Histone Deacetylase 6 Brain PET in Amyotrophic Lateral Sclerosis-Frontotemporal Spectrum Disorder
Funding: Financial support was provided through the Target ALS Foundation and AFTD, and a research grant to KU Leuven by Eikonizo Therapeutics.
Greet Vanderlinden and Charles Carron contributed equally to this work.
ABSTRACT
Objective
[18F]EKZ-001 is a positron emission tomography (PET) tracer targeting histone deacetylase 6 (HDAC6), an enzyme responsible for intracellular transport and clearance of misfolded proteins. HDAC6 modulation is a promising treatment strategy in neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS). Apart from motor symptoms, people with ALS (pwALS) can show a variable degree of cognitive impairment as part of the ALS-frontotemporal spectrum disorder (ALS-FTSD). This work assessed [18F]EKZ-001 binding in pwALS with variable involvement of FTSD.
Methods
Twenty-four pwALS (13M/11F, 61 ± 10 years) and 12 healthy controls (HC) (6M/6F, 58 ± 3 years) were included. Thirteen pwALS were cognitively normal (ALS-CN), and eleven pwALS presented with FTSD (ALS-FTSD) ranging from mild cognitive or behavioral impairment to FTD, according to their performance on the Edinburgh cognitive and behavioral ALS screen (ECAS). All subjects underwent dynamic PET-MR imaging with arterial sampling, and regional distribution volumes (VT) were calculated using a Logan graphical analysis.
Results
[18F]EKZ-001 VT was significantly lower in pwALS compared to HC. For ALS-CN, the largest reduction was found in the brainstem. For ALS-FTSD, reductions were more widespread in both gray and white matter. No differences in VT were found between pwALS with and without a C9orf72 mutation. [18F]EKZ-001 VT was not correlated with ECAS scores, age, or disease duration.
Interpretation
[18F]EKZ-001 binding is lower throughout the brain in pwALS compared to HC. This may be related to a compensatory mechanism to repair intracellular transport defects in ALS or to reduced HDAC6 enzyme availability for [18F]EKZ-001 binding due to sequestration of HDAC6 within protein aggregates.
1 Introduction
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative disorder affecting both upper and lower motor neurons. ALS also affects cognition, with up to 50% of people with ALS (pwALS) developing mild–severe cognitive or behavioral impairment, and concomitant frontotemporal degeneration (FTD) occurring in about 10% of pwALS [1].
Histone deacetylase 6 (HDAC6) is a class IIb HDAC paralog predominantly present in the cell cytoplasm. HDAC6 deacetylates α-tubulin, impacting microtubule dynamics and cell motility, and multiple additional cytoplasmic deacetylation substrates, such as cortactin, tau, and HSP90 [2-5]. HDAC6 also facilitates clearance of misfolded proteins through its ubiquitin binding domain by promoting aggresomal formation and autolysosome maturation. Consequently, HDAC6 is integral in protective responses to the aggregation of cytotoxic proteins and has also been implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer's and Parkinson's disease, where α-tubulin acetylation is decreased [6-8].
The role of HDAC6 in ALS is complex and discordant findings have been reported. HDAC6 mRNA is upregulated in fruit flies overexpressing human TAR DNA-binding protein 43 (TDP-43) mutations [9], and HDAC6 expression is reduced in fruit flies and in human cellular systems following loss of TDP-43 function [10]. Furthermore, in ALS induced pluripotent stem cell (iPSC)-derived motor neurons and skin fibroblasts, HDAC6 inhibition improved axonal transport defects, rescued defects in intracellular mitochondria distribution, reduced TDP-43 pathology, and enhanced neuromuscular junction formation [11-14]. In SOD1G93A mouse studies, HDAC6 deletion improved compound muscle action potential and neuromuscular junction innervation [15], and enhanced accumulation of mutant SOD1 [16], with conflicting results on motor neuron count and disease progression. Another study in SOD1G93A mice reported that decreased HDAC6 protein levels in the spinal cord were related to disease progression. When HDAC6 was overexpressed in SOD1G93 mice that exhibited decreased HDAC6 expression, motor neuron loss was ameliorated concomitant with increased clearance of toxic SOD1 protein [17]. These results align with observations of increased TDP-43 levels and SOD1 aggregation in cultured cells following HDAC6 knockdown [18, 19]. Taken together, these studies emphasize the importance of HDAC6 for toxic protein clearance in ALS, and support selective HDAC6 pharmacologic inhibition to rescue axonal transport defects, but also highlight the importance of maintaining HDAC6 protein clearance activity.
