Primary resistance of human patients to botulinum neurotoxins A and B
Funding Information:
The financial support of the “Interomics” CNR Project to C.M. is gratefully acknowledged.
Abstract
Botulinum neurotoxin serotypes A and B are successfully used to treat a variety of human diseases characterized by hyperactive peripheral nerve terminals. However, a number of patients are primary resistant to these pharmaceuticals, without having antitoxin-neutralizing antibodies. A straightforward explanation of this phenomenon posits that mutations of the toxin sites of interaction with their receptors or protein substrates prevent their neuroparalytic action. After a careful investigation of available human genomic databases, we conclude that it is very unlikely that humans are resistant to these two therapeutic neurotoxins because of mutations that would affect their binding or intracellular proteolytic actions.
Introduction
Botulinum neurotoxin type A (BoNT/A) and type B (BoNT/B) are the two serotypes most frequently causing the peripheral flaccid paralysis of botulism in humans.1 This neuroparalysis is caused by very potent three-domain neurotoxins of 150 kDa produced by different bacteria of the genus Clostridium.2 Their lethal dose 50% in mice is around 0.4 ng/kg body weight, while in humans it is estimated to be similar for BoNT/A but higher for BoNT/B.2
Such high toxicity results from the specific binding of BoNTs to the presynaptic membrane of peripheral cholinergic neurons and to their intraneuronal enzymatic activity. Neurospecific binding is mediated by a double interaction with a polysialoganglioside and with a protein receptor.3 The loop 4 (L4) of the luminal glycosylated domain of the synaptic vesicle protein SV2 and the helical segments 39-50 of synaptotagmin I and 47– 58 of synaptotagmin II have been identified as the protein receptors of BoNT/A and BoNT/B, respectively.3 The 50 kDa N-terminal domains, termed L chain, of BoNT/A and/B are metalloproteases that enter the nerve terminal cytosol and cleave one of the three SNARE proteins, thus blocking neurotransmitter release.2 The L chain of BoNT/B cleaves VAMP at the peptide bond Gln76-Phe77,4 while the L chain of BoNT/A cleaves SNAP-25 at its carboxyl terminal peptide bond Gln197-Arg198.5, 6 These single biochemical lesions lead to a long-lasting paralysis: 3–4 months at human skeletal muscles for BoNT/A and 1–2 months for BoNT/B. This paralysis is reversible upon degradation of the L chain and the affected muscle regains its full functional activity.7
Based on these three remarkable properties (neurospecificity, enzymatic activity, and reversibility), BoNT/A has become the therapeutic choice in the treatment of humans affected by a variety of syndromes that, independently from the primary cause, are characterized by a hyperactivity of cholinergic peripheral nerve terminals that in turn cause hyperfunction of the innervated cell.2, 8 The therapeutic and cosmetic uses of BoNT/A are continuously increasing. In addition, BoNT/B is commercially available.3, 9
BoNT/A is a poor antigen for humans, and rarely the repeated injections lead to immunization of patients with consequent acquired resistance to further BoNT/A treatments. BoNT/B is very useful in the case of formation of anti-BoNT/A antibodies.10, 11 For these patients, BoNT/C has been also proposed as a valid replacement.12
A still unexplained phenomenon is the existence of patients that are insensitive from the first injection, with consequent therapy failure.13 As discussed previously,13 this lack of sensitivity to BoNTs can be attributed to a number of reasons that go from inappropriate diagnosis to inappropriate target muscle selection or injection, from the use of an amount of BoNT insufficient to elicit the desired pharmacological outcome to the presence of antitoxin-neutralizing antibodies. Additionally, individuals carrying mutations at SNARE cleavage sites are expected to be BoNT insensitive. Yet, this latter hypothesis has been recently discarded because humans do not display mutations with significantly high frequency in the SNARE peptide bonds cleaved by BoNTs.14
Here, we have considered the possibility that the amino acid residues of the protein receptors involved in binding the two BoNTs are mutated. More precisely, we tested the possibility that some humans carry mutations at: (1) the toxin-binding sites located on the L4 loop of the synaptic vesicle luminal domain of SV2C, and of the related proteins SV2A and B, or (2) the luminal C-terminal segments of synaptotagmins 1 and 2. We pursued this hypothesis by analyzing the presently available databases of human mutations15 and the results obtained are described here.
Materials and Methods
Mutation frequency of the following human proteins: SV2 A, B, and C which are the receptors of BoNT/A, and synaptotagmins 1 and 2 which are the receptors of BoNT/B, were searched within the existing data bases on human genomes. We investigated the Exome Aggregation Consortium (ExAC), the Genome Aggregation Database (gnomAD) which include 126,216 exome sequences and 15,136 whole-genome sequences extracted from a variety of large-scale sequencing projects15 that were obtained from http://www.exac.broadinstitute.org/ and from http://gnomad.broadinstitute.org/. This bioinformatics search was performed following instructions contained in these URLs. Amino acid residues involved in toxin binding and proteolysis of their SNARE substrates were deduced from crystallographic data.3, 16, 17 Figures summarizing the frequency mutations of the human proteins mentioned above were made using http://www.uniprot.org/.
Results
The results of the searches of the available human genome databases for mutation within the luminal domain of the three isoforms of the synaptic vesicle protein SV2, which has been identified as the receptor-binding site of BoNT/A,2, 3, 16, 17 are given in Figures 1 and 2. We considered here only the protein-coding genetic variants (translated region) with respect to the canonical sequence of the corresponding protein.
