Native ancestry is associated with optic neuritis and age of onset in hispanics with multiple sclerosis
Abstract
Background and Objective
Hispanics with multiple sclerosis (MS) present younger and more often with optic neuritis (ON) as compared to Whites in the western United States. Regional differences related to Hispanic genetic admixture could be responsible. We investigated the association between global genetic ancestry and ON and age at onset of MS in Hispanics.
Methods
Data were obtained for 1033 self-identified Hispanics with MS from four MS-based registries from four academic institutions across the United States January 2016–April 2017. Multivariate regression models, utilizing genetic ancestry estimates for Native American (NA), African, and European ancestry, were used to assess the relationship between genetic ancestry and ON presentation and age of MS onset, defined as age at first symptom.
Results
Genetic ancestry and ON proportions varied by region where NA ancestry and ON proportions were highest among Hispanics in the southwestern United States (40% vs. 19% overall for NA and 38% vs. 25% overall for ON). A strong inverse correlation was observed between NA and European ancestry (r = −0.83). ON presentation was associated with younger age of onset (OR: 0.98; 95% CI: 0.96–0.99; P = 7.80 × 10−03) and increased NA ancestry (OR: 2.35 for the highest versus the lowest quartile of NA ancestry; 95% CI: 1.35–4.10; P = 2.60 × 10−03). Younger age of onset was found to be associated with a higher proportion NA (Beta: −5.58; P = 3.49 × 10−02) and African ancestry (Beta: −10.07; P = 1.39 × 10−03).
Interpretation
Ethnic differences associated with genetic admixture could influence clinical presentation in Hispanics with MS; underscoring the importance of considering genetic substructure in future clinical, genetic, and epigenetic studies in Hispanics.
Introduction
Multiple sclerosis (MS) is an immune-mediated disease where geography, ethnicity, and race play a role in disease susceptibility.1 The incidence rate of MS in Hispanic Americans has been reported to be 2.9–8.2/100,000, less than Whites but higher than Asians and Native Americans (NA).2, 3 The clinical presentation of MS is also said to vary between Hispanics and Whites. Studies from southern California have reported that Hispanics develop MS at an earlier age compared to Whites, in particular Hispanics born and raised in the United States.3, 4 Optic neuritis (ON) is a common initial clinical presentation of MS with a reported prevalence of 14–20% in predominantly Whites;5-7 however, in Hispanics, primarily of Mexican background, it has been reported to range between 32 and 54%.4, 8, 9 This finding mirrors Asian clinical reports of frequent optic neuritis as presenting symptom (42%), which is much greater when compared to studies conducted in White cohorts. Nonetheless, recent reports conducted in southeastern United States do not report ON as the most common presenting symptom which could be due to differences in Hispanic origin.10 Since both ancestry and MS have a known genetic component, we hypothesize that the underlying nature of these clinical observations may be explained by genetic ancestral differences between Hispanics; where the similarities to Asian reports may be explained by the genetic NA component.
Few studies have examined the relationship between global genetic ancestry (or average ancestry across the genome) and clinical characteristics of MS in admixed populations.11-13 Nevertheless, the examination of genetic ancestry in asthma and other autoimmune diseases has explained some of the genetic underpinnings behind clinical differences in ethnic groups.14-16 Recently, variations in genetic ancestry were also shown to explain clinical patterns of glaucoma and diabetic retinopathy among Hispanics.17, 18 Given these successes, assessing genetic ancestry in Hispanics with MS could aid in understanding clinical similarities/differences between Hispanics and developing future concept models for the genetic origins of MS presentation.
Utilizing the collection of Hispanic Americans within the Alliance for Research in Hispanic MS (ARHMS; www.arhms.org) consortium; our aim was to estimate global genetic ancestry and determine whether ancestry was an independent factor influencing age of onset and the presentation of ON as a first symptom in MS.
Methods
The Institutional Review Board at each institution approved this study and all patients provided informed consent prior to participation according to the principles outlined in the Declaration of Helsinki.
