Blended phenotype of TECPR2-associated hereditary sensory-autonomic neuropathy and Temple syndrome
Abstract
Uniparental isodisomy (UPiD) can cause mixed phenotypes of imprinting disorders and autosomal-recessive diseases. We present the case of a 3-year-old male with a blended phenotype of TECPR2-related hereditary sensory and autonomic neuropathy (HSAN9) and Temple syndrome (TS14) due to maternal UPiD of chromosome 14, which includes a loss-of-function founder variant in the TECPR2 gene [NM_014844.5: c.1319del, p.Leu440Argfs*19]. This case illustrates challenges associated with a mixed phenotype of ultra-rare disorders and underscores the importance of investigating recessive conditions in homozygosity regions when atypical clinical features occur in patients with well-characterized imprinting disorders.
Introduction
Chromosome 14 harbors an imprinted locus at 14q32. Uniparental isodisomy (UPiD) of maternal and paternal origins results in distinct phenotypes. Maternal UPiD, which leads to the absence of paternally expressed genes on chromosome 14q32, causes Temple syndrome (TS14). This rare syndrome results from dysregulation of imprinted genes in the 14q32 region, particularly affecting key developmental regulators, such as MEG3 and DLK1, and is characterized by dysmorphisms, pre- and postnatal growth restrictions, and feeding difficulties, hypotonia, and global developmental delay.1-4
TECPR2-related hereditary sensory and autonomic neuropathy (HSAN9) is an autosomal-recessive disorder caused by biallelic loss-of-function variants in the TECPR2 gene. The clinical spectrum includes an early-onset and progressive sensory and autonomic neuropathy, significant central apneas, hypotonia, impaired gait, global developmental delay with later intellectual disability, and bulbar dysfunction characterized by dysphagia and dysarthria with frequent recurring respiratory infections.5, 6 Two founder variants have been identified: c.3416delT (p.Leu1139ArgfsTer75) in Bukharian Jews (1.33% carrier frequency) and c.1319delT (p.Leu440ArgfsTer19) in the Ashkenazi Jewish background (0.65% carrier frequency).5-7
While homozygosity typically arises from consanguinity, UPiD can also generate homozygous genomic regions, therefore unmasking recessive genetic disorders. This mechanism creates an opportunity for the simultaneous occurrence of imprinting disorders and autosomal-recessive conditions. Here, we report a unique case of a 3-year-old male presenting with a blended phenotype of TECPR2-related HSAN9 and TS14 due to maternal UPiD of chromosome 14 (UPiD(14)mat).
Methods
Clinical data were collected as part of the Registry and Natural History Study for Early Onset Hereditary Spastic Paraplegia (NCT04712812), approved at Boston Children's Hospital (IRB-P00033016). Written consent and permission to publish the images in Figure 1 were obtained. A comprehensive genetic and clinical characterization was conducted, including physical and neurological examination, developmental assessments, and molecular testing, including a SNP array and whole exome sequencing.
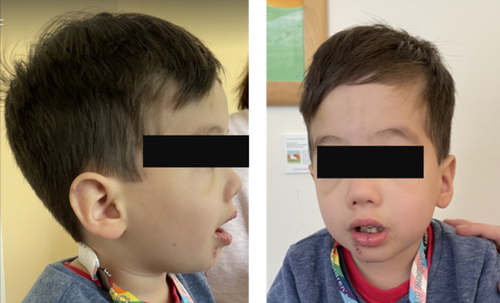
Case History
The patient, a 3-year-old male, was born as a dichorionic-diamniotic twin to a 35-year-old G1P0 mother with no reported maternal exposures. There was no reported consanguinity, and parents were of mixed ancestry (paternal: Vietnamese and Italian; maternal: Ashkenazi Jewish and Irish). The patient was born at 36 weeks' gestation via C-section due to intrauterine growth restriction and velamentous umbilical cord insertion. Birth was complicated by respiratory distress, briefly requiring non-invasive respiratory support. Physical examination in the neonatal period was notable for global hypotonia and distinctive facial features, including frontal bossing, micrognathia, a long triangular face, epicanthus, anteverted nares, and flat facial features. Anthropometric measurements indicated that the patient was small for age, with a birth weight of 1.48 kg (<10th percentile) and a length of 47 cm. Due to significant feeding difficulties, the patient was admitted to the NICU for 6 weeks, during which a gastrostomy tube (G-tube) was placed, and parenteral nutrition was initiated.
Newborn screening and metabolic screening laboratories were normal. However, given ongoing concerns for an underlying genetic etiology based on the distinctive facial features, a single-nucleotide polymorphism (SNP) array was performed. This revealed maternal isodisomy of chromosome 14q32 leading to a diagnosis of TS14, supported by the patient's characteristic phenotype.
Following discharge from the NICU, the patient was monitored by multiple specialists for short stature and failure to thrive. Nutritional support via the gastrostomy tube was continued, and growth hormone therapy was initiated at 6 months of age. Hypotonia and global developmental delay remained significant and were considered atypical in severity for TS14. Alongside profound hypotonia, the patient exhibited complete areflexia in the lower limbs, which is not typically seen in TS14. Dysphagia and hypotonia improved over time, and the patient's gastrostomy tube was removed at the age of 2 years, following a normal swallow study with no subsequent episodes of aspiration. At 2.5 years, he underwent bilateral strabismus correction. A sleep study indicated obstructive sleep apnea (but no central apneas), leading to the initiation of nocturnal BiPAP, which resolved after an adenoidectomy. By around the age of 3 years, he began walking short distances with assistance, using ankle-foot orthoses or a standing walker.
