Remote neurodegeneration in the lumbosacral cord one month after spinal cord injury: a cross-sectional MRI study
Abstract
Objective
To characterize structural integrity of the lumbosacral enlargement and conus medullaris within one month after spinal cord injury (SCI).
Methods
Lumbosacral cord MRI data were acquired in patients with sudden onset (<7 days) SCI at the cervical or thoracic level approximately one month after injury and in healthy controls. Tissue integrity and loss were evaluated through diffusion tensor (DTI) and T2*-weighted imaging (cross-sectional area [CSA] measurements). Associations with the degree of neurological impairment were assessed using linear mixed-effects models.
Results
Twenty-one patients with SCI showed lower white matter (WM) fractional anisotropy (FA) (≤−13.3%) and higher WM radial diffusivity (≤14.6%) compared to 27 healthy controls. Differences were most pronounced in the lateral columns of WM. CSA measurements revealed no group differences. For the lateral columns, lower FA values were associated with lower motor scores and lower amplitudes of motor evoked potentials. For the dorsal columns, lower FA values were associated with lower amplitudes of somatosensory evoked potentials from the lower extremities.
Interpretation
One month after SCI, first signs of WM degeneration were apparent, without indication of tissue loss. The more pronounced differences observed in the lateral column could be attributed to anterograde degeneration of the motor tracts. The variability among DTI measurements remote from the lesion site can be partially explained by the degree of the SCI-induced neurological impairment. Together with previous studies, our findings indicate that impaired tissue integrity precedes tissue loss. The presented techniques have potential applications in monitoring the progression of various neurological diseases.
Introduction
Injuries to the spinal cord (SC), whether traumatic (e.g., falls, traffic accidents) or non-traumatic (e.g., vascular or metabolic disorders), often result in motor and sensory dysfunction, along with impairment of lower urinary tract, sexual, and bowel functions.1-3 Besides the focal damage at the injury site, spinal cord injury (SCI) also triggers a cascade of secondary pathological processes which have been shown to result in degeneration above and below the level of injury, reaching even the lumbosacral cord.4, 5 Literature and preclinical evidence suggest that after SCI, efferent tracts (descending, e.g., corticospinal tract) predominantly undergo relatively fast and far-reaching anterograde (Wallerian) degeneration caudal to the lesion site. In contrast, retrograde degeneration of afferent tracts (ascending) has been observed only over short distances, typically within millimeters from the lesion site.6-9
The integrity of the lumbosacral cord is of particular interest as this region innervates the lower limbs10 and the lower urinary tract.11 Furthermore, it is a key relay station for neuromodulatory approaches applying electrical stimulation to the nerves in this region in order to improve or preserve, for example, lower urinary tract function.12-14 In how far neuromodulation can change the function and structure of this region is largely unknown and currently under further investigation.15-17
Atrophy of the SC can be quantified in vivo by measuring the cross-sectional area (CSA) of the SC, gray matter (GM), and white matter (WM) in axial images of the SC acquired by magnetic resonance imaging (MRI).18 Complementary information about WM tissue integrity can be gained by advanced MRI techniques, such as diffusion MRI.4, 5, 19-21 The feasibility of diffusion tensor imaging (DTI) and manual GM and WM segmentation has been demonstrated within the lumbosacral enlargement (LSE)22, 23 and conus medullaris (CM)24, 25 in healthy volunteers. Evidence of remote neurodegeneration within the LSE after cervical or thoracic SCI has been found4, 5, 21 in both the chronic (>1 year post-injury)5 and the subacute stage (2 months post-injury).4 However, the presence and degree of potential changes at earlier timepoints have not been investigated so far26 and neuropathological evidence suggests that early tissue loss may also occur below the level of the LSE.27 Investigations early after SCI would be of high relevance considering recent interventional studies (e.g., neuromodulative, neuroprotective, or neuroregenerative), which are often initiated in early stages after SCI.16, 28, 29 In this context, it is important to initiate treatment before further, often irreversible functional damage (e.g., detrusor overactivity) occurs, which is potentially caused by C-fiber mediated reflexes and with possible life-threatening effects such as kidney failure.
The aim of this study was to investigate the structural integrity of the LSE and CM one month after SCI. High-resolution T2*-weighted structural imaging in patients and healthy controls was acquired to obtain the CSA of SC, GM, and WM and spinal cord DTI to assess the integrity of WM columns. Furthermore, the relationship between structural measurements below the level of injury and neurological impairment due to the SCI was explored. To this end, we investigated the association of tract-specific DTI metrics in the lumbosacral cord to corresponding clinical and electrophysiological measures.
Considering anterograde degeneration, which begins shortly after injury,30, 31 we hypothesized that, remote from the injury site, initial impairments of structural integrity can be detected as early as one month after injury. Considering that axonal degeneration and myelin sheath disintegration occurs before the removal of axonal and myelin debris,32, 33 we primarily expect differences in DTI metrics rather than CSA measurements between patients and healthy controls.
Methods
Standard protocol approvals, registrations, and patient consents
The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich, BASEC ID: 2019-00074) and was conducted in accordance with the Declaration of Helsinki. Prior to participation, written informed consent was obtained from all study participants.