Study population
Participants were recruited from four MS tertiary care institutions that currently participate in ARHMS: University of Southern California (USC), University of Miami (UM), University of California San Francisco (UCSF) and San Juan MS Center in Puerto Rico. ARHMS was created in 2016 with a broad goal to improve our understanding of MS in the U.S. Hispanic population using existing epidemiological and biospecimen registries to investigate the genetic contributions to disease.10, 19 Inclusion criteria includes: (1) a diagnosis of MS, (2) self-report as Hispanic, (3) ability to complete a questionnaire using semistructured interview, and (4) consent to share medical records and a blood sample. Self-identified Hispanics were included using the NIH minority inclusion criteria; defined as a person who self-identifies as Hispanic or Latino of Cuban, Mexican, Puerto Rico, Southern or Central Americans, or other Spanish culture or origin, regardless of race.20 Questionnaire and medical records’ data are captured in CAFÉ (Common Application Framework Extensible; café.usc.edu) or RedCap (Research Electronic Data Capture; projectredcap.org).21 This combined cohort represents one of the largest well-characterized groups of U.S. Hispanics with MS.10, 19.
Clinical characteristics
Clinical characteristics abstracted from the registries were MS course (relapsing, progressive), family history of MS (yes/no), age of first symptom, age of diagnosis, ON (yes/no) as first symptom of MS, and walking status. Disease duration and lag time were calculated using age at first symptom and age at diagnosis. A neurological history was completed and an expanded disability status scale (EDSS)22 was assigned prior to enrollment by an MS specialist (LA, AC, MB, LT, MO, SD). An EDSS score of ≥6.0 was used to indicate individuals with ambulatory disability. All participants met the criteria for MS according to McDonald Criteria.23
Genotyping
DNA samples were genotyped using a custom Illumina genotyping array (inclusive of the ExomeChip content) via the Illumina Infinium platform. This array targeted >300,000 genetic markers and was designed by the International Multiple Sclerosis Genetics Consortium (IMSGC) to include both ancestry informative markers24 and other single-nucleotide polymorphisms (SNPs) specific to consortium member interests. Genotyping was conducted at the John P. Hussman Institute for Human Genomics at UM, School of Medicine.
Quality control
In total, DNA samples for 1118 MS cases from USC, UM, UCSF, and Puerto Rico were genotyped. Samples were excluded based on genotyping call rate ≤98% (n = 5), discrepancy between self-reported and genotyped gender (n = 9), excess autosomal heterozygosity >3 standard deviations from the mean (n = 22), identity by descent indicative of sample duplication or relatedness (n = 42), and population outliers determined using EIGENSTRAT25 as exceeding 6 standard deviations from the mean for the first 10 principal components (n = 7). Of the remaining 1033, only 905 MS cases had clinical data available on either age of onset or presentation of ON.
SNPs were removed due to low genotyping call rate (≤99.5% for SNPs with minor allele frequency (MAF) ≤5%; ≤99% for SNPs with MAF ≤10% and >5%; and ≤98% for SNPs with MAF > 10%) and deviations from Hardy–Weinberg equilibrium (P ≤ 1.0 × 10−05). Lastly, SNPs were removed if discordance was observed across the 34 plate controls which were included in the overall genotyping experiment.
