions through genome engineering or the introduction of specific components into the microenvironment, thereby producing reasonably accurate physiological proxies.74, 75 To this end, brain organoids are finding increasing use in modeling the dynamic evolution of the tissue microenvironment, including changes in the population and distribution of neural cells, protein and metabolite content, structural organization and tissue plasticity.76 The impact of exogenous patterning factors and their spatiotemporal gradients on the tissue microenvironment itself, particularly cellular composition and organization, too can be probed using the platform.22, 77, 78 Still, despite their immense utility as an investigational tool, brain organoids nonetheless are incomplete systems since they are almost exclusively composed of NSCs, NPCs, and their progeny. Not only must the missing cell types such as endothelial and immune cells be introduced in order to increase the relevance of brain organoids as a model for studying phenomena such as cortical layering, but also this must be achieved in a manner that is physiologically accurate. We anticipate that the next wave of methodological advances in the production of brain organoids will be made toward this end.
6 Unraveling the Causes of Improper Neuronal Development
Neurodevelopmental disorders and neurodegenerative diseases such as AD, chronic traumatic encephalopathy, and frontotemporal dementia incur high caregiver costs and significantly diminish economic output.79 Treating these debilitating diseases is an urgent priority. Unfortunately, though, there are no treatments against these diseases. The discovery of drugs has been stymied primarily by the absence of reliable preclinical drug screening platforms, particularly disease models for the identification of promising lead compounds. For instance, 2D cultures of cortical neurons, the most commonly employed in vitro model, do not incorporate the BBB; and the tissue microenvironment within mice and rodent brains, arguably the most common animal model for studying neurodevelopmental disorders and neurodegeneration,80 bears little resemblance to that of the human brain.81 As a consequence, results from preclinical testing do not translate well to the clinic, and nearly all drug candidates, particularly those that target neurodegeneration, fail when tested in real patients on account of poor safety and/or efficacy. The use of brain organoids to investigate the mechanisms through which these diseases occur, identify therapeutic targets and identify promising leads could result in improved graduation rates of drug candidates in clinical testing. The past 5 years have witnessed the establishment and validation of protocols for differentiating induced PSCs into glutamatergic neurons, presynaptic and postsynaptic cortical neurons, GABAergic interneurons of the forebrain, dopaminergic neurons of the midbrain, and neurons in the hindbrain,82, 83 as well as methods to engineer the PSCs themselves.84 These protocols can be easily extended to generate disease-specific organoids for use in drug screening. Lancaster and co-workers used RNA interference to engineer patient-derived induced PSCs, which were then differentiated into brain organoids that exhibited the pathophysiology of microcephaly.23 The gene expression pattern in cells within the organoid accurately mimicked actual tissue and recapitulated biochemical events as they occur in vivo.85, 86 In similar vein, a team at Johns Hopkins University led by Guo-li Ming and Hongjun Song exposed forebrain organoids to the Zika virus to trace the progression of microcephaly in the tissues and potentially develop antiviral drugs.25, 87 The organoids reproduce the organization of the progenitor zone, neurogenesis and gene expression of native tissue. In fact, just like the developing human brain, the outer layer of the organoids is comprised exclusively of radial glial cells, and exposure to the Zika virus induces results in increased cell death and reduced proliferation, which reduces the volume of the neuronal cells within the tissues.87
Brain organoids have also been used to model synaptogenesis and identify mechanisms that destabilize the process, thereby causing developmental disorders.6, 88 For instance, Paşca and co-workers investigated the migration of GABAergic (γ-aminobutyric-acid releasing) neurons from the ventral to dorsal forebrain in spheroids produced from human PSCs in the absence of ECM patterning factors.27 The human PSCs were genetically reprogrammed to harbor a mutation in the CaV1.2 calcium channel.27 Differentiated neurons expressing the mutated channel exhibit abnormal migratory saltations, which is the pathophysiology of a neurodevelopmental disorder called Timothy's Syndrome. Paşca and his team observed that the spheroids comprise distinct neuronal subtypes interspersed with astrocytes, and that this architecture closely mimics the in vivo physiology.77, 89 Induced PSC-derived brain organoids have also been used to reproduce the defective synaptic conductivity that is observed in autistic spectrum disorders.88 In one study, for example, up-regulation of the transcription factor, the Forkhead box protein G1 (FOXG1), resulted in an overproduction of GABAergic neurons, which subsequently increased synaptogenesis and dendrite outgrowth.90 The neurons in the organoids also exhibited an accelerated cell cycle. These observations are consistent with clinical data.