Study participants
Twenty-one patients with SCI (14 traumatic [67%], 8 females [38%], 13 males [62%]), age (median [range]: 58 [18–81] years), time since injury (median [range]: 33 [28–42] days) and 27 healthy controls (13 females [48%], 14 males [52%], age: 37 [20–70] years) were included for this analysis. The type of injury was classified according to the International Spinal Cord Injury Core Data Set (version 3.0).34 Demographic and clinical information of the patients with SCI is listed in Table 1. All patients with SCI were recruited from the Spinal Cord Injury Center at Balgrist University Hospital, Zürich, Switzerland, between December 2019 and April 2023. The patient cohort was part of a randomized controlled trial investigating the effect of transcutaneous tibial nerve stimulation on the emergence of neurogenic lower urinary dysfunction following SCI (TASCI, ClinicalTrials.gov identifier: NCT03965299).16 All data presented in this study were acquired at baseline before the onset of potential treatment effects of the intervention. The main inclusion criteria were: no contraindications for MRI and ≥18 years of age; for healthy controls, no known medical condition, signs or symptoms of central or peripheral nervous system disorder based on history taking and a comprehensive neurophysiology assessment; for patients: traumatic or non-traumatic SCI with cervical and/or thoracic lesions with a sudden onset (<7 days), and no serious neurodegenerative disease (e.g., multiple sclerosis, Parkinson's disease). For a more comprehensive list of inclusion and exclusion criteria specific to patients with SCI, refer to Birkhäuser et al. 2020.16
| ID | Sex | Categorized agec | Neurological level of injury | AIS | Type of injury | Etiology | UEMS (max. 50) | LEMS (L/R) (max. 25/25) | LELT (L/R) (max. 16/16) | LEPP (L/R) (max. 16/16) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 18–30 | T2 | A | Traumatic | Dislocation fracture (C1, T8-9) | 50 | 0/0 | 0/0 | 0/0 |
| 2 | M | 18–30 | T5 | A | Traumatic | Dislocation fracture (T7-T8) | 50 | 0/0 | 0/0 | 0/0 |
| 3 | F | 46–60 | T6 | B | Traumatica | Contusion (T8-T10) | NA (50) | 0/0 | 8/7 | 1/4 |
| 4 | M | 31–45 | T2 | C | Traumatic | Epidural hematoma (C7-T4) | 50 | 18/1 | 8/8 | 0/0 |
| 5 | M | 61–75 | C1 | D | Traumatic | Contusion (C3-5) | 29 | 16/10 | 8/8 | 8/8 |
| 6 | F | 61–75 | C3 | D | Traumatic | Contusion (C4-6) | 30 | 14/15 | 8/8 | 8/0 |
| 7 | M | 31–45 | T6 | D | Traumaticb | Tumor resection (T3-T9) | 50 | 22/15 | 8/8 | 8/8 |
| 8 | F | 76+ | C5 | D | Traumatic | Epidural hematoma (C2-T4) | 27 | 17/23 | 12/16 | 8/16 |
| 9 | F | 46–60 | C4 | D | Traumaticb | Intramedullar edema (C5-C6) | 46 | 25/23 | 8/8 | 8/8 |
| 10 | M | 18–30 | C6 | D | Traumatic | Dislocation fracture (C5-6)/Contusion (T3-4) | 39 | 23/25 | 16/16 | 16/14 |
| 11 | M | 46–60 | C1 | D | Traumatic | Dislocation fracture (C1) | 41 | 25/24 | 8/8 | 0/8 |
| 12 | F | 61–75 | C3 | D | Traumatic | Contusion (C5-6) | 41 | 24/25 | 16/16 | 16/16 |
| 13 | M | 31–45 | C3 | D | Traumatic | Contusion (C4-7) | NA (47) | 25/25 | 8/8 | 8/8 |
| 14 | F | 18–30 | C4 | D | Traumatic | Dislocation fracture (C4-C5) | 45 | 25/25 | 16/16 | 16/16 |
| 15 | M | 46–60 | T3 | B | Non-traumatic | Ischemia (T3) | 50 | 0/0 | 8/1 | 0/0 |
| 16 | F | 46–60 | C1 | D | Non-traumatic | Epidural hematoma (C1-L5) | 49 | 20/20 | 8/8 | 4/2 |
| 17 | M | 61–75 | T5 | D | Non-traumatic | Ischemia (T5) | 50 | 18/25 | 8/16 | 8/16 |
| 18 | F | 61–75 | C5 | D | Non-traumatic | Epidural abscess (C2-7) | 35 | 19/24 | 16/16 | 16/16 |
| 19 | M | 61–75 | C1 | D | Non-traumatic | Subdural hematoma (C3-T10) | 50 | 24/20 | 8/8 | 3/7 |
| 20 | M | 18–30 | T6 | D | Non-traumatic | Acute myelitis | 50 | 25/22 | 8/8 | 8/8 |
| 21 | M | 76+ | C5 | D | Non-traumatic | Contusion (C3-5) | 46 | 25/25 | 16/16 | 1/1 |
- AIS, American Spinal Injury Association Impairment Scale; F, female; L/R, left/right; LEMS, International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) lower extremity motor score; LELT, ISNCSCI lower extremity light touch score; LEPP, ISNCSCI lower extremity pinprick score; M, male; NA, not available due to upper extremity injury (values assessed 3 months after SCI); UEMS, ISNCSCI upper extremity motor score.
- a Diffusion MRI not available.
- b Excluded from restrictive traumatic group analysis due to a known history of neurological symptoms prior to the spinal cord injury.
- c Age is shown categorized according to the recommendations of the International Spinal Cord Society.34
Clinical examination
The neurological impairment, including neurological level and SCI severity (using the American Spinal Injury Association (ASIA) Impariment Scale (AIS) grade) of patients with SCI was assessed according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) protocol.35 Motor scores between the neurological levels L2 and S1 were summed and are referred to as the lower extremity motor score (LEMS). Sensory scores between the neurological levels L2 and S4-5 were summed and are referred to as lower extremity light touch (LELT) and pinprick scores (LEPP).
Electrophysiological assessments
Cortical responses of somatosensory evoked potentials (SSEPs) were recorded with the active electrode at Cz’ and referenced to Fz according to the international 10–20 electroencephalography system. The electrode impedance was kept below 5 kΩ. Electrical stimulation of the tibial nerve was applied at the medial malleolus (left and right leg) and the pudendal nerve (using a square wave with a pulse duration of 0.2 ms and a frequency of 3.1 Hz). A minimum of 2 runs of 200 stimuli each were averaged and superimposed. The latency was measured from the stimulation onset until the P40 peak of the primary complex. The peak-to-peak amplitude P40N50 was calculated as the difference between the P40 and N50 peak.
Motor evoked potentials (MEPs) of the abductor hallucis and tibialis anterior were simultaneously acquired for each leg by applying single-pulse transcranial magnetic stimulation using a biphasic stimulus duration of 0.2 ms. The coil was placed centrally over Cz. A sampling frequency of 2000 Hz and a band-pass filter of 10 Hz–2 kHz were used. Latency of MEPs was determined as the time from the stimulation until the onset of the muscle response. The amplitude of MEPs was measured from baseline to the highest peak of the potential.