Population structure and global ancestry estimates
ADMIXTURE26 was utilized to estimate the global ancestry proportions of NA, African, and European ancestry within each individual. Reference population data for 108 NA from the Human Genome Diversity Project (HGDP)27 were available from the Illumina 650Y, including populations of Surui and Karitiana from Brazil, and Maya and Pima from Mexico. A total of 120 Yoruba DNA samples were obtained from the NHGRI Sample Repository for Human Genetic Research at the Coriell Institute for Medical Research, and 232 Spaniard control DNA samples were obtained from the Centre d'Esclerosi Múltiple de Catalunya (Cemcat) in Barcelona, Spain. Both the Yoruba and Spaniard DNA samples were genotyped on the custom Illumina array described above. Using identity by descent analyses, it was found that only 46 NA were unrelated (PI_HAT ≤ 0.2), and so these were used as the NA reference panel. To avoid bias in the reference panel sizes, we randomly selected 46 unrelated Yorubans and Spaniards which passed all sample QC to represent the African and European reference panels, respectively. SNP QC was done in each reference set separately, and then the overlap assessed with the variants passing QC in the Hispanic sample. In total, 14,731 autosomal variants which overlapped (the custom designed Illumina array and the Illumina 650Y array) were independent (R2 ≤ 0.1), had MAF > 0.01, were not in extended regions of linkage disequilibrium, and were not within 1 MB of any established MS risk loci28 were used in ADMIXTURE analyses.
Statistical analysis
Demographics and clinical characteristics were compared between ascertainment sites using ANOVA and Chi-square tests as appropriate. Tukey's method or Chi-square testing was used for pairwise comparison between sites. Among all participants, we tested for a correlation between each of the European, NA, and African ancestral proportions.
To examine the relationship between genetic ancestry and ON, a multivariable logistic regression model was used. Ancestry was modeled by inclusion of both the proportion of NA and African ancestry, and covariates included age of first symptom and sex. Linearity between the independent variables and the log odds was confirmed. To investigate the relationship of younger age of onset to genetic ancestry, a multivariable linear regression model was used, adjusting for sex and family history of MS. Linear model assumptions including linearity between the independent and dependent variables, homoscedasticity, and normality were assessed.
To determine if ascertainment site or HLA-DRB1*15:01 (rs3135388),29 HLA-A*02:01 (rs2517840, rs2523822, rs9295825), HLA-B*44:02 (rs9266773), HLA-B*55:01 (rs3819284), HLA-DQA1*01:01 (rs13193645), HLA-DQB1*03:02 (rs3998159 and rs3957146), HLA-DRB1*08:01 (rs4713586 and rs7775055), and rs2229092 as well as rs9277565 which are all MHC variants which have all been associated with MS Risk in European populations30 would be considered confounders in a test of association between either ON or age at first symptom and genetic ancestry, we tested for a nonzero partial correlation between ON and age at first symptom with each site (modeled using indicator variables for each site) and each HLA tag, after controlling for ancestry. Partial correlations which were statistically different from zero would suggest that analyses be stratified by the confounding variable and subsequently meta-analyzed using inverse variance meta-analysis. The I2 statistic was used to determine the degree of heterogeneity across studies defined according to the stratification variable.
For each model, association of phenotype with genetic ancestry was assessed using both a joint test of NA and African ancestry as well as calculation of ancestry specific parameter estimates. For the analysis of ON, ancestry specific quartiles were then calculated and included in our multivariable logistic regression model to further examine the contribution of ancestry to ON presentation. All statistical analyses were performed using SAS 9.4 and R 3.0.2.
Results
Demographics
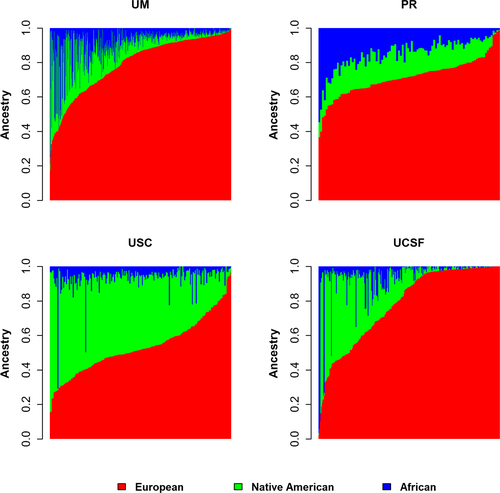
The cohort consisted of 1033 self-identified Hispanic cases and passed all QC. They were primarily female (77%, n = 791) with a mean age of ~44 (Table 1). The distribution of genetic ancestry proportions significantly varied among the Hispanic population by region (Fig. 1). The contribution of each ancestry had a wide range: NA 0–81% (mean: 19%, SD: 18%), European 2–100% (mean: 72%, SD: 20%), and African 0–97% (mean: 9%, SD: 11%). The NA proportions are significantly higher among Hispanics ascertained in Southern California (mean 40% compared to 19% overall, P = 1.49 × 10−98 for differences between sites), while higher African proportions (mean 15% compared to 9% overall, P = 3.04 × 10−09 for differences between sites) predominate in individuals ascertained in Puerto Rico. A strong inverse correlation was observed between NA and European ancestry (r = −0.83) and a moderate inverse correlation between African and European (r = −0.47) (Table S1).