Quantifying HDAC6 in the human brain is therefore of great interest to investigate disease pathogenesis, progression, and possible treatment response in ALS. [18F]EKZ-001 ([18F]Bavarostat) is a PET tracer with specific affinity for HDAC6. [18F]EKZ-001 has favorable kinetic properties for in vivo evaluation of HDAC6 binding [20, 21] and can be quantified by Logan graphical analysis (LGA) modeling with appropriate test–retest reproducibility in healthy volunteers [22]. The aim of this study was to assess [18F]EKZ-001 binding in the brain of pwALS with variable involvement of frontotemporal spectrum disorder (FTSD) compared to HC using PET-MR. Additionally, the relationship of [18F]EKZ-001 binding with C9orf72 mutation status, as it is the most common genetic mutation in ALS, was explored as well as the correlation with neuropsychological assessment, age, and disease duration.
2 Methods
2.1 Study Participants
pwALS were prospectively recruited at the Motor Neuron Disorder Clinic of the neurology department at the University Hospital Leuven between 2019 and 2024. Eligible patients were diagnosed with “probable, laboratory supported ALS,” “probable ALS,” “possible ALS,” and “suspected ALS” according to the El Escorial criteria [23]. Main exclusion criteria for pwALS consisted of other major internal pathology (e.g., cancer) and contra-indications for MRI or for PET (e.g., pregnancy). As part of the routine clinical work-up, all pwALS underwent genetic testing for C9orf72, TARDBP, GRN and FUS. The revised ALS functional rating scale (ALSFRS-R) and the Edinburgh cognitive and behavioral ALS screen (ECAS), evaluated by an experienced clinical neuropsychologist, were acquired in all pwALS. pwALS were classified as ALS-CN or ALS-FTSD [24] according to their performance on the ECAS and behavioral assessment [25]. Cerebrospinal fluid (CSF) neurofilament light chain (NfL) levels were determined for a subset of pwALS either as part of the routine clinical work-up or through participation in other research studies. No other biomarkers (e.g., Alzheimer's disease biomarkers) were determined. HC were recruited as previously reported [22].
2.2 Data Acquisition
[18F]EKZ-001 PET-MR acquisitions were performed on a 3T GE Signa PET-MR scanner (General Electric, Milwaukee, MI, USA). PET scans were performed in list mode and were reconstructed using ordered subset expectation maximization (28 subsets, 6 iterations) with isotropic Gaussian post-smoothing (4 mm full width at half maximum [FWHM]), including corrections for decay, scatter, deadtime, and randoms and time-of-flight detector response. Zero echo time MR-based attenuation correction was applied [26].
HC fasted for at least 4 h prior to the PET scan and underwent a full 120-min dynamic [18F]EKZ-001 acquisition with arterial blood activity and radiometabolite sampling. Coffee-break imaging protocols (CBP) were installed and validated to improve feasibility of [18F]EKZ-001 PET in pwALS [22]. PwALS did not fast prior to the PET scan. Ten pwALS underwent a coffee-break PET acquisition consisting of a 60-min dynamic acquisition followed by a 30-min scan from 90 to 120 min post injection (coffee-break protocol 1 [CBP1]). This was extended to a longer coffee-break protocol for some pwALS, with an initial 30-min dynamic scan followed by a second 30-min scan from 90 to 120 min (coffee-break protocol 2 [CBP2]). Quantitative validation of the different coffee-break protocols in healthy volunteers was performed (Data S1). The average bias introduced by CBP2 compared to the full dynamic acquisition ranged from 1.7% in the occipital cortex to 2.6% in the white matter (Data S1). Arterial sampling and radiometabolite analysis were performed according to the same protocol as for HC.