For MEPs and SSEP analysis, patients without any recordable potential were assigned an amplitude of 0 mV/μV, while the latency values remained missing.
MRI acquisition
All MRI scans were performed using a 3T Siemens Prisma scanner (Siemens Healthineers, Erlangen, Germany) equipped with a body transmit coil and a standard 32-channel spine matrix coil. As an anatomical reference of the lumbosacral cord, a sagittal T2-weighted turbo spin echo sequence was acquired with 15 slices of 4 mm thickness (10% slice gap), in-plane resolution of 0.7 × 0.7 mm2, field of view (FOV) of 330 × 330 mm2, repetition time (TR) of 3 s, echo time (TE) of 89 ms, flip angle of 154°, and an acquisition time of 00:59 min (Fig. 1A).
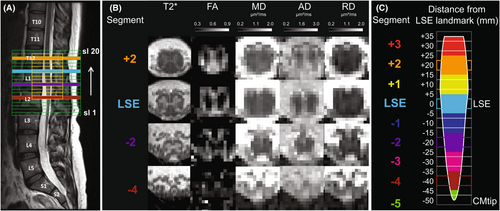
Subsequently, axial T2*-weighted images were acquired using a 3D spoiled multi-echo gradient-echo sequence (Siemens FLASH) with 20 axial-oblique slices and 5 mm thickness (no gap) (Fig. 1B). To account for the positional variation of the lumbosacral cord in relation to the vertebral levels,36 the FOV was not fixed to certain vertebral levels. Instead, it was set such that the sixth most rostral slice corresponded with the maximum width of the spinal cord as observed in the sagittal T2-weighted image, as described in our previous publications,25, 37 ensuring coverage of both the LSE and CM. The slice stack was positioned perpendicularly to the longitudinal axis of the cord (Fig. 1A). Sequence parameters were: in-plane resolution 0.5 × 0.5 mm2, in-plane FOV of 192 × 192 mm2, TR of 38 ms, echo train length of 5, first echo time of 6.85 ms, echo spacing of 4 ms, flip angle of 8°, 8 repetitions, GRAPPA 2×, no partial Fourier, anterior–posterior phase-encoding direction, no navigator echoes, bandwidth of 260 Hz/pixel, and an acquisition time of 17:56 min.37
Diffusion MRI consisted of 180 diffusion weighted (b = 800 s/mm2) and 6 T2-weighted (b = 0 s/mm2) images and was acquired using a reduced-FOV single-shot spin-echo echo planar imaging sequence. The slice stack of 15 slices, each with a 5 mm thickness and no gap, was arranged so that the central slice coincided with the ninth most rostral slice of the axial T2*-weighted scan, given the smaller size of the diffusion MRI slice stack (Fig. 1A). Sequence parameters were: in-plane resolution of 0.9 × 0.9 mm2, in-plane FOV of 86 × 32 mm2, TR of 440 ms, TE of 56 ms, no GRAPPA, 7/8 phase partial Fourier in the anterior–posterior phase-encoding direction, interleaved slice acquisition scheme, and bandwidth of 1270 Hz/pixel. The acquisition was cardiac gated (3 slices per cycle, trigger delay of 120 ms) and lasted around 12 min, depending on the individual heart rate.
Processing of ME-GRE images
For each repetition, the first three echoes of the ME-GRE scan were combined via root-mean-squares summation, as we previously demonstrated it to be the optimal combination for segmenting the SC and GM within the same image.37 The resulting combined echoes were then averaged across repetitions. The SC and GM were manually segmented by an experienced rater (S.B.), who was provided with a pseudo-randomized and pseudonymized set of images. Segmentation was performed according to a standard operating procedure, accessible on GitHub (https://github.com/NeuroimagingBalgrist/LumbosacralCordMRI), using the sub-voxel segmentation tool in JIM 7.0 (Xinapse systems), providing corresponding CSA values. WM CSA was obtained by subtracting GM CSA from SC CSA.
The slice with the greatest GM CSA, referred to as the “LSE landmark,” was used as a neuroanatomical landmark, as recommended in our previous study.25 To determine the LSE landmark, the curve of slice-wise GM CSA values was smoothed using moving window averaging across three consecutive slices (corresponding to 15 mm). The “CMtip landmark” was determined by extrapolating the curve of slice-wise SC CSA values to zero. Note that the most caudal slice where SC was segmented was usually 1–2 slices above the CMtip landmark.
Processing of diffusion MRI
The diffusion MRI images were processed using the SPM-based ACID toolbox.38 After cropping all images to an in-plane FOV of 32 × 32 mm2, eddy current and motion correction was applied using ECMOCO,39 consisting of a 3-degrees-of-freedom (DOF) volume-wise registration (translation along x and y and scaling along y, with x and y being the frequency- and phase-encoding direction, respectively) followed by a 2-DOF slice-wise registration (translation and scaling along y). Images underwent adaptive smoothing using msPOAS. The diffusion tensor model was fitted on the corrected images using a robust tensor fitting algorithm40, 41 to generate maps of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD).
The mean ECMOCO-corrected diffusion-weighted (b = 800 s/mm2) image was manually segmented for spinal cord in FSLeyes. The same image was spatially normalized to the PAM50 spinal cord template using the sct_register_to_template function,42 yielding both forward (native to template space) and backward (template to native space) warping fields. Normalization involved aligning the LSE and CMtip landmarks with corresponding labels in the PAM50 space. In particular, the tip of the spinal cord was matched with “label 60” of the PAM50 template, while the LSE landmark was matched with a custom-added label (“label 59”) located at slice 143 (z = −490.34 mm). This slice corresponds to the mid-T12 vertebral level and the caudal end of neurological level L3, according to the PAM50 neurological segments, which rely on a previously published review.43
The probabilistic WM atlas of the PAM50 template was then warped into the native space using the obtained backward warping field. To account for registration errors, probabilistic WM masks were manually inspected and corrected if necessary. Weighted average values of DTI metrics were extracted slice-wise within the entire SC, GM, and WM, as well as specific WM subregions such as the dorsal, lateral, and ventral WM columns, using sct_extract_metric from the Spinal Cord Toolbox (v.6.2).44 DTI metrics were extracted only from the rostral half of the CM, as the PAM50 template does not encompass the caudal half.