| Characteristics | UM | PR | USC | UCSF | Total | Comparison between sites | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | F-test P | Pairwise P < 0.05 | |
| Ancestry | ||||||||||||
| African | 0.10 (0.11) | 548 | 0.15 (0.09) | 98 | 0.06 (0.07) | 189 | 0.07 (0.14) | 198 | 0.09 (0.11) | 1033 | 3.04 × 10−09 | M:P, M:SC, M:SF, P:SC, P:SF |
| European | 0.79 (0.17) | 548 | 0.71 (0.11) | 98 | 0.54 (0.16) | 189 | 0.72 (0.24) | 198 | 0.72 (0.20) | 1033 | 1.15 × 10−54 | M:P, M:SC, M:SF, P:SC, P:SF, SC:SF |
| Native American | 0.11 (0.13) | 548 | 0.14 (0.04) | 98 | 0.40 (0.16) | 189 | 0.21 (0.20) | 198 | 0.19 (0.18) | 1033 | 1.49 × 10−98 | M:SC, M:SF, P:SC, P:SF, SC:SF |
| Age at Ascertainment | 46.3 (12.1) | 512 | 43.2 (11.6) | 80 | 43.0 (12.0) | 189 | 40.3 (17.0) | 198 | 44.2 (13.4) | 979 | 5.84 × 10−07 | M:SC, M:SF |
| Age at Diagnosis | 37.5 (11.0) | 505 | 33.5 (10.5) | 80 | 33.2 (11.5) | 186 | 31.6 (11.9) | 116 | 35.5 (11.4) | 887 | 1.08 × 10−08 | M:P, M:SC, M:SF |
| Age at 1st Symptom | 33.7 (10.5) | 508 | 28.8 (10.0) | 79 | 30.4 (11.0) | 186 | 28.6 (11.3) | 126 | 31.9 (10.9) | 899 | 8.59 × 10−08 | M:P, M:SC, M:SF |
| Disease Duration | 12.5 (9.9) | 508 | 14.3 (9.4) | 79 | 12.7 (7.6) | 186 | 18.4 (12.3) | 126 | 13.5 (10.0) | 899 | 2.68 × 10−08 | M:SF, P:SF, SC:SF |
| Lag time | 3.7 (6.1) | 502 | 4.6 (6.6) | 79 | 2.5 (4.2) | 186 | 3.5 (6.1) | 115 | 3.5 (5.8) | 882 | 3.80 × 10−02 | P:SC |
| n (%) | N | n (%) | N | n (%) | N | n (%) | N | n (%) | N | Chi-Square P | Pairwise P < 0.05 | |
| Gender (Female) | 447 (81.6) | 548 | 75 (76.5) | 98 | 116 (61.4) | 189 | 153 (77.3) | 198 | 791 (76.6) | 1033 | 5.20 × 10−07 | M:SC, P:SC, SC:SF |
| HLA-DRB1*15:01 taga | 171 (15.6) | 1096 | 26 (13.3) | 196 | 41 (10.9) | 378 | 81 (20.5) | 396 | 319 (15.7) | 2066 | 2.33 × 10−03 | M:SC, M:SF, P:SF, SC:SF |
| Family history of MS | 75 (13.7) | 548 | 13 (17.3) | 75 | 13 (7.0) | 187 | 34 (13.2) | 198 | 135 (13.4) | 1008 | 1.72 × 10−02 | M:SC, P:SC, SC:SF |
| Relapsing MS | 419 (81.8) | 512 | 68 (85.0) | 80 | 158 (83.6) | 189 | 130 (80.8) | 161 | 775 (82.3) | 942 | 8.11 × 10−01 | |
| EDSS≥6 | 148 (29.2) | 507 | 17 (22.4) | 76 | 58 (31.0) | 187 | 32 (27.4) | 117 | 255 (28.8) | 887 | 5.44 × 10−02 | |
| Optic neuritis | 90 (18.2) | 495 | 26 (32.5) | 80 | 71 (38.0) | 187 | 26 (26.0) | 100 | 213 (24.7) | 862 | 6.04 × 10−07 | M:P, M:SC, SC:SF |
- For pairwise comparisons: M = UM, P = PR, SC = USC, SF = UCSF where significant comparisons are listed as Site1:Site2.