3D T1-weighted and fluid-attenuated inversion recovery (FLAIR) MRI scans were acquired using a vendor-supplied 8-channel brain-phased array head coil on the same PET-MR scanner. FLAIR images were used to determine Fazekas scores by a nuclear medicine specialist in training (C.C.).
2.3 Image Processing
Reconstructed [18F]EKZ-001 images were corrected for motion using a rigid frame-by-frame co-registration in PMOD v4.1 (PMOD technologies, Zurich, Switzerland). Next, PET images were co-registered to the structural T1-weighted MRI scans. MRI segmentation was performed by the CAT12 toolbox of SPM12 (Statistical Parametric Mapping, Welcome Trust Centre for Neuroimaging, University College, London, UK) [27]. Ten composite bilateral volumes-of-interest (VOIs) were defined from the Neuromorphometrics atlas: frontal cortex (excluding primary motor cortex), parietal cortex, occipital cortex, temporal cortex, primary motor cortex, striatum, thalamus, brainstem, cerebellum, and cerebral white matter. The LGA algorithm from the PKIN tool (PMOD v4.1) was used to calculate VT for all VOIs, as was previously validated [22]. The time t* in an LGA can be described as the time after which the plotted samples can be fitted by a linear regression and no further significant increase in the slope is observed. To enable direct comparisons between all groups, regardless of the imaging protocol, t* was set to 26 min for all subjects since this was the latest available timepoint before the coffee break for subjects who were scanned with CBP2. For group comparisons, the PXMOD tool (PMOD v4.1) was used to construct parametric VT images based on the same LGA. Parametric VT maps were analyzed both with and without partial volume correction (PVC), using a region-based voxel-wise (RBV) algorithm, as described previously [28] with PET resolution modeling with a Gaussian kernel of 5 mm FWHM. Data were also processed using the two-tissue compartment model, as validated previously [22], to obtain VOI-based K1 values.
Gray matter probability maps for voxel-based morphometry (VBM) and parametric VT images (both with and without PVC) were normalized to MNI space using the CAT12 toolbox of SPM12 and were smoothed by an isotropic Gaussian kernel of 8 mm FWHM.
2.4 Statistical Analysis
General statistical analyses were performed in GraphPad Prism v10 (GraphPad Software, La Jolla, CA). Normality was assessed using Shapiro–Wilk tests. VBM and voxel-based group comparisons were performed using an unpaired t-test in SPM12 (cluster height pFWE < 0.05, peak height puncorrected < 0.001). For VOI-based comparisons, when comparing more than two groups, ANOVA tests were performed with post hoc two-sample t-tests. For these post hoc two-sample t-tests, p-values lower than 0.005 were considered significant (Bonferroni correction for 10 VOIs). To assess the influence of age on group differences in [18F]EKZ-001 VT, linear regression models were tested per VOI where [18F]EKZ-001 VT was assessed as the dependent variable and group (HC/ALS-CN/ALS-FTSD) and age as independent variables. To assess the influence of white matter lesions on [18F]EKZ-001 VT in the ALS group specifically, linear regression models were tested where [18F]EKZ-001 VT was assessed as the dependent variable and Fazekas (0/1/2/3) as an independent variable.
Associations of [18F]EKZ-001 VT PVC with scores of the neuropsychological assessment (ECAS), CSF NfL levels, age, and disease duration were assessed through Pearson or Spearman correlation analyses as appropriate. ECAS cognitive subdomains include an ALS-specific part (language, verbal fluency, and executive domains) and an ALS non-specific part (memory and visuospatial domains). For correlation analyses, residual scores for each cognitive domain were used. These residual scores were calculated using predicted values for each participant based on age, level of education, and sex [25]. pwALS for whom there was more than 3 months between ECAS assessment and the PET scan were excluded from the correlation analyses (17/24 included). Due to the exploratory character of the correlation analyses, no correction for multiple comparisons was performed.