Adjustment for the individual length of the conus medullaris
To adjust for the individual CM length, we divided the CM, that is, the space between the LSE and CMtip landmarks, into segments of equal thickness in each participant, as previously described.25 One segment is centered at the LSE landmark (segment LSE), another one at the tip of the spinal cord (segment LSE-5), and the space between them is covered by four segments. Using the same segment thickness, the spinal cord rostral to the LSE landmark is also divided into three segments (Fig. 1C). Without reslicing the images, we extracted CSA values and DTI metrics within each segment, computed as a weighted average of the slice-wise values, where the weights represent the spatial contribution of each slice to the particular segment. If a value for a slice which contributes more than 25% to the segment was not available, the value for that segment was not calculated.
Statistical analysis
We conducted normality tests (histograms, Q–Q plots, Shapiro–Wilk test) and subsequently applied appropriate statistical measures. Analysis of variance (ANOVA) on linear mixed-effects models was used to assess between-group differences in CSA (SC, GM, WM) and DTI metrics, including the main effects of group (between-subject) and segment (within-subject, categorical), their interaction, and the effect of age and CM length as covariates. Participants were treated as random effects to account for repeated within-subject measurements. Furthermore, segment-wise mean, standard deviation, and effect size (Cohen's d) are reported.
In patients with SCI, linear mixed-effects models were used to investigate the relationship between MRI outcomes, clinical scores, and electrophysiological measures. To mitigate the effects of outliers and variability of the amplitude of MEPs and SSEPs, clinical scores and electrophysiological measures were converted to ranks using the fractional ranks method. Ties were accounted for by averaging the ranks of tied values. Latency analysis of the electrophysiological data was not performed due to the considerable number of missing data in patients.
Since outcome measures from the ISNCSCI exam broadly inform about spinal tract deficits,35 they were correlated to DTI metrics of the corresponding WM column within the spinal cord (LEMS to lateral columns on the ipsilateral side, LELT to dorsal columns on the ipsilateral side, and LEPP to lateral and ventral columns on the contralateral side). Electrophysiological measures are more tract-specific and probe ascending and descending spinal cord pathways.45 Since MEPs provide information about the integrity of the corticospinal tract, the ranked amplitudes were correlated to DTI metrics from the lateral columns. SSEPs provide information about the integrity of sensory tracts and ranked amplitudes were therefore correlated to DTI metrics from the dorsal columns. In total WM analyses, the means across the values from both sides of the body (left and right) were used for DTI metrics, clinical, and electrophysiological measures.
To investigate segment-wise trajectories, an additional analysis was performed on a subgroup of participants with DTI values available for all investigated segments (complete case group). To explore the effect of a purely mechanical insult to the spinal cord, another subgroup analysis was performed on patients with traumatic SCI and no known history of neurological symptoms prior to SCI (restrictive traumatic group).
Results
The characteristics of the patient and control groups were similar in terms of sex (Fischer's exact test, P = 0.565), age (Mann–Whitney U test, P = 0.101), and the length of the CM (from the LSE landmark to the CMtip; median [range] in patients with SCI: 45 [35–60] mm, in healthy controls: 45 [35–55] mm; Mann–Whitney U test, P = 0.725). An overview of the electrophysiological characteristics of patients with SCI and controls is shown in the supplementary material (Table S1).
Diffusion tensor imaging
In one patient with SCI, diffusion-weighted images could not be acquired due to a metal implant in close proximity to the lumbosacral cord. Because of registration errors, a single slice was excluded from the DTI analysis in five participants (2 patients, 3 healthy controls).
The ANOVA on the linear mixed-effects model revealed that patients had lower FA (F(1, 42.60) = 13.91, P = 0.001) and higher RD (F(1, 43.63) = 5.14, P = 0.028) in the WM compared to healthy controls. Differences were most pronounced in the lateral WM columns (FA: F(1, 42.69) = 36.57, P < 0.001; AD: F(1, 43.22) = 6.45, P = 0.015); RD: (F(1, 43.54) = 5.37, P = 0.025) followed by the dorsal WM columns (FA: F(1, 42.52) = 6.11, P = 0.018) (detailed description in Table 2). Furthermore, there was a significant main effect of segment for FA, AD, and RD. The interaction between group and segment was significant for FA in the WM and lateral WM columns, indicating that the differences between groups varied across segments (Table S3). The effect size between patients with SCI and healthy controls was the highest rostral to the LSE landmark and decreased towards caudal segments (Table 2). Subgroup analyses for the complete case group (Fig. 2) and restrictive traumatic group (Table S2) yielded comparable results.