- a rs3135388 was used as the tag for HLA-DRB1*15:01 (de Bakker, Nature Genetics, 2006).
Clinical characteristics
The mean age of first symptom was 31.9 (SD: 10.9) and for diagnosis (mean: 35.5, SD: 11.4), with significantly older age of first symptom seen in cases ascertained in the southeastern United States compared to west coast (P = 8.59 × 10−08 for differences between sites). Family history of MS was estimated at 13.4% (of n = 1008), with some differences seen between sites (P = 1.72 × 10−02). ON was frequently more common in individuals ascertained in southern California compared to the other sites (mean 38% compared to 24.7% overall, P = 6.04 × 10−07 for differences between sites). However, no differences were seen in type of MS and ambulatory disability (Table 1).
Ascertainment site was identified as a potential confounder in a test of association between genetic ancestry and both age at first symptom and ON (P < 5.0 × 10−02 for partial correlations of each phenotype and site after controlling for genetic ancestry, data not shown). Therefore, regression analyses were done within each site, and parameter estimates were combined across sites using an inverse variance meta-analysis. HLA was not determined to be a confounder for the test of association between genetic ancestry and either age at first symptom or ON (P > 5.0 × 10−02 for partial correlations of each phenotype and different HLA tags after controlling for genetic ancestry, data not shown), and so no further analytical consideration for HLA was necessary.
Optic neuritis
Genetic ancestry was found to be significantly associated with ON as the presenting symptom, after adjusting for sex and age at first symptom (joint test of NA and African ancestry after controlling for site, P = 3.01 × 10−03). This ancestral association was primarily explained by NA ancestry (OR = 6.06, 95% CI = 1.94–18.96, P = 1.95 × 10−03, Table 2). To further explain the association between NA ancestry and ON presentation, the distribution of ON by quartiles of NA ancestry was examined (Table 2). From the lowest to highest quartiles of NA ancestry, the frequency of ON as the presenting symptom was statistically different (16%, 21%, 25%, and 38%, respectively with P = 3.45 × 10−07). To analyze all quartiles simultaneously, ascertainment site was included as a covariate in the regression model, in addition to sex and age at first symptom. We found that those in the highest quartile of NA ancestry had a ~2.4 increased likelihood of ON (OR = 2.35, 95% CI = 1.35–4.10, P = 2.6 × 10−03) compared to those in the lowest quartile (Table 3).