At last follow-up at the age of 3.5 years, developmental milestones remained significantly delayed in all domains. The patient's fine motor assessment indicated that he was beginning to develop the ability to perform a mature pincer grasp and provide some assistance with dressing. Expressive language was delayed, with only a few spoken words and approximately 10 words in sign language. Significant concerns included behavioral issues, such as daily aggressive episodes, self-injurious behavior (e.g., head banging), and impulsive behavior. The severity of behavioral dysregulation was notable and beyond what is commonly observed in TS14.
On physical examination, the patient presented with distinct facial features, most prominently micrognathia (Fig. 1). Motor examination showed significant axial hypotonia with joint hyperlaxity in major joints, including elbow and knees. There was no spasticity or dystonia. Deep tendon reflexes were absent in upper and lower limbs. Babinski sign was present bilaterally. The family also described a history of high pain tolerance suggesting impaired pain sensitivity, sensitivity to temperature fluctuations, easy fatigability, and frequent viral infections compared to his twin brother. On gait assessment, there was pronounced ataxia.
Given the atypical severity of symptoms and the combination of features not fully explained by TS14 alone, additional diagnostic investigations were pursued. Clinical exome sequencing was performed, revealing a pathogenic variant in the TECPR2 gene (NM_014844.5: c.1319del, p.Leu440Argfs*19) and maternally inherited in a homozygous state (chr14:102432030). This single base-pair deletion in exon 8 caused a frameshift and a premature stop codon 19 amino acids downstream, leading to an absent or truncated protein product. This variant, common in the Ashkenazi Jewish population, had been reported in multiple unrelated individuals with features of autosomal-recessive HSAN9.5, 6 Exome sequencing also confirmed the maternal origin of the UPiD previously observed on the SNP array.
This confirmed a diagnosis of TECPR2-related HSAN9 and TS14 with a blended phenotype. Table 1 summarizes the clinical features of TS14 and HSAN9 present in our patient. Notably, while the patient exhibited features of both TS14 and HSAN9, the severity of symptoms typically associated with TECPR2 mutations appeared milder than often reported in the literature.
| TECPR2 (HSAN9) | Temple syndrome (TS14) |
|---|---|
|
|
- Manifestations present in the patient are indicated in bold letters.
Discussion
This case highlights a unique case of a blended phenotype resulting from co-occurrence of TS14 and HSAN9, caused by maternally inherited UPiD of chromosome 14 covering a loss-of-function variant in TECPR2 (Table 1). TS14, a rare imprinting disorder characterized by growth restriction, hypotonia, and developmental delays, results from dysregulation of imprinted genes in the 14q32 region.2-4
Briefly, the clinical features of TS14 observed included intrauterine growth restriction (IUGR) and failure to thrive, hypotonia, global developmental delays, and characteristic facial features. Manifestations of TECPR2-associated HSAN9 included an early-onset sensory and autonomic neuropathy, behavioral dysregulation, and strabismus.
The dual diagnosis required a multidisciplinary approach to management. Growth hormone therapy was initiated to address growth deficiencies associated with TS14, while nutritional support and monitoring were essential due to feeding difficulties and dependence on G-tube and BiPAP. The patient's developmental delays necessitated ongoing early interventions, including speech, occupational, and physical therapies.
More broadly, this case illustrates the importance of comprehensive genomic testing, such as exome sequencing, in patients with atypical or severe presentations of known syndromes.8 Notable other examples include the coexistence of Prader–Willi syndrome with hereditary spastic paraplegia type 11 (SPG11) due to UPiD of chromosome 15, and Silver–Russell syndrome with hereditary spastic paraplegia type 50 (SPG50) stemming from UPiD of chromosome 7.9, 10 In our patient, the identification of the TECPR2 mutation was crucial in understanding the full spectrum of the patient's symptoms and informed a targeted management plan. The blended phenotype resulting from TS14 and HSAN9 contributes to the growing recognition of diseases arising from multilocus genomic variations. This possibility is especially notable for cases of imprinting disorders where autosomal-recessive disorders can be unmasked when clinical features extend beyond well-described syndrome and particularly for cases in populations with known founder mutations.
Acknowledgments
The authors thank the patient and the family for their support. Research in the Ebrahimi-Fakhari Laboratory is supported by the Spastic Paraplegia Foundation, the Boston Children's Hospital Translational Research Program, and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (K08NS123552). Vicente Quiroz received a fellowship from the International Parkinson and Movement Disorder Society. Luca Schierbaum received fellowship from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, 536105452).
Author Contributions
D.E.-F. designed the study and provided supervision. U.Z., K.Y., L.S., A.T., N.B., J.R., V.Q. collected and analyzed data. U.Z. and D.E.-F. drafted the original manuscript, with contributions from other authors. All authors contributed to the final draft of the manuscript.
Funding Information
Research in the Ebrahimi-Fakhari Laboratory is supported by the Spastic Paraplegia Foundation, the Boston Children's Hospital Translational Research Program, and the National Institute of Neurological Disorders and Stroke (K08NS123552). Vicente Quiroz received a fellowship from the International Parkinson and Movement Disorder Society. Luca Schierbaum received fellowship from the Deutsche Forschungsgemeinschaft (German Research Foundation, 536105452).
Conflicts of Interest
The authors declare no conflict of interest exists.
Open Research
Data Availability Statement
Data are available from the corresponding author upon reasonable request.