| Segment | Fractional anisotropy | Mean diffusivity (10−3 mm2/s) | Axial diffusivity (10−3 mm2/s) | Radial diffusivity (10−3 mm2/s) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients mean ± SD | Controls mean ± SD | Diff (%) | Effect size | Patients mean ± SD | Controls mean ± SD | Diff (%) | Effect size | Patients mean ± SD | Controls mean ± SD | Diff (%) | Effect size | Patients mean ± SD | Controls mean ± SD | Diff (%) | Effect size | ||
| White matter | +3 | 0.51 ± 0.07 | 0.58 ± 0.04 | −13.3 | −1.41 | 1.02 ± 0.16 | 0.99 ± 0.06 | 2.7 | 0.23 | 1.64 ± 0.19 | 1.74 ± 0.13 | −5.8 | −0.64 | 0.71 ± 0.17 | 0.62 ± 0.06 | 14.6 | 0.77 |
| +2 | 0.49 ± 0.06 | 0.55 ± 0.05 | −11.6 | −1.23 | 0.99 ± 0.07 | 0.96 ± 0.07 | 2.7 | 0.36 | 1.57 ± 0.10 | 1.64 ± 0.14 | −3.9 | −0.51 | 0.69 ± 0.08 | 0.62 ± 0.06 | 11.5 | 0.99 | |
| +1 | 0.46 ± 0.05 | 0.51 ± 0.04 | −8.2 | −0.87 | 0.95 ± 0.10 | 0.95 ± 0.07 | 0.5 | 0.05 | 1.48 ± 0.13 | 1.55 ± 0.11 | −4.0 | −0.52 | 0.69 ± 0.10 | 0.65 ± 0.07 | 5.8 | 0.45 | |
| LSE | 0.44 ± 0.05 | 0.47 ± 0.04 | −6.5 | −0.69 | 0.93 ± 0.12 | 0.93 ± 0.07 | 0.8 | 0.08 | 1.42 ± 0.15 | 1.46 ± 0.12 | −2.8 | −0.31 | 0.69 ± 0.11 | 0.66 ± 0.07 | 4.9 | 0.36 | |
| −1 | 0.42 ± 0.05 | 0.44 ± 0.04 | −4.5 | −0.41 | 0.93 ± 0.10 | 0.92 ± 0.08 | 0.9 | 0.09 | 1.37 ± 0.12 | 1.40 ± 0.15 | −1.7 | −0.17 | 0.71 ± 0.10 | 0.68 ± 0.06 | 3.5 | 0.30 | |
| −2 | 0.40 ± 0.06 | 0.42 ± 0.08 | −4.5 | −0.27 | 0.93 ± 0.10 | 0.91 ± 0.08 | 1.3 | 0.12 | 1.34 ± 0.15 | 1.36 ± 0.14 | −1.1 | −0.10 | 0.72 ± 0.09 | 0.69 ± 0.09 | 3.6 | 0.27 | |
| WM dorsal | +3 | 0.58 ± 0.09 | 0.65 ± 0.07 | −10.8 | −0.90 | 1.06 ± 0.20 | 1.03 ± 0.09 | 3.2 | 0.23 | 1.83 ± 0.20 | 1.91 ± 0.13 | −4.4 | −0.52 | 0.68 ± 0.22 | 0.59 ± 0.11 | 15.6 | 0.55 |
| +2 | 0.58 ± 0.07 | 0.63 ± 0.06 | −8.3 | −0.79 | 1.02 ± 0.11 | 0.98 ± 0.09 | 3.7 | 0.37 | 1.78 ± 0.17 | 1.81 ± 0.15 | −1.9 | −0.22 | 0.64 ± 0.11 | 0.57 ± 0.10 | 12.6 | 0.69 | |
| +1 | 0.57 ± 0.07 | 0.60 ± 0.06 | −4.8 | −0.46 | 0.96 ± 0.10 | 0.94 ± 0.06 | 1.8 | 0.21 | 1.66 ± 0.15 | 1.69 ± 0.11 | −1.4 | −0.19 | 0.60 ± 0.11 | 0.57 ± 0.08 | 6.5 | 0.41 | |
| LSE | 0.53 ± 0.07 | 0.56 ± 0.06 | −4.9 | −0.45 | 0.93 ± 0.11 | 0.92 ± 0.09 | 0.7 | 0.07 | 1.55 ± 0.13 | 1.58 ± 0.13 | −2.1 | −0.25 | 0.62 ± 0.12 | 0.59 ± 0.09 | 4.4 | 0.25 | |
| −1 | 0.49 ± 0.06 | 0.51 ± 0.05 | −3.6 | −0.32 | 0.89 ± 0.09 | 0.89 ± 0.11 | −0.4 | −0.04 | 1.42 ± 0.14 | 1.45 ± 0.18 | −1.8 | −0.16 | 0.62 ± 0.09 | 0.61 ± 0.09 | 1.3 | 0.09 | |
| −2 | 0.44 ± 0.06 | 0.47 ± 0.08 | −6.6 | −0.44 | 0.93 ± 0.15 | 0.91 ± 0.09 | 2.5 | 0.19 | 1.41 ± 0.20 | 1.42 ± 0.17 | −0.6 | −0.04 | 0.69 ± 0.13 | 0.65 ± 0.09 | 5.7 | 0.35 | |
| WM lateral | +3 | 0.47 ± 0.07 | 0.59 ± 0.04 | −19.5 | −2.07 | 0.99 ± 0.16 | 0.96 ± 0.08 | 3.6 | 0.28 | 1.54 ± 0.19 | 1.69 ± 0.14 | −8.6 | −0.87 | 0.72 ± 0.16 | 0.60 ± 0.08 | 20.7 | 1.06 |
| +2 | 0.44 ± 0.07 | 0.54 ± 0.06 | −18.4 | −1.65 | 0.98 ± 0.10 | 0.96 ± 0.10 | 2.2 | 0.21 | 1.49 ± 0.12 | 1.61 ± 0.16 | −7.8 | −0.87 | 0.73 ± 0.11 | 0.64 ± 0.09 | 14.9 | 0.93 | |
| +1 | 0.42 ± 0.06 | 0.49 ± 0.05 | −14.1 | −1.31 | 0.97 ± 0.13 | 0.97 ± 0.11 | −0.1 | −0.00 | 1.44 ± 0.15 | 1.55 ± 0.15 | −7.2 | −0.73 | 0.74 ± 0.12 | 0.69 ± 0.10 | 8.0 | 0.51 | |
| LSE | 0.40 ± 0.05 | 0.46 ± 0.05 | −13.4 | −1.35 | 0.95 ± 0.16 | 0.97 ± 0.11 | −1.4 | −0.10 | 1.37 ± 0.19 | 1.48 ± 0.15 | −7.6 | −0.66 | 0.74 ± 0.14 | 0.71 ± 0.10 | 5.2 | 0.30 | |
| −1 | 0.38 ± 0.05 | 0.42 ± 0.05 | −8.6 | −0.73 | 0.97 ± 0.14 | 0.98 ± 0.12 | −1.4 | −0.11 | 1.37 ± 0.18 | 1.46 ± 0.20 | −5.8 | −0.45 | 0.76 ± 0.13 | 0.74 ± 0.10 | 2.9 | 0.19 | |
| −2 | 0.39 ± 0.06 | 0.42 ± 0.09 | −7.4 | −0.40 | 0.96 ± 0.15 | 0.96 ± 0.15 | 0.6 | 0.04 | 1.38 ± 0.21 | 1.42 ± 0.21 | −2.5 | −0.17 | 0.75 ± 0.14 | 0.73 ± 0.15 | 3.6 | 0.18 | |
| WM ventral | +3 | 0.45 ± 0.07 | 0.50 ± 0.05 | −9.4 | −0.81 | 0.99 ± 0.16 | 0.98 ± 0.09 | 0.9 | 0.07 | 1.52 ± 0.22 | 1.59 ± 0.19 | −4.6 | −0.36 | 0.72 ± 0.15 | 0.67 ± 0.06 | 7.4 | 0.46 |
| +2 | 0.43 ± 0.06 | 0.46 ± 0.04 | −7.2 | −0.65 | 0.95 ± 0.08 | 0.93 ± 0.09 | 2.1 | 0.23 | 1.44 ± 0.12 | 1.47 ± 0.18 | −1.9 | −0.18 | 0.71 ± 0.08 | 0.67 ± 0.06 | 6.5 | 0.59 | |