| Optic neuritis | Overall | UM | PR | USC | UCSF | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P a | Het Pc | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| African ancestry | 0.35 (0.07–1.87) | 2.21 × 10−01 | 9.80 × 10−01 | 0.24 (0.02–2.87) | 0.62 (0.00–b) | 0.48 (0.00–95.80) | 0.45 (0.03–7.78) |
| Native American ancestry | 6.06 (1.94–18.96) | 1.95 × 10−03 | 8.52 × 10−01 | 3.99 (0.75–21.2) | 0.51 (0.00–b) | 11.71 (1.46–93.99) | 6.62 (0.60–73.58) |
| Sex (Female vs. Male) | 1.19 (0.79–1.77) | 4.04 × 10−01 | 2.72 × 10−01 | 1.98 (1.00–3.90) | 0.62 (0.21–1.87) | 0.98 (0.53–1.83) | 1.02 (0.28–3.80) |
| Age at first symptom | 0.98 (0.97–0.99) | 1.48 × 10−02 | 1.37 × 10−01 | 0.99 (0.97–1.01) | 0.94 (0.88–0.99) | 0.99 (0.96–1.01) | 0.95 (0.90–0.99) |
| Age at First Symptom | BETA (SE) | P a | Het Pb | BETA (SE) | BETA (SE) | BETA (SE) | BETA (SE) |
| African ancestry | −10.07 (3.15) | 1.39 × 10−03 | 1.11 × 10−02 | −1.09 (4.41) | −14.26 (11.32) | −3.37 (12.07) | −23.78 (5.37) |
| Native American ancestry | −5.58 (2.65) | 3.49 × 10−02 | 3.27 × 10−02 | −6.96 (3.74) | −69.00 (24.83) | 2.05 (5.40) | −7.27 (5.31) |
| Sex (Female vs. Male) | −0.81 (0.85) | 3.43 × 10−01 | 6.71 × 10−01 | −1.52 (1.18) | −0.51 (2.65) | −0.72 (1.68) | 1.88 (2.49) |
| Family history | 0.43 (1.05) | 6.84 × 10−01 | 2.04 × 10−01 | 1.06 (1.35) | −3.51 (3.01) | −3.38 (3.26) | 3.34 (2.55) |
- Bold indicates NA ancestry increases the risk of ON presentation while both NA and AA increases the risk of presenting with MS at a younger age independent of sex and age of first symptom.
- PR, San Juan MS Center in Puerto Rico; UM, University of Miami; USC, University of Southern California; UCSF, University of California, San Francisco.
- a P indicates significance of variable with phenotype association, after controlling for other variables in the model.
- b Indicates OR > 100.
- c Het P indicates significance of a test for heterogeneity of effect across sites.
| Variables in model | OR (95% CI) | P |
|---|---|---|
| Native American ancestry | ||
| 2nd vs. 1st Quartile | 1.27 (0.77–2.10) | 3.49 × 10−01 |
| 3rd vs. 1st Quartile | 1.29 (0.76–2.19) | 3.44 × 10−01 |
| 4th vs. 1st quartile | 2.35 (1.35–4.10) | 2.60 × 10−03 |
| African ancestry | 0.48 (0.10–2.41) | 3.74 × 10−01 |
| Sex (Female vs. Male) | 1.20 (0.82–1.77) | 3.46 × 10−01 |
| Age at first symptom | 0.98 (0.96–0.99) | 7.80 × 10−03 |
| Site | ||
| PR vs. UM | 1.98 (1.11–3.53) | 2.00 × 10−02 |
| USC vs. UM | 1.65 (1.00–2.71) | 4.88 × 10−02 |
| UCSF vs. UM | 1.33 (0.79–2.25) | 2.83 × 10−01 |
- Bold indicates the highest quartile of NA ancestry increases the likelihood of ON presentaiton by more than 2 fold.
- PR, San Juan MS Center in Puerto Rico; UM, University of Miami; USC, University of Southern California; UCSF, University of California, San Francisco.
Age of onset
When we evaluated the association of genetic ancestry with age at first symptom using multivariate linear regression we observed a significant association between genetic ancestry and age of first symptom (P = 6.37 × 10−04 for the joint test of NA and African ancestry). Both an increase in African (beta = −10.07, P = 1.39 × 10−03) and NA (beta = −5.58, P = 3.49 × 10−02) ancestry contributed to a younger age at first symptom (Table 2, Fig. S1), although heterogeneity across sites was observed. The presence of any family history or sex does not appear to significantly impact the likelihood of presenting with MS at a younger age of onset. A sensitivity analysis confirmed that limiting family history to first-degree relatives also showed no association with age of onset (P = 0.342).