7 Toward an Informative Model for Investigating Neurodegeneration
While brain organoids are excellent models to study neurodevelopment, their high fidelity to developing brain tissue is precisely why they have found limited use as a model for neurodegeneration since nearly all the brain organoids developed thus far can only recapitulate the fetal brain. Specifically, brain organoids lack the extensive vasculature that is essential for the sustenance of older neurons. Vascularization in the developing human brain commences in the fourth week of development and originates from the pia mater, the innermost layer of the meninges.91 Not only are these timelines incongruent with the culturing times for organoids, but also the absence of the meninges precludes angiogenesis from occurring in the manner it does within the brain. One way to overcome these challenges is to generate organ buds by mixing induced PSC-derived neurons with endothelial and mesenchymal stem cells and later plating the mixture onto a presolidified matrix that has a stiffness that corresponds with that of native tissue.92, 93 The cells then form a 3D mass within 1–2 days. Nevertheless, the impact that dissociating neurons from a connected network (as is the case with 2D cultures) and subsequently reassociating them has on the synapses is unclear and merits further investigation. On the other hand, Lancaster and co-workers generated enhanced, 3D brain tissues using a mesh of microfilamentous poly(lactide-co-glycolide) as a floating scaffold.94 Not only did the resulting cerebral organoids display pronounced neuroectoderm formation and improved cortical development, but since the floating scaffold also provides a basement membrane, the tissues also form a radially oriented dense cortical plate, much like a mature cerebral cortex.
Some researchers are also turning to bioengineering to vascularize the brain organoids. Some approaches that have been considered include forming the organoids around a network of microchannels that can be lined with collagen for attachment of endothelial cells that comprise the vasculature.95-97 using permissive hydrogels supported by sacrificial materials such as polydimethylsiloxane98, 99; layer-by-layer assembly of 2D vascularized tissue to form a 3D network99; bioprinting100; and incorporating angiogenesis-inducing cues an such as vascular endothelial growth factor,101 angiopoetin,99 platelet-derived growth factor,102 and laminins and integrins.96 Of these, the use of microfluidics is arguably at the most advanced stage, and researchers at Leiden University recently used the technique to generate vascularized zebrafish embryoid bodies.103 Separately, the integration of bioengineering with the brain organoid technology is also benefitting the study of physicochemical processes that affect neurogenesis, oxygen transfer.104 For instance, Peter Carmeliet and his team at Leuven University recently showed that expansion and differentiation of radial glial cells in the developing cerebral cortex are attenuated by low oxygen levels, and that relief of hypoxia through the ingrowth of blood vessels coincided spatially and temporally with differentiation of NSCs.104 Carmeliet's team later used this insight to introduce microfluidic and axial patterning within the tissue to ensure a healthy supply of nutrients and oxygen. In particular, the elucidation of the spatial and temporal concentration profile of oxygen within neural tissue has significant implications for the design of a vasculature within the brain organoids.