| +1 | 0.40 ± 0.05 | 0.42 ± 0.05 | −4.7 | −0.40 | 0.92 ± 0.11 | 0.93 ± 0.08 | −0.5 | −0.05 | 1.35 ± 0.15 | 1.39 ± 0.13 | −3.0 | −0.30 | 0.71 ± 0.09 | 0.69 ± 0.07 | 2.0 | 0.17 | |
| LSE | 0.40 ± 0.05 | 0.40 ± 0.05 | 0.9 | 0.07 | 0.92 ± 0.12 | 0.88 ± 0.06 | 3.9 | 0.38 | 1.35 ± 0.17 | 1.31 ± 0.13 | 2.8 | 0.25 | 0.71 ± 0.11 | 0.67 ± 0.05 | 5.0 | 0.43 | |
| −1 | 0.39 ± 0.07 | 0.39 ± 0.05 | 0.2 | 0.01 | 0.92 ± 0.11 | 0.87 ± 0.08 | 5.4 | 0.50 | 1.33 ± 0.14 | 1.27 ± 0.16 | 4.7 | 0.40 | 0.71 ± 0.11 | 0.67 ± 0.06 | 6.1 | 0.48 | |
| −2 | 0.37 ± 0.09 | 0.36 ± 0.09 | 2.9 | 0.12 | 0.88 ± 0.12 | 0.86 ± 0.07 | 1.4 | 0.13 | 1.24 ± 0.14 | 1.23 ± 0.15 | 0.9 | 0.08 | 0.70 ± 0.13 | 0.68 ± 0.07 | 1.9 | 0.13 | |
- A positive segment indicates a rostral direction from the lumbosacral enlargement (LSE) landmark. Due to missing values, DTI metrics were not available for all participants in certain segments. The number of participants for segment “LSE+3” was as follows: patients n = 16 and controls n = 23; for segment “LSE+2”: patients n = 18 and controls n = 27. Effect sizes (Cohen's d) of 0.2, 0.5, and 0.8 represent small, medium, and large effects, respectively.
- Diff, difference; DTI, diffusion tensor imaging; SD, standard deviation; WM, white matter.
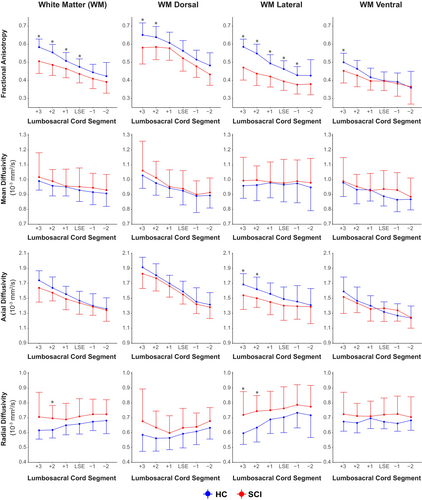
Segment-wise DTI results for patients (N = 20) and controls (N = 27) are shown in Table 2. Corresponding line plots for the complete case group are shown in Fig. 2. Further details on the results of linear mixed-effects model analysis are reported in the Table S3.
Cross-sectional area
Cross-sectional area analysis revealed no statistically significant differences between patients with SCI and healthy controls for SC, GM, and WM at any lumbosacral cord segment (Table 3, Table S4). Supplementary analyses restricted to the complete case group (Fig. 3) and focused on the restrictive traumatic group also showed no statistically significant group differences (Table S5).
| Segment | Patients | Controls | Diff (%) | Effect size | |||
|---|---|---|---|---|---|---|---|
| CSA (mm2) mean ± SD | (N) | CSA (mm2) mean ± SD | (N) | ||||
| Spinal Cord | +3 | 50.0 ± 5.3 | (16) | 49.2 ± 6.2 | (27) | 1.7 | 0.14 |
| +2 | 54.7 ± 6.5 | (19) | 54.8 ± 6.1 | (27) | −0.2 | −0.02 | |
| +1 | 57.9 ± 6.5 | (21) | 59.3 ± 5.9 | (27) | −2.5 | −0.24 | |
| LSE | 57.9 ± 6.2 | (21) | 58.6 ± 6.4 | (27) | −1.1 | −0.10 | |
| −1 | 48.1 ± 5.7 | (21) | 47.5 ± 6.6 | (27) | 1.2 | 0.09 | |
| −2 | 30.5 ± 6.9 | (21) | 28.6 ± 6.3 | (27) | 6.8 | 0.30 | |
| −3 | 16.2 ± 4.3 | (21) | 14.2 ± 3.6 | (27) | 13.9 | 0.50 | |
| −4 | 7.8 ± 2.5 | (21) | 6.2 ± 1.8 | (27) | 25.1 | 0.74 | |
| −5 | 5.1 ± 2.3 | (21) | 3.9 ± 1.2 | (27) | 30.6 | 0.66 | |
| Gray Matter | +3 | 16.8 ± 2.4 | (14) | 16.0 ± 2.5 | (26) | 4.7 | 0.31 |
| +2 | 19.2 ± 3.2 | (18) | 19.4 ± 2.9 | (27) | −1.4 | −0.09 | |
| +1 | 22.4 ± 3.1 | (21) | 22.9 ± 3.1 | (27) | −2.3 | −0.17 | |
| LSE | 24.9 ± 3.3 | (21) | 25.8 ± 3.1 | (27) | −3.5 | −0.28 | |
| −1 | 21.2 ± 2.6 | (21) | 21.9 ± 3.1 | (27) | −3.1 | −0.24 | |
| −2 | 13.3 ± 2.9 | (21) | 12.9 ± 3.3 | (27) | 3.3 | 0.13 | |
| −3 | 6.9 ± 2.0 | (21) | 6.1 ± 1.9 | (27) | 12.9 | 0.40 | |
| −4 | 2.8 ± 1.0 | (21) | 2.2 ± 0.9 | (27) | 30.8 | 0.68 | |
| −5 | 1.5 ± 0.9 | (20) | 1.1 ± 0.6 | (27) | 42.2 | 0.60 | |
| White Matter | +3 | 33.1 ± 3.6 | (14) | 32.9 ± 4.4 | (26) | 0.5 | 0.04 |
| +2 | 35.4 ± 4.0 | (18) | 35.3 ± 3.8 | (27) | 0.2 | 0.02 | |
| +1 | 35.4 ± 3.8 | (21) | 36.4 ± 3.7 | (27) | −2.6 | −0.25 | |
| LSE | 33.0 ± 3.7 | (21) | 32.8 ± 4.0 | (27) | 0.8 | 0.07 | |
| −1 | 26.9 ± 3.7 | (21) | 25.6 ± 4.0 | (27) | 4.9 | 0.33 | |
| −2 | 17.2 ± 4.2 | (21) | 15.7 ± 3.2 | (27) | 9.8 | 0.42 | |
| −3 | 9.3 ± 2.5 | (21) | 8.1 ± 1.9 | (27) | 14.7 | 0.54 | |
| −4 | 5.0 ± 1.7 | (21) | 4.1 ± 1.0 | (27) | 23.2 | 0.70 | |
| −5 | 3.6 ± 1.6 | (20) | 2.8 ± 0.8 | (27) | 28.7 | 0.66 | |
- A positive segment indicates a rostral direction from the lumbosacral enlargement (LSE) landmark. N = number of participants for each segment. Effect sizes (Cohen's d) of 0.2, 0.5, and 0.8 represent small, medium, and large effects, respectively.