Discussion
Using 14,731 independent genetic markers, we estimated global ancestry among the largest collection of self-identified Hispanics with MS in the United States. We found significant associations between increasing NA ancestry and both clinical presentation of ON and earlier age of disease onset in MS. In addition, we observed that Hispanics with MS are admixed and as expected derive from the original indigenous natives of America and both European and African settlers.31 These findings are in line with previous reports that reflect the expected settlers in the regions ascertained. The Hispanic population is diversely distributed, with persons of Mexican origin mostly living in the southwestern parts of the United States. Those of South American, Puerto Rico, and Cuban origins reside primarily along the East Coast of the United States.32, 33 Notable differences have been reported in ancestral admixture: those of Mexican origin tend to have higher proportions of NA, while the same is true of Puerto Rico and African ancestry.34, 35 These findings highlight the importance of incorporating population genetic structure in clinical MS studies.
The most common pathologic basis for optic neuritis is likely to be inflammatory demyelination of the optic nerve occurring at presentation in ~20% of all MS cases and typically presenting with a subacute painful visual loss.36 The few reports comparing Hispanics and non-Hispanic Whites with MS indicate this to be most common in Hispanics of Mexican and Central American background, alluding to their common Asian heritage as a potential source.3, 4, 8 An early study from Mexico reported ON as presenting symptom in 33%, a much higher proportion when compared to White historical cohorts.8 The observations that any deviation in the presentation and clinical progression of MS in Hispanics from Whites could be influenced by genetic admixture, until now, were simply speculative.4, 8, 10, 37
While GWAS studies in MS have been successful in providing us with >200 candidate genes of interest outside of the MHC in Whites,38 we have little information regarding candidate genes in other populations nor their candidacy related to the regional distribution of where MS plaques or demyelination occur. The possibility exists that genetic and to some extent environmental factors in MS related to ethnicity are playing a role in characterizing the process of axonal loss and neurodegeneration, neuroprotection, and perhaps neurorestoration as well. Studies of other chronic diseases, such as Type 2 Diabetes, have observed that Hispanics have a higher prevalence of diabetic retinopathy compared to Whites.39 In a recent study using genetic admixture in Hispanics, having a higher proportion of Native American ancestry was found to be associated with severe diabetic retinopathy, alluding to the possible genetic effect on diabetes’ manifestation.18 Studies of systemic lupus erythematous have also identified an increase in autoantibody reactivity in non-Whites,40 leading to shifts in the understanding that genetically determined ancestry is involved in the biological processes that increase lupus prevalence and disease severity. Thus, we propose that future studies should incorporate multiethnic cohorts and genetic ancestry to better address markers of disease process in both ON and MS.
We find that global ancestry estimates partly account for the observed ON presentation in Hispanics with MS and could be responsible for the predominant observation of optic nerve and motor involvement in MS across Hispanics in the United States and Latin America.41, 42 However, sex and age of onset had less of an effect on the risk of ON as a first symptom of MS. Of note, because not all patients presenting with ON will go on to develop MS43, our results suggest more broadly that studies of ON in non-MS cohorts may benefit from studies of admixed populations.
Predominantly White natural history cohorts have suggested a favorable prognosis for presentation with ON as a first symptom.44, 45 However, recent reports have demonstrated that this may not necessarily indicate a phenotypic advantage.46, 47 Differences in ON recovery and severity of episodes have been observed in African Americans when compared to Whites.46, 47 Future studies on the relationship between ON presentation, ancestry, and severity would be beneficial.