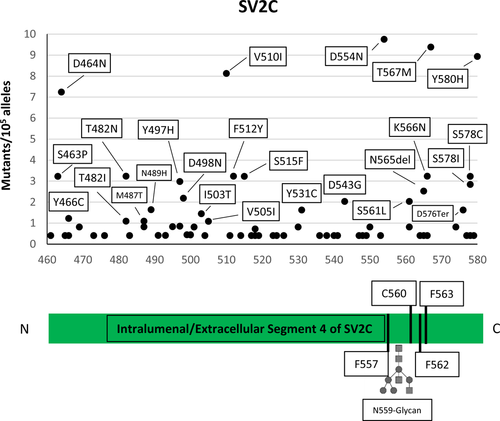
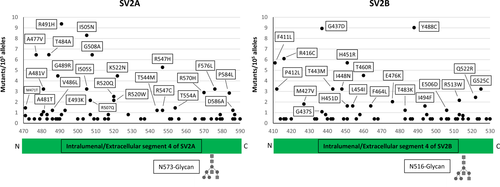
When compared to proteins known to carry high-sequence variability without functional consequences, for example, major histocompatibility complex proteins (HLA-A/B/C) or olfactory receptors, the number of amino acid substitutions in the L4 segments of the SV2 A, B, and C proteins is dramatically lower.18 Very significantly, no mutants of the aminoacid residues directly involved in BoNT/A binding, including Asn 573 (SV2A), Asn 516 (SV2B), and Asn 559 (SV2C), whose posttranslational glycosylation plays an essential role in the high-affinity interaction with BoNT/A-binding domain17, 19 were found. This observation suggests that this domain, located inside the synaptic vesicle lumen, and exposed to the extracellular medium only upon vesicle fusion with the presynaptic membrane during neurotransmitter release, is extremely conserved and cannot be altered without deleterious consequences for human physiology.
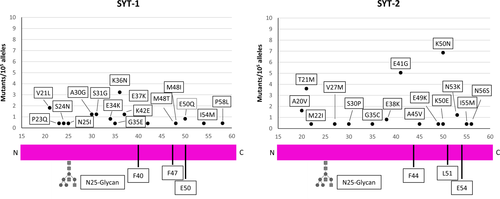
The N-terminal part of synaptotagmin isoforms Syt-1 and Syt-2 projects in the interior of synaptic vesicles and becomes exposed to the extracellular fluid when the vesicles fuse with the presynaptic membrane to release their neurotransmitter content. At this moment, BoNT/B, already bound to the extracellular leaflet of the membrane via a polysialoganglioside molecule, can bind to the N-terminal segment of Syt-1 and Syt-2.20 The three residues of Syt governing the interaction with BoNT/B are two Phe and one Glu residues, located on the same side of an alpha-helical segment (Fig. 3). Only one mutant has been identified (left panel of Fig. 3): Glu50 of Syt-1 is changed in Gln and this is expected to impact negatively on the binding affinity of BoNT/B as the ionic interaction with Lys1192 is lost.6 However, it should be considered that the mutation is heterozygous and therefore it should not affect the overall BoNT/B binding and sensitivity.
Discussion
Here, we found that none of the residues of the L4 loop of the intravesicular domains of the three proteins SV2 (A, B, and C), essential for BoNT/A binding, are mutated in the human genome databases that presently include >120,000 genomes. The key residues of the protein receptor SV2C for the binding of BoNT/A are conserved in mice and in humans and indeed the sensitivity of these two species to BoNT/A is very similar.
In the case of BoNT/B, only one mutation in one of the three residues important for BoNT/B binding to its protein receptor Syt-1 has been observed. However, the frequency of this E50Q mutation is very low and it has been detected in heterozygosis. Moreover, the main protein receptor of BoNT/B at the neuromuscular junction is Syt-2, which is the Syt isoform expressed in all motor axon terminals, differently from Syt-1 that is present only in a subpopulation of motoneurons.21 We found no mutants of the key residues of Syt-2 essential for BoNT/B binding. These residues are partially conserved among mice and humans. In fact, Phe 54 in mouse synaptotagmin-2 is substituted by a Leu (L51) in human Syt-2.22 This variant, present only in humans and in chimpanzees, eliminates one of three major interactions between Syt-2 and BoNT/B and, together with the low expression of Syt-1 at the NMJ, it explains why BoNT/B has to be administered in 40-fold higher dosages than BoNT/A to treat muscle disorders.3, 9
We have recently performed an analysis of the human mutations at the cleavage sites of all the botulinum neurotoxins and have found no mutations at the BoNT/B cleavage site in human VAMP-1 and -2.14 In the same analysis, we found only two heterozygous mutants at the BoNT/A cleavage site of SNAP-25 out of >120,000 genomes. These findings do not support the possibility that primary resistance to BoNT/A or BoNT/B are due to mutations at the cleavage sites of the two proteins target of the metalloproteolytic actions of these neurotoxins.
Considering the size of the data bases analyzed, the lack of mutations affecting either binding or substrate cleavage of BoNT/A and BoNT/B strongly suggest that patients primary resistant to the BoNTs do not carry mutations that prevent binding to their receptors or cleavage of their substrates.
Acknowledgments
The present work is in partial fulfillment of the Doctorate thesis of S.C. at the University of Ulm. The financial support of the “Interomics” CNR Project to C.M. is gratefully acknowledged.
Conflict of Interest
None declared.
Author Contributions
C.M., M.P., O.R., S.C., and H. B. conceived the project; S.C. and M.P. performed the databases searches; C.M. and M.P. wrote the paper with contributions of all coauthors.