- CSA, cross-sectional area; Diff, difference; SD, standard deviation.

Infralesional MRI metrics in relation to clinical and functional tract-specific impairment
The segment with the most robust group differences and no missing values (LSE+1) was used to assess the relationship between DTI metrics and clinical and electrophysiological outcome measures in patients with SCI. In the lateral WM columns, higher FA values were positively associated with ranked LEMS (b = 0.0022, 95% CI: 0.0011, 0.0034, P < 0.001, marginal R2 = 0.17) (Fig. 4A). Also, if accounting for other variables such as age, sex, and LEPP (marginal R2 = 0.32), LEMS remained the largest contributor in explaining variability in FA within the lateral columns (Table S6). As an alternative to LEMS, we also tested MEPs reflecting the integrity of the lateral corticospinal tract. The ranked amplitude of tibial nerve MEPs explained a statistically significant amount of the variance among FA values (b = 0.0016, CI: 0.0004, 0.0028, P = 0.014, marginal R2 = 0.07) (Fig. 4B), and the ranked amplitude of abductor hallucis MEPs showed a trend (b = 0.0011, CI: −0.0002, 0.0024, P = 0.098, marginal R2 = 0.04). Explorative analysis showed that the highest amount of variance in FA explained by fixed factors was achieved when incorporating ranked LEMS, age, and sex into the model (marginal R2 = 0.33).
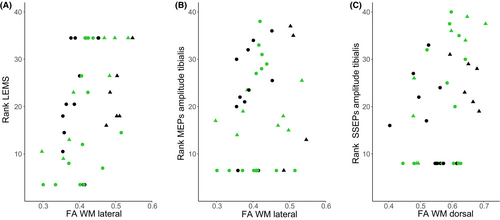
In the dorsal WM columns, higher FA values were positively associated with ranked amplitude of tibial nerve SSEPs (b = 0.0021, CI: 0.0001, 0.0041, P = 0.048, marginal R2 = 0.11) (Fig. 4C) and showed a trend for ranked LELT (b = 0.0021, CI: −0.0001, 0.0047, P = 0.051, marginal R2 = 0.11). The model with the highest amount of variance explained by multiple fixed factors included ranked amplitude of tibial nerve SSEPs, ranked LELT, age, and sex (marginal R2 = 0.30).
Within the whole WM, higher FA values were positively associated with ranked LEMS (b = 0.0043, CI: 0.0005, 0.0081, P = 0.028, adjusted R2 = 0.20) and showed a trend for ranked LELT (b = 0.0040, CI: −0.0003, 0.0082, P = 0.068, adjusted R2 = 0.13). For electrophysiological measures, a positive association was shown for the ranked amplitude of tibial nerve SSEPs for FA (b = 0.0040, CI: 0.0000, 0.0080, P = 0.048, adjusted R2 = 0.16) and a negative association for RD (b = −0.0081, CI: −0.0157, −0.0004 × 10−3 mm2/s, P = 0.040, adjusted R2 = 0.17). In addition, explorative analysis for the highest proportion of variance in FA explained by multiple fixed factors showed that the highest value was for the combination of ranked LEMS, age and sex (adjusted R2 = 0.33).
Discussion
We investigated remote degeneration in the lumbosacral cord one month after cervical or thoracic spinal cord injury (SCI) with a sudden onset. We observed signs of neurodegeneration using diffusion MRI in the lateral and dorsal white matter (WM) columns at and rostral to the center of the lumbosacral enlargement (LSE), but not yet any indication of atrophy using cross-sectional area (CSA) measurements. The tract-specific tissue integrity was associated with corresponding SCI-induced impairment reflected by clinical and electrophysiological measures.