3 Results
3.1 Demographics
Demographic variables are summarized in Table 1. A total cohort of 24 pwALS (ALS-T) (13M/11F, mean 61 ± 10 y/o, range 44–80) was included. This ALS-T cohort consisted of two people with “probable ALS, laboratory supported,” six people with “probable ALS,” 14 people with “possible ALS” and two people with “suspected ALS” at time of diagnosis, which were later confirmed with laboratory supported ALS. The mean disease duration at the time of scanning was 2.2 ± 2.2 years (range 0.7–7.9). Thirteen pwALS were categorized as ALS-CN (7M/6F, mean 57 ± 9 y/o, range 44–80) and 11 as ALS-FTSD (6M/5F, mean 65 ± 9 y/o, range 51–80). Of the ALS-FTSD cohort, three met the diagnostic criteria for concomitant FTD [24], five had a cognitive impairment (CI), one presented with isolated behavioral impairment (BI), and two showed both cognitive and behavioral impairment (CBI). ALSFRS-R was acquired with an average of 58 ± 53 days (range 1–166) between ALSFRS-R and scan acquisition. ECAS was acquired in 21 out of 24 pwALS with a mean of 43 ± 49 days (range 0–176) between ECAS and scan acquisition. ALS-FTSD had significantly lower ECAS scores than ALS-CN (mean difference of 21 ± 6.0, p < 0.01). Compared to ALS-CN, ALS-FTSD had a longer disease duration (mean difference of 2.2 ± 0.8 years, p < 0.01) and were older (mean difference of 8.4 ± 3.7 years, p = 0.03). ALS-FTSD were also significantly older compared to HC (mean difference of 7.1 ± 2.7 years, p = 0.02).
| ALS-T | ALS-CN | ALS-FTSD | HC | |
|---|---|---|---|---|
| N | 24 | 13 | 11 (3 FTD, 5 CI, 1 BI, 2 CBI) | 12 |
| Age at scan (years) | 61 ± 10 (44–80) | 57 ± 9 (44–80) | 65 ± 9 (51–80) | 58 ± 4 (52–63) |
| Male/female (N) | 13/11 | 7/6 | 6/5 | 6/6 |
| Disease duration (years) | 2.2 ± 2.2 (0.7–7.9) (n = 23) | 1.2 ± 0.7 (0.7–3.3) (n = 13) | 3.4 ± 2.8 (0.7–7.9) (n = 10) | NA |
| ALS-FRS-R (0–48) | 38.0 ± 6 (19–46) | 39 ± 7 (19–44) | 37 ± 6 (28–46) | NA |
| Time between ALS-FRS-R and HDAC6 PET (days) | 58 ± 53 (1–166) | 50 ± 50 (2–166) | 67 ± 57 (1–144) | NA |
| ECAS (0–136) | 98 ± 17 (41–116) (n = 21) | 109 ± 7 (97–116) (n = 10) | 88 ± 17 (41–99) (n = 11) | NA |
| Time between ECAS and HDAC6 PET (days) | 43 ± 49 (0–176) (n = 21) | 47 ± 61 (0–176) (n = 10) | 38 ± 33 (6–109) (n = 11) | NA |
| Bulbar/spinals (side) onset | 11/13 (9L4R) | 7/6 (3L3R) | 4/7 (6L1R) | NA |
| Mutations |
7 C9orf72 1 TARDBP 1 GRN |
4 C9orf72 1 TARDBP |
3 C9orf72 1 GRN |
NA |
| Injected activity (MBq) | 126 ± 14 (102–169) | 127 ± 16 (104–169) | 125 ± 13 (102–145) | 138 ± 33 (116–201) |
- Note: Values as mean ± SD (range).
- Abbreviations: ALS, amyotrophic lateral sclerosis; CI, cognitively impaired; CN, cognitively normal; HC, healthy controls; L, left; NA, not applicable; R, right; T, total.