Mature glial cells also produce myelin, a lipid-rich substance that coats the axons of some nerve cells. The insulating coat, or sheath, facilitates axonal conduction through the transfer of action potentials. The formation of the myelin sheath is referred to as myelination and is critically important to the nervous system. Damage to the myelin sheath or demyelination is a common pathological characteristic in conditions such as stroke, post-traumatic brain injury and several neurodegenerative conditions.105 A recent study at the University of Luxembourg observed myelination of neurites in human midbrain-specific organoids derived from neuroepithelial stem cells.64 The spatial organization of dopaminergic neurons within the organoids mimicked that of brains exhibiting Parkinson's disease, and the study revealed that cyclic nucleotide phosphodiesterase (CNPase), a myelin-associated enzyme, is primarily responsible for ensheating neurons. This discovery suggests that organoids can reasonably recapitulate complex processes such as myelination, which makes them effective models to study neurodegeneration. Myelination within in vitro tissue was also demonstrated by a team at the University of Konstanz in 3D co-cultures of mature neurons and glial cells that had been derived from induced PSCs.106 The Konstanz team observed as much as 40% overall myelination after 8 weeks of differentiation, which is quite high for an in vitro model, and used their system to successfully investigate neuron-neuroglia function and pathogenic mechanisms in neurotoxicology.
An alternative approach to generate pathophysiologically accurate brain organoids involves the use of PSCs derived directly from patients, as well as CRISPR/Cas-based genome engineering. The former method offers the advantage of capturing the pathology of the disease in an age-dependent manner, and was recently used to generate organoids that model Alzheimer's disease.107 In fact, when the AD-bearing organoids were treated with known inhibitors of γ-secretase and β-secretase 1 (BACE1), the number and size of amyloid beta (Aβ) plaques in the tissues reduced in a dose dependent manner, suggesting that the AD organoids could be effectively used for screening drugs that reverse neurodegeneration.
The complexity of the human brain and limited availability of human brain tissue for investigation have hitherto limited comprehensive studies on diseases affecting the brain. This situation is now changing owing to the ease of generation and widespread availability of iPSC-derived organoids.108 The organoid platform is already reaping rich dividends in applications where animal model are uninformative or do not suffice.66 For instance, whole brain organoids were successfully used to investigate microcephaly and the effect the virus infection of the development of the brain.48, 87 In another study, the forebrain organoids were used to model Zika virus exposure.25, 109 The reduction in tissue size and other pathophysiological signatures were similar in the in vivo and organoid models.
8 Emerging Frontiers in Brain Organoid Engineering
Brain organoids are excellent models to investigate fundamental mechanisms that guide neurodevelopment. Nevertheless, they are incomplete systems. Since they are comprised exclusively of cells with a neural lineage, cross-tissue communication or communication with non-neural cells, which influences morphology significantly in vivo, is entirely absent in brain organoids.74 The spatial patterning cues that brain organoids receive from their environments during development too are distinct from those that the developing brain receives in vivo.31 For instance, brain organoids cannot establish body axes to guide configuration of the posterior-anterior and dorsoventral axes. As a consequence, these tissues fail to assume the physical appearance and characteristics of native tissue. Spatial patterning of biomaterials and growth factors is commonly employed to assemble heterogeneous tissues comprising cells of a diversity of lineages but the method has not yet been successfully used to assemble brain organoids.110 Genome editing could overcome these challenges, and the method has been applied to great effect to modify cellular composition within tissue and reprogram signalling cascades that govern assembly and regulation within the organoids.111 Tissue heterogeneity arises from transcriptomic, proteomic, and metabolomic diversities, and differences between tissues are directly related to differences between their transcriptomic, proteomic, and metabolomic states. While cells having different lineages will have very different states, cells with the same lineage but in different parts of the brain organoid too exhibit differences in their biochemical states owing to spatiotemporal chemical gradients within the tissue. To this end, the generation of organoids of specific regions of the brain in a controllable and repeatable manner is far from being a solved question. Current protocols utilize self-patterning and self-organization of the cells to form a particular region of the brain but the process is highly variable since control over the spatiotemporal chemical gradients within the brain organoids is not as stringent as native tissue.112, 113