Remote neurodegeneration along the spinal cord axis
At and rostral to the LSE landmark, we found decreased axial diffusivity (AD) and increased radial diffusivity (RD), with an overall reduction of fractional anisotropy (FA) in the WM of patients with SCI compared to healthy controls. Diffusion tensor imaging (DTI) metrics are sensitive but not specific markers of neurodegenerative processes; however, a decrease in AD has been shown to be more indicative of axonal degeneration, while an increase in RD is more indicative of demyelination.46-49
Although the lumbosacral cord segments used here do not correspond one-to-one to individual neurological levels, they allow for a consistent comparison of levels across participants. Considering literature values, it can be approximated that the segments from the LSE landmark and rostral to it incorporate the L1 to L4 neurological levels.43 Based on our results, this suggests that WM columns at neurological levels important for locomotion and lower urinary tract function undergo neurodegeneration early after SCI. The degree of neurodegeneration within the first month after injury, without actual tissue loss, was in line with previous studies, showing remote neurodegeneration of WM columns at the LSE landmark approximately 2 months after injury4 and also in the chronic stage.5
In healthy controls, FA, AD, and RD show a rostral-caudal gradient with higher FA and AD and lower RD values in rostral segments. It has been hypothesized that this gradient is due to greater proportions of densely organized axons with large diameters in more rostral regions.50
We found no group differences in CSA, suggesting that the amount of gray matter (GM) and WM tissue remote from the injury site is still preserved in a cohort of relatively mildly impaired patients after SCI (>75% AIS D). This finding is not surprising, considering the early timepoint after SCI; the earliest evidence of atrophy in the lumbosacral cord following a cervical or thoracic injury has been demonstrated approximately two months post-injury.4 The observation that differences in DTI metrics are detectable indicates that DTI is more sensitive than CSA measurements in detecting early neurodegenerative processes after SCI, and that neurodegeneration remote from the injury site precedes tissue loss. Indeed, preclinical and post-mortem studies found that axonal degeneration and myelin sheath disintegration occur before the removal of axonal and myelin debris by phagocythosis.32, 33
Remote neurodegeneration in the spinal cord cross-section
In lumbosacral cord segments at the LSE landmark and rostral to it, we observed the most pronounced differences in the lateral WM columns (FA: −13.4% to −19.5%, AD: −7.2% to −8.6%, RD: 5.2% to 20.7%), where predominantly efferent motor tracts such as the corticospinal tract are located. In contrast, differences in the dorsal WM columns, where predominantly afferent sensory afferent tracts are located, were less pronounced (FA: −4.8% to −10.8%, AD: −1.4% to −4.4%, RD: 4.4% to 15.6%). These findings are consistent with preclinical evidence, which suggests that after SCI, efferent tracts (descending, e.g., corticospinal tract) predominantly undergo relatively fast and far-reaching anterograde (Wallerian) degeneration caudal to the lesion site. Mechanisms of degeneration in afferent (ascending) tracts are less established but might include retrograde axonal dieback. However, since axonal dieback has been reported to be rather limited to short distances from the injury site,6-9 other processes of remote degeneration might be involved. Overall, the extent of neurodegeneration was comparable to a study that reported DTI metrics in patients with SCI at a later timepoint (2 months after injury), which showed a −10.3% difference in FA in the lateral WM columns at the LSE landmark.4
Relationship between MRI metrics, clinical, and tract-specific outcomes
Despite the early timepoint of the investigation following SCI, we demonstrated an association between impaired tract-specific WM tissue integrity (e.g., lower FA) within the lumbosacral cord and lower motor and sensory scores of the lower extremities. Similar associations between remote changes in diffusivity at the LSE landmark and clinical impairment have also been reported in patients with SCI at the chronic stage.5 Regarding electrophysiological measures, somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs) are considered objective readouts for the functional integrity of spinal pathways.45 Indeed, we showed that impaired tissue integrity in the lateral and dorsal columns was associated with lower amplitudes of MEPs and SSEPs, respectively. The relation between amplitudes of SSEPs and dorsal column FA values at the LSE landmark has been previously reported in patients with chronic cervical SCI.51
Furthermore, a considerable amount of variance was explained by sex even when controlling for severity scores such as the lower extremity motor score (LEMS). This is most likely an effect specific to our sample, where female patients with SCI showed higher FA values than males, while no such association was observed in healthy controls. All female patients were mildly impaired (AIS D), which excludes the possibility of using the AIS scale to control for the severity of SCI.
Limitations
The study included a rather heterogeneous cohort of patients in terms of type (traumatic and non-traumatic) and level of injury (cervical and thoracic). Hence, additional analyses of more homogenous subgroups (excluding non-traumatic and patients with preexisting neurological symptoms) were performed, which showed comparable results. This points towards the robustness of our results; however, further studies are needed to replicate these results. Furthermore, clinical scores, such as LEMS, were on the upper end of the scale, close to the values of healthy volunteers, which limits the effectiveness of correlation analyses with MRI metrics and constrains the generalizability of the findings. For instance, we would anticipate more pronounced remote neurodegeneration in a cohort with more severely affected patients (e.g., AIS A). Additionally, DTI metrics could not be extracted from the caudal half of the CM, as this region is not covered by the PAM50 atlas.
Conclusion
In conclusion, this MRI investigation of the lumbosacral cord in patients with SCI demonstrates first signs of remote degeneration, without tissue loss, as early as one month after injury. Together with existing evidence, it highlights that remote degeneration precedes tissue loss. Our results suggest relevant structural deteriorations in remote areas, below the level of lesion, which may highlight the importance of early interventions before gross anatomical changes potentially lead to irreversible neuroplastic alterations. The MRI measures applied in this study can be utilized to monitor neuroplasticity (e.g., neurodegenerative and regenerative processes) throughout neurorehabilitation and in relation to therapeutic or neuromodulatory interventions, such as tibial nerve stimulation.
Author Contributions
S.B., T.K., P.F., G.D., and M.L. contributed to the conception and design of the study. S.B., C.A., V.B., O.G., C.K., L.L., N.M., U.M., R.R., S.S., S.V., C.Z., G.D., and M.L. contributed to the acquisition and analysis of data. S.B., G.D., and M.L. contributed to drafting a significant portion of the manuscript and preparing the figures. All authors reviewed the manuscript critically for intellectual content and approved the final version.
Acknowledgements
We thank all the volunteers who participated in this study. We also thank the entire clinical team of the Department of Neuro-Urology, Balgrist University Hospital, University of Zürich for recruitment and clinical assessments. Additionally, we thank all members of the TASCI Study Group for their support. Imaging was performed with support of the Swiss Center for Musculoskeletal Imaging, SCMI, Balgrist Campus AG, Zürich. This work was financially supported by the Swiss National Science Foundation (SNSF, grant number: 33IC30_179644) and the Swiss Paraplegic Foundation. PF was funded by an SNSF Eccellenza Professorial Fellowship grant (PCEFP3_181362/1).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