The ALS group was exploratively divided into pwALS with known genetic mutations (“mutation carriers”) and those without (“no mutation carriers”), regardless of their cognitive status. In ALS “mutation carriers,” 4 out of 9 pwALS were categorized as cognitively impaired (3 CI and 1 CBI). In pwALS “no mutation carriers” this was 7 out of 15 (3 FTD, 2 CI, 1 BI and 1 CBI).
3.2 Group Comparison of Gray Matter Atrophy
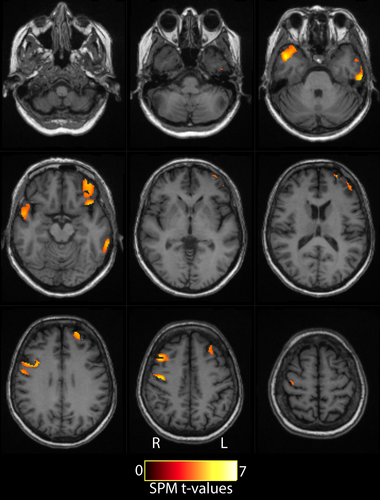
VBM showed multiple clusters of significantly lower gray matter concentration in pwALS compared to HC, with clusters in the right precentral gyrus, left inferior temporal gyrus, left superior frontal gyrus, right temporal pole, and left posterior orbital gyrus (Figure 1). Therefore, group comparisons and correlation analyses were performed with PVC. Results without PVC can be found in the Supporting Information.
3.3 Group Comparison of [18F]EKZ-001 Binding
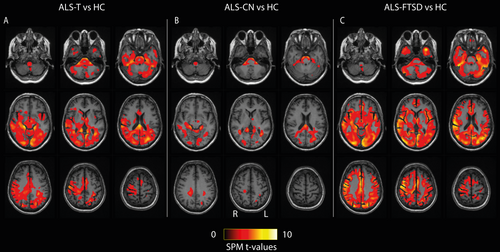
Group mean [18F]EKZ-001 VT images as well as individual example [18F]EKZ-001 VT images are shown in Figures S1 and S2. Voxel-based group analyses showed a widespread cluster, most prominent in the right parietotemporal and occipital regions, with significantly lower [18F]EKZ-001 VT in ALS-T compared to HC. [18F]EKZ-001 VT was lower in ALS-CN compared to HC in the brainstem, splenium of the corpus callosum, occipital white matter, and superior occipital gyrus. Even greater reductions in [18F]EKZ-001 VT were evident in ALS-FTSD compared to HC throughout most gray and white matter regions, with peak voxels in the postcentral gyrus (Figure 2). The voxel-based analysis showed no significant differences in [18F]EKZ-001 VT between ALS-FTSD and ALS-CN. Additional results, with and without PVC, can be found in Tables S1–S7 and Figure S3.
VOI-based analyses showed significantly lower [18F]EKZ-001 VT values in the ALS-T group compared to HC in the brainstem, cerebellum, and thalamus as well as in the occipital, parietal, and temporal cortex (Figure S4). The most pronounced reduction was found in the brainstem (22%). Despite widespread trends of lower tracer binding across brain regions in ALS-CN compared to HC, [18F]EKZ-001 VT values were only significantly lower in the brainstem VOI with a mean reduction of 20% (Figure 3). When comparing ALS-FTSD with HC, we found significantly lower tracer binding in all VOIs except for the frontal cortex and white matter (Figure 3). The largest reductions were found in the brainstem (24%) and in the occipital and temporal cortex VOIs (both 23%). Comparing the ALS-FTSD to the ALS-CN group showed widespread trends of lower tracer binding (Figure 3). Percent differences are summarized in Table 2 (with PVC) and Table S8 (without PVC). Group differences in [18F]EKZ-001 VT remained significant after correction for age. [18F]EKZ-001 VT levels were significantly associated with Fazekas scores in the cerebellum VOI (p = 0.02). However, this association did not remain significant after Bonferroni correction for multiple comparisons.