Our finding that higher proportions of NA and African ancestry increase the chance of presenting with MS at a younger age is of great interest as well. A recent study that compared Hispanics and African Americans to Whites ascertained in the Northeastern United States, with similar socioeconomic background, indicated that Hispanics have a younger age of onset compared to Whites and a similar age of onset to African Americans.37 Previous studies have also observed an even more pronounced age of onset difference between U.S. and non-U.S. born Hispanics, suggesting that geographical and environmental changes are also likely to influence clinical onset.4, 48 This could in part explain the heterogeneity of the observed ancestral effects across sites. Unfortunately, because factors related to place of birth and age of migration to the United States are not currently available for this cohort, we are unable to incorporate these factors. Nevertheless, because we were concerned about the potential residual confounding that geographical variation could pose to our investigation we included site of ascertainment in our models. In the future, as we examine disease severity by race and ethnicity, an investigation of the relationship of genetic ancestry and sociodemographic factors15 in MS will be important. In order to pinpoint the genetic regions underlying our observed effects of increased NA ancestry, future studies may focus on local ancestry and include an assessment of the previously reported relationship between HLA-DRB1 positivity and an earlier age of onset.49, 50
Limitations and strengths
There are several limitations to our study including the small, geographically limited, reference NA dataset available from HGDP, which curtails the degree of precision in our NA ancestry estimates and our ability to infer causal relationships. We acknowledge that self-reported ethnicity has relevance in health disparity and that sociocultural and socioeconomic (SES) factors may influence who seeks care for ON, potentially resulting in over- or underreporting. Whether SES is a confounder in the analysis of genetic ancestry and phenotypic presentation of MS is unknown. Nevertheless, while our study did not specifically adjust for SES, we did adjust for recruitment site intentionally to account for any potential residual confounding. Research supports that lower SES is associated with poor access to care and non-European ancestry,15 and that MS risk in Hispanics is associated with greater European ancestry,12 then it is possible that the recruitment is biased toward those that have greater access to care, have higher SES, and greater European proportions would be expected since ON is a common presentation in Hispanics.4 Thus, the possibility that our results are due to SES makes it less likely and any concern for an overestimation of ON in Hispanics with high Native American ancestry biasing our results should be lessen. Nevertheless, our study should follow with more granular analysis specific to ON outcomes (i.e., severity, recovery, OCT measures) and SES measurements (insurance status, language barriers, time to diagnosis, medication compliance, level of education, cultural differences, etc.). In addition, registry-based participation and the confirmation of diagnosis with ON via medical record lessens the potential of overreporting. Yet, there are several important strengths which includes the large sample size, the priori definitions used to assess Hispanic and the consistency of data collection used across MS centers which combined are representative of the broad and diverse Hispanic U.S. population. The potential for generalizability is also a strength that should encourage future studies of genetic predispositions for modifiable factors using genetic ancestry. Leveraging genetic ancestry may prove helpful in uncovering novel disease mechanisms underlying clinical outcomes in MS; something that has not previously been successful in even the largest MS genetic datasets of European ancestry.
Conclusion
The integration of global ancestry estimates in our assessment of ON as first symptom and age of onset with MS in Hispanics provides a framework for utilizing admixture to elucidate genetic factors contributing to the observed clinical variation in MS. The recent formation of the first U.S.-based Hispanic MS consortium, ARHMS will serve as a fundamental resource to better understand how clinical, genetic, and environmental factors interplay in MS risk, disability, and clinical features within the Hispanic population.
Acknowledgments
The authors thank Ana Palomeque and Jose Aparicio (USC), Stacy Caillier, and Adam Santaniello (UCSF) for their contributions to data collection/recruitment and the individuals from the diverse MS registries across sites for their participation in the study. We also acknowledge the Center for Genome Technology within the University of Miami John P. Hussman Institute for Human Genomics for generating the genotype data for this project.
Author Contributions
Dr. Amezcua had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Amezcua, Beecham, Oksenberg, McCauley. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Amezcua, Beecham, Oksenberg, McCauley. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Amezcua, Conti, Beecham. Obtained funding: Amezcua, Oksenberg, McCauley. Administrative, technical, or material support: Manrique, Gomez, Lund. Study supervision: Amezcua, McCauley.
Conflict of Interests
The authors report no relevant conflict of interests.