The immature vasculature compounds this problem further since the transport of oxygen, nutrients, and morphogenetic and growth factors to the inner core is limited and nonuniform.104, 114-116 The organ buds described earlier appear to offer a compelling solution for vascularization, and revealing the biochemical states of the their constituent cells could lead to the development of better protocols for stricter control of chemical gradients. Nevertheless, organ buds of a specific region of the brain would still have to be combined with buds of other regions to generate a physiologically relevant, connected, heterogeneous system. To this end, the organoid platform will benefit tremendously from the influx of insights and methodologies from tissue engineering and regenerative medicine. For instance, scaffolding could be used to create 3D structures that are patterned with differentiation and/or morphogenesis factors in order to co-culture brain organoids with support cells that have a role in vascularization.66, 74, 117, 118 Beyond patterning, microfabrication technologies can also be used for engineering 3D chambers to culture brain organoids in a manner that uniquely facilitates the study of biophysical phenomena that influences development and aging of the brain. For instance, a team at the Weizmann Institute of Science recently investigated the dynamics of surface wrinkling in human brain organoids that were cultured in a specially constructed micro-compartment that could be imaged in situ over a period of weeks.119 The team observed that convolutions and nuclear strain only emerge after a critical density of cells within the organoids is reached, and that the dynamic interplay between cytoskeletal contraction within the core of the organoid and cell-cycle-dependent nuclear expansion at the perimeter governs folding. A more recent study by Fred Gage and his team at the Salk Institute showed functional vascularization in the hiPSC-derived brain organoids upon in vivo engraftment of the organoids in the mouse brain.9 Although the blood vessels in this case were derived from the rodent, their demonstration is a significant milestone for generating vascularized organoids with synaptic connectivity to the host brain. Their work also sheds some light on the integration of brain organoid transplants with the host brain and their potential for repair/rescue of degenerative brain tissue. Likewise, earlier this year, Ben Waldau and his team at the University of California Davis Medical Center achieved vascularization of brain organoids using a double Matrigel embedding technique.120; The ensuing vascularization resembled that of the brain at very early, fetal stages, and the whole-brain organoid was the first to include patient-derived blood vessels.
There is also a critical need to develop methodologies for incorporating the BBB into the brain organoids. The BBB is instrumental in maintaining the specialized microenvironment of the central nervous system (CNS), and changes to the BBB have been implicated in several CNS pathologies.121 Presently, brain organoids do not bear this critical tissue. Absence of the BBB precludes the use of brain organoids in the investigation of neurovascular dysfunction or as a screening platform to identify anti-neurodegenerative drugs that can be delivered through the circulatory system. Beyond these applications, inclusion of the BBB into brain organoids could also facilitate use of the platform as a model for investigating systemic inflammation of neural tissue in syndromes such as sickness behavior, delirium, septic encephalopathy, as well as neurological conditions such as Alzheimer's disease and multiple sclerosis.86, 122 Induced PSCs can be reliably differentiated into inflammatory cells such as macrophages and microglia, and these cells can colonize neural organoids to create a heterogeneous population.123 However, new protocols will need to be developed in order to form the BBB and subsequently replicate intercellular interactions between endothelial cells, inflammatory cells and neural cells.124