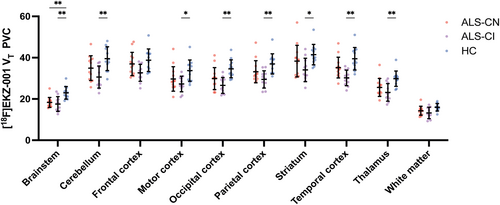
| VOI | HC | ALS-T | Diff | p-value | ALS-CN | Diff | p-value | ALS-FTSD | Diff | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Brainstem | 23.0 ± 3.1 | 18.0 ± 3.0 | 22% | < 0.001 | 18.3 ± 2.4 | 20% | < 0.001 | 17.5 ± 3.6 | 24% | < 0.001 |
| Cerebellum | 39.5 ± 5.8 | 33.0 ± 6.0 | 17% | 0.004 | 34.9 ± 6.0 | 12% | 0.064 | 30.6 ± 5.3 | 22% | 0.001 |
| Frontal cortex | 38.8 ± 5.4 | 35.0 ± 5.3 | 10% | 0.055 | 37.0 ± 5.6 | 5% | 0.418 | 32.7 ± 4.1 | 16% | 0.007 |
| Motor cortex | 33.7 ± 5.2 | 28.6 ± 5.1 | 15% | 0.008 | 29.7 ± 5.9 | 12% | 0.085 | 27.3 ± 3.8 | 19% | 0.003 |
| Occipital cortex | 34.6 ± 4.3 | 28.3 ± 4.9 | 18% | < 0.001 | 29.9 ± 5.3 | 14% | 0.023 | 26.6 ± 3.8 | 23% | < 0.001 |
| Parietal cortex | 37.0 ± 4.9 | 31.5 ± 5.1 | 15% | 0.004 | 33.2 ± 5.3 | 10% | 0.079 | 29.5 ± 4.2 | 20% | < 0.001 |
| Striatum | 41.5 ± 5.0 | 36.4 ± 7.0 | 12% | 0.031 | 38.3 ± 7.7 | 8% | 0.238 | 34.1 ± 5.7 | 18% | 0.003 |
| Temporal cortex | 39.5 ± 5.5 | 32.9 ± 5.1 | 17% | 0.001 | 35.2 ± 5.0 | 11% | 0.053 | 30.2 ± 3.8 | 23% | < 0.001 |
| Thalamus | 29.8 ± 3.7 | 24.5 ± 4.4 | 18% | < 0.001 | 25.6 ± 4.3 | 14% | 0.015 | 23.2 ± 4.2 | 22% | < 0.001 |
| White matter | 16.0 ± 1.8 | 13.8 ± 2.5 | 14% | 0.011 | 14.3 ± 2.2 | 11% | 0.047 | 13.2 ± 2.8 | 17% | 0.010 |
- Abbreviations: ALS-CN, ALS-cognitively normal; ALS-FTSD, ALS-cognitively impaired; ALS-T, total ALS group; Diff, relative difference; HC, healthy controls; VOI, volume-of-interest; VT, distribution volume.
[18F]EKZ-001 VT was not significantly different between “mutation carriers” and “no mutation carriers” (all p > 0.05) (Figure S5). Two-tissue compartment model K1 values were not significantly different between ALS-T and HC, nor between ALS-CN, ALS-FTSD and HC (Figure S6).
3.4 Correlation of [18F]EKZ-001 Binding With Neuropsychological Measures
[18F]EKZ-001 VT was not significantly correlated with residual scores of the cognitive domains from the neuropsychological assessment, age, or disease duration (all p ≥ 0.07). Correlations between [18F]EKZ-001 VT and neuropsychological assessment domain residual scores are summarized by a correlation matrix (Figure S7). For a subset of 14 pwALS, CSF NfL levels were determined, but these were not significantly associated with [18F]EKZ-001 VT (puncorrected between 0.08 and 0.3 for all VOIs).
4 Discussion
The goal of this study was to investigate the binding of the HDAC6 PET tracer, [18F]EKZ-001, in pwALS compared to HC. Our primary finding is that [18F]EKZ-001 binding as measured by VT was lower in pwALS compared to HC in most VOIs, including the brainstem, occipital, parietal, and temporal cortex, thalamus, and cerebellum. This suggests that HDAC6 expression may be lower in pwALS compared to HC, in line with reduced HDAC6 expression observed in SOD1G93A transgenic mice [17]. However, reduced [18F]EKZ-001 binding may not be indicative of reduced enzymatic activity, as HDAC6 can be modulated by GSK3β-mediated phosphorylation to increase deacetylase activity [29]. We did not find evidence that known underlying genetic forms of ALS (specifically a C9orf72 expansion) influence [18F]EKZ-001 binding. The group comparison of K1 values showed no significant differences, indicating that [18F]EKZ-001 influx is comparable between groups and cannot explain the differences in VT between pwALS and HC. In the ALS-T group, cerebellar [18F]EKZ-001 VT was significantly associated with Fazekas scores, but this association was not significant in any other VOI and would not have survived correction for multiple comparisons, indicating that differences in tracer binding were not likely to be driven by white matter lesions. There was a trend towards an association of [18F]EKZ-001 VT with CSF NfL levels in the ALS-T group. However, these NfL levels were only available for a subset of 14 pwALS, resulting in limited statistical power for this analysis and warranting the need for replication in larger cohorts.
HDAC6 downregulation in SOD1G93A mice is progressive and occurs in older mice, as they become symptomatic [17], suggesting that HDAC6 expression may be dynamic in ALS. In presymptomatic disease stages, there may be a transient increase in HDAC6 expression to aid in the clearance of toxic proteins, such as TDP-43 and SOD1. Consistently, HDAC6 is upregulated in acute models of Parkinson's disease [30, 31]. Reduced HDAC6 expression in symptomatic disease stages could be a compensatory response to the excessive deacetylating activity of the enzyme [32-34]. Future longitudinal [18F]EKZ-001 PET studies in pre-symptomatic carriers would be interesting to gain further insight into HDAC6 dynamics throughout the disease course of ALS. Alternatively, increased demand for HDAC6-dependent autophagic protein aggregate clearance may lead to sequestration of HDAC6 within protein aggregates [35, 36], rendering the HDAC6 catalytic domain 2 inaccessible to the binding of [18F]EKZ-001. Finally, the difference in [18F]EKZ-001 signal could be due to off-target binding of the tracer to metallo-beta-lactamase domain-containing protein 2 (MBLAC2), a palmitoyl-CoA hydrolase involved in endo- and exocytosis. Hydroxamate-based HDAC6 inhibitors, including EKZ-001 (unpublished results), inhibit MBLAC2 in vitro with nanomolar potency [37]. Although the relevance and role of MBLAC2 in ALS is unknown, differences in MBLAC2 expression between ALS and HC might account for the observed findings. To further characterize potential [18F]EKZ-001 off-target binding, quantitative binding assays and autoradiography in healthy and ALS brain samples are needed.
These findings may have implications for the development of disease-modifying therapies for ALS. Therapeutic development currently focuses on small-molecule HDAC6 inhibitors. Importantly, selective inhibition of HDAC6 catalytic domain 2, which has broad substrate specificity and is the primary target of HDAC6 inhibitors [38], does not impede the c-terminal zinc-finger ubiquitin binding domain activities of HDAC6 in protein aggregate clearance but instead stimulates autophagic flux within neurons [39]. Thus, despite the apparent lower HDAC6 expression in ALS compared to HC described here, inhibition of the catalytic activity of the existing pool of HDAC6 in pwALS has the potential to (1) facilitate intracellular transport by increasing α-tubulin acetylation and (2) stimulate autophagic clearance of toxic protein aggregates like TDP-43 in ALS. In support of this possibility, HDAC6 inhibition is efficacious in preclinical animal and cellular models of ALS and FTD [11, 13, 14, 40]. Furthermore, in line with preclinical evidence, HDAC6 overexpression combined with HDAC6 catalytic domain 2 inhibition could be beneficial through interactions with the ubiquitin-proteasome and autophagy-lysosome systems, which could mitigate neuronal toxicity caused by TDP-43 pathology [17-19].