Emerging technologies such as 3D bioprinting could have an important role to play in the production of more physiologically accurate organoids. 3D bioprinting or organ printing has attracted a lot of attention in recent years, largely owing to the work of Anthony Atala at the Wake Forest Institute for Regenerative Medicine,125 and the global bioprinting market is expected to touch $2 billion within the next 2 years.126 Bioprinting employs 3D printers to assemble living tissues, and the technology is a modification of ink-jet printing. A mixture of living cells and biomaterials known as a :bio-ink” is patterned onto a flat surface layer by layer, and such stacking eventually produces 3D tissues that can survive for weeks.127 3D bioprinting offers tight control of the spatiotemporal distribution of the cells within the tissue,128 and the technology has been used to fabricate multi-layered neural circuits for studying the physiology of learning and memory formation, as well as understanding initiation and progression of traumatic brain injury.129 In another study, when “bio-blocks” comprising NSCs embedded within polyurethane hydrogels were printed and subsequently injected into a zebrafish embryo neural injury model and adult zebrafish with traumatic brain injury, the authors observed that the impaired nervous systems were rescued in both tests.130 3D bioprinting could be employed to reliably and rapidly assemble vascularized organ buds of distinct regions of the brain into a single, connected tissue construct. However, bio-inks representing each region would need to be developed, and, if the former is successful, the printer heads would need to be modified in order to ensure that viability of the neuronal cells, as well as integrity and connectivity between the neurite outgrowths is sufficiently preserved.29 Additionally, connecting organoids representing subregions of the brain into a single, spatially heterogeneous mass necessitates tight control of the interregional interactions, which has not been achieved effectively in vitro. Nevertheless, some research groups are taking early steps toward achieving this goal. For instance, Jürgen Knoblich's team recently fused together two region-specific organoids and assessed the construct by investigating neuronal migration between the two regions.26 Paşca and co-workers went further and also identified the transcriptional changes associated with neuronal migration between the regions.27
9 Concluding Remarks
In closing, brain organoids are an attractive investigational model to study the biochemical and genetic mechanisms that underpin brain structure, function, and disease. Although they are far from perfect systems, brain organoids are nevertheless highly useful to study biophysical processes such as neuronal differentiation, migration, and connection; tissue morphology, shape, and patterning; cortical folding; and response to physicochemical stimuli. These processes have proven to be challenging to study owing to the absence of good investigational models. When combined with technologies such as CRISPR/Cas-based genome engineering, optogenetics, massively parallel single-cell RNA sequencing, metabolomics and proteomics, and next-generation imaging modalities, brain organoids could be used to map the human brain up from the level of individual cells in unprecedented chemical and genetic detail. A few technical challenges remain to be addressed in order to make brain organoids even more physiologically accurate, most notably, integration of immune cells such as microglia. However, recent examples of constructing vascularized brain organoids using in vivo transplantation or using a patient's own iPSC-derived endothelial cells offer significant encouragement. We hope that the roadmap for technology development that is presented herein will appeal to both, neuroscientists and bioengineers; motivate greater collaboration between the two groups; and prove to be the seed for maturation of brain organoids into a truly transformative biomedical technology.
Conflict of Interest
The authors declare no conflict of interest.
Biographies

Roza Vaez Ghaemi obtained her B.Sc. degree in biomedical engineering at Amirkabir University of Technology (Tehran Polytechnic) in 2011. She is now a doctoral candidate in biomedical engineering at the University of British Columbia. Her current research focuses on the assembly of brain organoids for use as a model for investigating neurotrauma. She is particularly interested in establishing quantitative relationships between trauma and microglial activation. The ultimate goal of her research is to develop a more realistic in vitro model of traumatic brain injury.

Ileana L. Co is currently pursuing her doctorate degree at the Institute of Biomaterials and Biomedical Engineering at the University of Toronto. Her research focuses on tissue engineering, drug discovery, and immune-oncology. She graduated in 2017 from the University of British Columbia with a B.A.Sc. degree in chemical and biological engineering and a minor in commerce. At UBC, she conducted research on brain organoids.

Matthew C. McFee graduated from the University of British Columbia with a B.A.Sc. degree in chemical and biological engineering. Under the mentorship of Dr. Vikramaditya Yadav, Matthew conducted research in the fields of tissue and biomaterials engineering. Upon graduation, he worked in the biotechnology sector with Boston Scientific. Matthew is now a biomedical engineering doctoral candidate at the University of Toronto working in Dr. Penney Gilbert's research group. The focus of his research is muscle stem cell bioengineering.