To date, one previous study explored differences in HDAC binding in pwALS using [11C]Martinostat PET, a pan-HDAC tracer that binds to paralogs 1, 2, and 3, as well as to HDAC6. The authors reported no significant differences in [11C]Martinostat binding [41]. Since HDAC6 is significantly different in pwALS compared to HC, we hypothesize that HDAC6 may have a more specific role compared to the other HDAC paralogs in ALS. In contrast, in patients with Alzheimer's disease, reduced HDAC levels as measured by [11C]Martinostat were associated with elevated amyloid-β and tau PET levels [42]. Moreover, [11C]Martinostat binding was shown to mediate the deleterious effects of amyloid-β and tau on brain atrophy and cognitive impairment. To the best of our knowledge, [18F]EKZ-001 PET has not been studied in other neurodegenerative disorders and a head-to-head comparison of [11C]Martinostat and [18F]EKZ-001 is still lacking.
The lack of correlation with neuropsychological assessment, age, and disease duration, in combination with the observed difference in group comparisons for ALS-FTSD and ALS-CN, could suggest differences in underlying disease pathophysiology between ALS-FTSD and ALS-CN. Future longitudinal evaluation should be conducted to elucidate the possible differential effect related to extra-motor involvement. It would also be interesting to compare cerebral [18F]EKZ-001 binding with plasma assessments of TDP-43 and NfL, as it was recently shown that both plasma TDP-43 and NfL are associated with disease severity in ALS, as measured by ECAS and ALSFRS-R [43, 44].
Limitations of this study include a relatively small sample size, particularly for the various genetic variants and FTSD, as well as the necessity of progressive coffee-break protocols for the acquisition of the [18F]EKZ-001 PET scans due to the inability of advanced pwALS to undergo long dynamic scanning. To ensure that group differences were not driven by differences in processing, all images were processed using the same t* values. In HC, the average bias introduced by the minimal coffee-break protocol (CBP2) compared to the full dynamic acquisition ranged from 1.7% in the occipital cortex to 2.6% in the white matter (Data S1), indicating minimal influence on the obtained results. The pwALS sample included in this study is not entirely representative of the ALS population since we included a relatively large subsample of C9orf72 mutation carriers. Moreover, the recruited C9orf72 mutation carriers were in an early disease stage, associated with a lower prevalence of cognitive impairment in this subcohort. Furthermore, due to the small sample size per group, we did not include age and sex as covariates in the analyses even though sex differences have been shown previously for [18F]EKZ-001 PET [22]. However, the number of males and females per group was matched to ensure sex differences would not influence the findings.
To conclude, we observed that HDAC6 tracer binding is lower throughout the brain in pwALS compared to HC, as measured by [18F]EKZ-001 PET. The reduction was more pronounced in ALS-FTSD and was not influenced by C9orf72 mutation status or related to performance on cognitive domains of the ECAS, age, or disease duration.
Author Contributions
K.V.L., P.V.D., D.V.W., T.M.G., and J.E.K. contributed to the study concept and design. G.V., C.C., J.D.V., F.O., P.M., C.D.W., and P.V.D. contributed to participant recruitment. G.V., C.C., and C.D.W. contributed to data acquisition. G.V., C.C., M.K., R.E.J., F.A.S., J.M.H., T.M.G., and K.V.L. contributed to data analysis and interpretation. G.V., C.C., and K.V.L. drafted the manuscript. Manuscript revision was performed by all authors.
Acknowledgments
We thank the participants for their willingness to participate in this study. We thank the PET-MR technologists for their contribution to data acquisition. We thank the PET radiopharmacy team and nuclear medicine medical physics team for their skilled contributions. Financial support was provided through the Target ALS Foundation and AFTD, and a research grant to KU Leuven by Eikonizo Therapeutics.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Anonymized data will be deposited in an access-controlled file server used by the academic research PET imaging group, which can be shared upon reasonable request from any qualified investigator on approval by the Ethics Committee of the local university hospital.




